
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vernon W. Dolinsky | -- | 2953 | 2023-08-21 02:56:23 | | | |
| 2 | Rita Xu | Meta information modification | 2953 | 2023-08-21 05:48:31 | | |
Video Upload Options
Pregnancy involves a range of metabolic adaptations to supply adequate energy for fetal growth and development. Gestational diabetes (GDM) is defined as hyperglycemia with first onset during pregnancy. GDM is a recognized risk factor for both pregnancy complications and long-term maternal and offspring risk of cardiometabolic disease development. Pregnancy changes maternal metabolism, GDM can be viewed as a maladaptation by maternal systems to pregnancy, which may include mechanisms such as insufficient insulin secretion, dysregulated hepatic glucose output, mitochondrial dysfunction and lipotoxicity. Adiponectin is an adipose-tissue-derived adipokine that circulates in the body and regulates a diverse range of physiologic mechanisms including energy metabolism and insulin sensitivity. In pregnant women, circulating adiponectin levels decrease correspondingly with insulin sensitivity, and adiponectin levels are low in GDM.
1. Metabolic Adaptations during Pregnancy
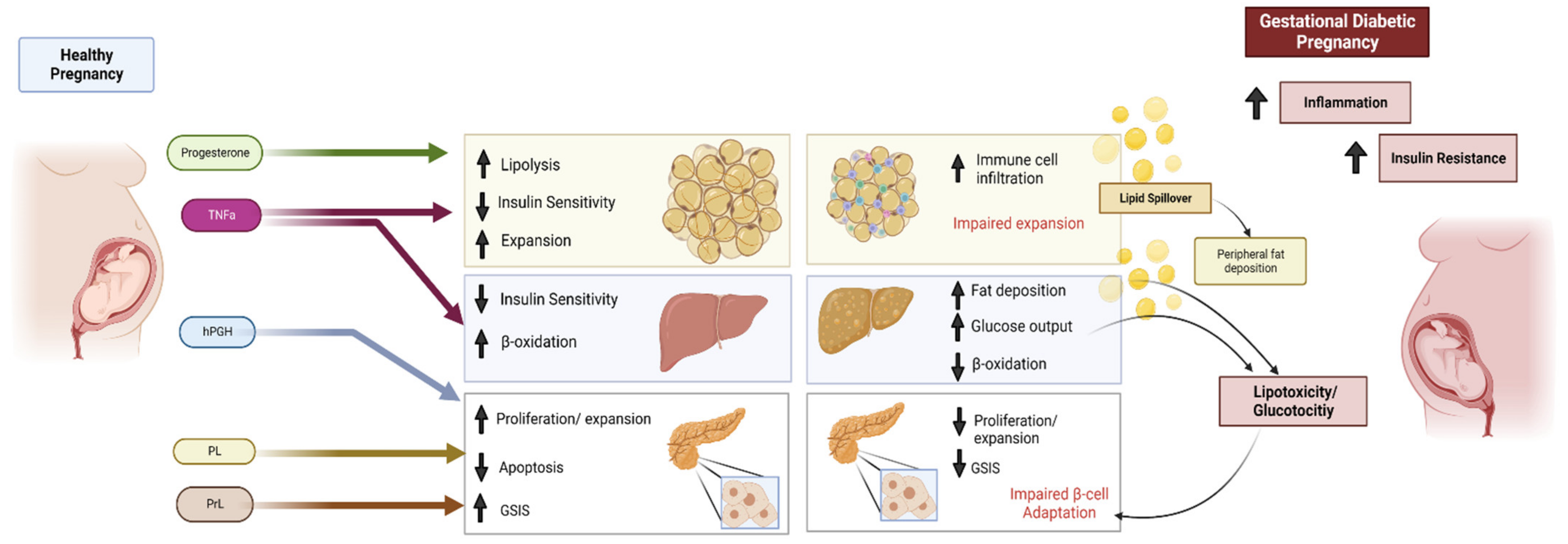
1.1. The Endocrine Pancreas and Its Adaptation to Pregnancy
1.2. White Adipose Tissue in Pregnancy
1.3. Adaptations of Liver Metabolism during Pregnancy
2. Gestational Diabetes Mellitus
2.1. Impact of GDM on the Liver
2.2. β-Cell Dysfunction in GDM
2.3. Impact of GDM on Adipose Tissue
References
- Soma-Pillay, P.; Catherine, N.-P.; Tolppanen, H.; Mebazaa, A.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94.
- Hillerer, K.M.; Jacobs, V.R.; Fischer, T.; Aigner, L. The Maternal Brain: An Organ with Peripartal Plasticity. Neural Plast. 2014, 2014, 574159.
- Vivas, Y.; Diez-Hochleitner, M.; Izquierdo-Lahuerta, A.; Corrales, P.; Horrillo, D.; Velasco, I.; Martinez-Garcia, C.; Campbell, M.; Sevillano, J.; Ricote, M.; et al. Peroxisome proliferator activated receptor gamma 2 modulates late pregnancy homeostatic metabolic adaptations. Mol. Med. 2016, 22, 724–736.
- Wharfe, M.D.; Wyrwoll, C.; Waddell, B.J.; Mark, P.J. Pregnancy-induced changes in the circadian expression of hepatic clock genes: Implications for maternal glucose homeostasis. Am. J. Physiol. Metab. 2016, 311, E575–E586.
- Elliott, J.A. The effect of pregnancy on the control of lipolysis in fat cells isolated from human adipose tissue. Eur. J. Clin. Investig. 1975, 5, 159–163.
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S.
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. Ann. Nutr. Metab. 2017, 70, 59–65.
- Herrera, E. Lipid Metabolism in Pregnancy and its Consequences in the Fetus and Newborn. Endocrine 2002, 19, 43–56.
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929.
- Handwerger, S.; Freemark, M. The Roles of Placental Growth Hormone and Placental Lactogen in the Regulation of Human Fetal Growth and Development. J. Pediatr. Endocrinol. Metab. 2000, 13, 343–356.
- Angueira, A.R.; Ludvik, A.E.; Reddy, T.E.; Wicksteed, B.; Lowe, W.L., Jr.; Layden, B.T. New Insights Into Gestational Glucose Metabolism: Lessons Learned From 21st Century Approaches. Diabetes 2015, 64, 327–334.
- Butler, A.E.; Cao-Minh, L.; Galasso, R.; Rizza, R.A.; Corradin, A.; Cobelli, C.; Butler, P.C. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 2010, 53, 2167–2176.
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409.
- Kirwan, J.P.; Varastehpour, A.; Jing, M.; Presley, L.; Shao, J.; Friedman, J.E.; Catalano, P.M. Reversal of Insulin Resistance Postpartum Is Linked to Enhanced Skeletal Muscle Insulin Signaling. J. Clin. Endocrinol. Metab. 2004, 89, 4678–4684.
- Moyce, B.L.; Dolinsky, V.W. Maternal beta-cell adaptations in pregnancy and placental signalling: Implications for gestational diabetes. Int. J. Mol. Sci. 2018, 19, 3467.
- Kalkhoff, R.K. Metabolic effects of progesterone. Am. J. Obstet. Gynecol. 1982, 142, 735–738.
- Lacasa, D.; Le Liepvre, X.; Ferre, P.; Dugail, I. Progesterone stimulates adipocyte determination and differentiation 1/sterol regulatory element-binding protein 1c gene expression. potential mechanism for the lipogenic effect of progesterone in adipose tissue. J. Biol. Chem. 2001, 276, 11512–11516.
- Nielsen, J.H. Beta cell adaptation in pregnancy: A tribute to Claes Hellerström. Upsala J. Med. Sci. 2016, 121, 151–154.
- Nielsen, J.H.; Galsgaard, E.D.; Møldrup, A.; Friedrichsen, B.N.; Billestrup, N.; Hansen, J.A.; Lee, Y.C.; Carlsson, C. Regulation of beta-cell mass by hormones and growth factors. Diabetes 2001, 50 (Suppl. S1), S25.
- Billestrup, N.; Nielsen, J.H. The stimulatory effect of growth hormone, prolactin, and placental lactogen on beta-cell proliferation is not mediated by insulin-like growth factor-I. Endocrinology 1991, 129, 883–888.
- Williams, C.; Coltart, T.M. Adipose tissue metabolism in pregnancy: The lipolytic effect of human placental lactogen. Br. J. Obstet. Gynaecol. 1978, 85, 43–46.
- Xu, J.; Zhao, Y.H.; Chen, Y.P.; Yuan, X.L.; Wang, J.; Zhu, H.; Lu, C.M. Maternal Circulating Concentrations of Tumor Necrosis Factor-Alpha, Leptin, and Adiponectin in Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Sci. World J. 2014, 2014, 926932.
- Wei, W.; Zhang, X. Expression of ADP and TNF-α in patients with gestational diabetes mellitus and its relationship with pregnancy outcomes. Exp. Ther. Med. 2020, 20, 2184–2190.
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.Y.; Jun, H.S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384.
- Azzu, V.; Vacca, M.; Virtue, S.; Allison, M.; Vidal-Puig, A. Adipose Tissue-Liver Cross Talk in the Control of Whole-Body Metabolism: Implications in Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 158, 1899–1912.
- Baetens, D.; Malaisse-Lagae, F.; Perrelet, A.; Orci, L. Endocrine Pancreas: Three-Dimensional Reconstruction Shows Two Types of Islets of Langerhans. Science 1979, 206, 1323–1325.
- Kim, A.; Miller, K.; Jo, J.; Kilimnik, G.; Wojcik, P.; Hara, M. Islet architecture: A comparative study. Islets 2009, 1, 129–136.
- Wieczorek, G.; Pospischil, A.; Perentes, E. A comparative immunohistochemical study of pancreatic islets in laboratory animals (rats, dogs, minipigs, nonhuman primates). Exp. Toxicol. Pathol. 1998, 50, 151–172.
- Van Assche, F.A.; Aerts, L.; De Prins, F. A morphological study of the endocrine pancreas in human pregnancy. BJOG Int. J. Obstet. Gynaecol. 1978, 85, 818–820.
- Ernst, S.; Demirci, C.; Valle, S.; Velazquez-Garcia, S.; Garcia-Ocaña, A. Mechanisms in the adaptation of maternal β-cells during pregnancy. Diabetes Manag. 2011, 1, 239–248.
- Sferruzzi-Perri, A.N.; Vaughan, O.R.; Haro, M.; Cooper, W.N.; Musial, B.; Charalambous, M.; Pestana, D.; Ayyar, S.; Ferguson-Smith, A.C.; Burton, G.J.; et al. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J. 2013, 27, 3928–3937.
- Zhang, Z.; Piro, A.L.; Dai, F.F.; Wheeler, M.B. Adaptive Changes in Glucose Homeostasis and Islet Function During Pregnancy: A Targeted Metabolomics Study in Mice. Front. Endocrinol. 2022, 13, 852149.
- Bonner-Weir, S.; Guo, L.; Li, W.-C.; Ouziel-Yahalom, L.; Weir, G.C.; Sharma, A. Islet Neogenesis: A Possible Pathway for Beta-Cell Replenishment. Rev. Diabet. Stud. 2012, 9, 407–416.
- Aye, I.L.; Powell, T.L.; Jansson, T. Review: Adiponectin--the missing link between maternal adiposity, placental transport and fetal growth? Placenta 2013, 34, S40–S45.
- Rawn, S.M.; Huang, C.; Hughes, M.; Shaykhutdinov, R.; Vogel, H.J.; Cross, J.C. Pregnancy Hyperglycemia in Prolactin Receptor Mutant, but Not Prolactin Mutant, Mice and Feeding-Responsive Regulation of Placental Lactogen Genes Implies Placental Control of Maternal Glucose Homeostasis1. Biol. Reprod. 2015, 93, 75.
- Vasavada, R.C.; Garcia-Ocaña, A.; Zawalich, W.S.; Sorenson, R.L.; Dann, P.; Syed, M.; Ogren, L.; Talamantes, F.; Stewart, A.F. Targeted Expression of Placental Lactogen in the Beta Cells of Transgenic Mice Results in Beta Cell Proliferation, Islet Mass Augmentation, and Hypoglycemia. J. Biol. Chem. 2000, 275, 15399–15406.
- Rieck, S.; Kaestner, K.H. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010, 21, 151–158.
- Søstrup, B.; Gaarn, L.W.; Nalla, A.; Billestrup, N.; Nielsen, J.H. Co-ordinated regulation of neurogenin-3 expression in the maternal and fetal pancreas during pregnancy. Acta Obstet. Gynecol. Scand. 2014, 93, 1190–1197.
- Qiao, L.; Saget, S.; Lu, C.; Zang, T.; Dzyuba, B.; Hay, J.W.W.; Shao, J. The Essential Role of Pancreatic α-Cells in Maternal Metabolic Adaptation to Pregnancy. Diabetes 2022, 71, 978–988.
- Luyckx, A.S.; Gerard, J.; Gaspard, U.; Lefebvre, P.J. Plasma glucagon levels in normal women during pregnancy. Diabetologia 1975, 11, 549–554.
- Morriseau, T.S.; Doucette, C.A.; Dolinsky, V.W. More than meets the islet: Aligning nutrient and paracrine inputs with hormone secretion in health and disease. Am. J. Physiol. Metab. 2022, 322, E446–E463.
- Coltart, T.M.; Williams, C. Effect of insulin on adipose tissue lipolysis in human pregnancy. BJOG Int. J. Obstet. Gynaecol. 1976, 83, 241–244.
- Pujol, E.; Proenza, A.; Llado, I.; Roca, P. Pregnancy effects on rat adipose tissue lipolytic capacity are dependent on anatomical location. Cell. Physiol. Biochem. 2005, 16, 229–236.
- Jayabalan, N.; Nair, S.; Nuzhat, Z.; Rice, G.E.; Zuñiga, F.A.; Sobrevia, L.; Leiva, A.; Sanhueza, C.; Gutiérrez, J.A.; Lappas, M.; et al. Cross Talk between Adipose Tissue and Placenta in Obese and Gestational Diabetes Mellitus Pregnancies via Exosomes. Front. Endocrinol. 2017, 8, 239.
- Rojas-Rodriguez, R.; Lifshitz, L.M.; Bellvé, K.D.; Min, S.Y.; Pires, J.; Leung, K.; Boeras, C.; Sert, A.; Draper, J.T.; Corvera, S.; et al. Human adipose tissue expansion in pregnancy is impaired in gestational diabetes mellitus. Diabetologia 2015, 58, 2106–2114.
- Rojas-Rodriguez, R.; Ziegler, R.; DeSouza, T.; Majid, S.; Madore, A.S.; Amir, N.; Pace, V.A.; Nachreiner, D.; Alfego, D.; Mathew, J.; et al. PAPPA-mediated adipose tissue remodeling mitigates insulin resistance and protects against gestational diabetes in mice and humans. Sci. Transl. Med. 2020, 12, eaay4145.
- Hoffstedt, J.; Arner, E.; Wahrenberg, H.; Andersson, D.P.; Qvisth, V.; Löfgren, P.; Rydén, M.; Thörne, A.; Wirén, M.; Palmér, M.; et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 2010, 53, 2496–2503.
- Knopp, R.H.; Herrera, E.; Freinkel, N. Carbohydrate metabolism in pregnancy. 8. Metabolism of adipose tissue isolated from fed and fasted pregnant rats during late gestation. J. Clin. Investig. 1970, 49, 1438–1446.
- Palacín, M.; Lasunción, M.A.; Asunción, M.; Herrera, E. Circulating metabolite utilization by periuterine adipose tissue in situ in the pregnant rat. Metab. Clin. Exp. 1991, 40, 534–539.
- Kirwan, J.P.; Hauguel-De Mouzon, S.; Lepercq, J.; Challier, J.-C.; Huston-Presley, L.; Friedman, J.E.; Kalhan, S.C.; Catalano, P.M. TNF-alpha Is a Predictor of Insulin Resistance in Human Pregnancy. Diabetes 2002, 51, 2207–2213.
- Lain, K.Y.; Catalano, P.M. Metabolic changes in pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948.
- Towler, M.C.; Hardie, D.G. AMP-Activated Protein Kinase in Metabolic Control and Insulin Signaling. Circ. Res. 2007, 100, 328–341.
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342.
- Guariguata, L.; Linnenkamp, U.; Beagley, J.; Whiting, D.; Cho, N. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 2014, 103, 176–185.
- Brown, J.; Alwan, N.A.; West, J.; Brown, S.; McKinlay, C.J.; Farrar, D.; Crowther, C.A. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst. Rev. 2017, 2017, CD011970.
- Hui, A.L.; Sevenhuysen, G.; Harvey, D.; Salamon, E. Barriers and coping strategies of women with gestational diabetes to follow dietary advice. Women Birth 2014, 27, 292–297.
- Buchanan, T.A.; Xiang, A.; Page, K.A. Gestational diabetes mellitus: Risks and management during and after pregnancy. Nat. Rev. Endocrinol. 2012, 8, 639–649.
- Sherifali, D.; Rabi, D.M.; McDonald, C.G.; Butalia, S.; Campbell, D.J.T.; Hunt, D.; Leung, A.A.; Mahon, J.; McBrien, K.A.; Palda, V.A.; et al. Methods. Can. J. Diabetes 2018, 42, S6–S9.
- Herath, H.; Herath, R.; Wickremasinghe, R. Gestational diabetes mellitus and risk of type 2 diabetes 10 years after the index pregnancy in Sri Lankan women—A community based retrospective cohort study. PLoS ONE 2017, 12, e0179647.
- Meek, C.L.; Lewis, H.B.; Patient, C.; Murphy, H.R.; Simmons, D. Diagnosis of gestational diabetes mellitus: Falling through the net. Diabetologia 2015, 58, 2003–2012.
- Hung, T.-H.; Hsieh, T.-T. The Effects of Implementing the International Association of Diabetes and Pregnancy Study Groups Criteria for Diagnosing Gestational Diabetes on Maternal and Neonatal Outcomes. PLoS ONE 2015, 10, e0122261.
- Rani, P.R.; Begum, J. Screening and Diagnosis of Gestational Diabetes Mellitus, Where Do We Stand. J. Clin. Diagn. Res. 2016, 10, QE01–QE04.
- Shah, B.R.; Sharifi, F. Perinatal outcomes for untreated women with gestational diabetes by IADPSG criteria: A population-based study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 116–122.
- Lowe, W.L., Jr.; Scholtens, D.M.; Kuang, A.; Linder, B.; Lawrence, J.M.; Lebenthal, Y.; McCance, D.; Hamilton, J.; Nodzenski, M.; Talbot, O.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 372–380.
- Murphy, H.R. 2020 NICE guideline update: Good news for pregnant women with type 1 diabetes and past or current gestational diabetes. Diabet. Med. 2021, 38, e14576.
- Yamamoto, J.M.; Donovan, L.E.; Feig, D.S.; Berger, H. Urgent Update—Temporary Alternative Screening Strategy for Gestational Diabetes Screening During the COVID-19 Pandemic. Can. J. Diabetes 2022, 65, 37–54.
- Keating, N.; Carpenter, K.; McCarthy, K.; Coveney, C.; McAuliffe, F.; Mahony, R.; Walsh, J.; Hatunic, M.; Higgins, M. Clinical Outcomes Following a Change in Gestational Diabetes Mellitus Diagnostic Criteria Due to the COVID-19 Pandemic: A Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 1884.
- McIntyre, H.D.; Gibbons, K.; Ma, R.C.; Tam, W.H.; Sacks, D.A.; Lowe, J.; Madsen, L.R.; Catalano, P.M. Testing for gestational diabetes during the COVID-19 pandemic. An evaluation of proposed protocols for the United Kingdom, Canada and Australia. Diabetes Res. Clin. Pract. 2020, 167, 108353.
- Agarwal, P.; Morriseau, T.S.; Kereliuk, S.M.; Doucette, C.A.; Wicklow, B.A.; Dolinsky, V.W. Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit. Rev. Clin. Lab. Sci. 2018, 55, 71–101.
- Guillemette, L.; Wicklow, B.; Sellers, E.A.; Dart, A.; Shen, G.X.; Dolinsky, V.W.; Gordon, J.W.; Jassal, D.S.; Nickel, N.; Duhamel, T.A.; et al. Intrauterine exposure to diabetes and risk of cardiovascular disease in adolescence and early adulthood: A population-based birth cohort study. Can. Med. Assoc. J. 2020, 192, E1104–E1113.
- Franks, P.W.; Looker, H.C.; Kobes, S.; Touger, L.; Tataranni, P.A.; Hanson, R.L.; Knowler, W.C. Gestational Glucose Tolerance and Risk of Type 2 Diabetes in Young Pima Indian Offspring. Diabetes 2006, 55, 460–465.
- Sellers, E.A.; Dean, H.J.; Shafer, L.A.; Martens, P.J.; Phillips-Beck, W.; Heaman, M.; Prior, H.J.; Dart, A.B.; McGavock, J.; Morris, M.; et al. Exposure to Gestational Diabetes Mellitus: Impact on the Development of Early-Onset Type 2 Diabetes in Canadian First Nations and Non–First Nations Offspring. Diabetes Care 2016, 39, 2240–2246.
- Dabelea, D.; Hanson, R.L.; Lindsay, R.S.; Pettitt, D.J.; Imperatore, G.; Gabir, M.M.; Roumain, J.; Bennett, P.H.; Knowler, W.C. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes 2000, 49, 2208–2211.
- Dart, A.B.; Ruth, C.A.; Sellers, E.A.; Au, W.; Dean, H.J. Maternal Diabetes Mellitus and Congenital Anomalies of the Kidney and Urinary Tract (CAKUT) in the Child. Am. J. Kidney Dis. 2015, 65, 684–691.
- Do, V.; Eckersley, L.; Lin, L.; Davidge, S.T.; Stickland, M.K.; Ojala, T.; Serrano-Lomelin, J.; Hornberger, L.K. Persistent Aortic Stiffness and Left Ventricular Hypertrophy in Children of Diabetic Mothers. CJC Open 2021, 3, 345–353.
- Yu, Y.; Arah, O.A.; Liew, Z.; Cnattingius, S.; Olsen, J.; Sorensen, H.T.; Qin, G.; Li, J. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: Population based cohort study with 40 years of follow-up. BMJ 2019, 367, l6398.
- Yu, Y.; Soohoo, M.; Sørensen, H.T.; Li, J.; Arah, O.A. Gestational Diabetes Mellitus and the Risks of Overall and Type-Specific Cardiovascular Diseases: A Population- and Sibling-Matched Cohort Study. Diabetes Care 2022, 45, 151–159.
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392.
- Blais, K.; Arguin, M.; Allard, C.; Doyon, M.; Dolinsky, V.W.; Bouchard, L.; Hivert, M.F.; Perron, P. Maternal glucose in pregnancy is associated with child’s adiposity and leptin at 5 years of age. Pediatr. Obes. 2021, 16, e12788.
- White, S.L.; on behalf of the UPBEAT Consortium; Pasupathy, D.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; Briley, A.L.; Seed, P.T.; Welsh, P.; Poston, L. Metabolic profiling of gestational diabetes in obese women during pregnancy. Diabetologia 2017, 60, 1903–1912.
- Choi, S.S.; Diehl, A.M. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr. Opin. Lipidol. 2008, 19, 295–300.
- Hershman, M.; Mei, R.; Kushner, T. Implications of Nonalcoholic Fatty Liver Disease on Pregnancy and Maternal and Child Outcomes. Gastroenterol. Hepatol. 2019, 15, 221–228.
- Lavrentaki, A.; Thomas, T.; Subramanian, A.; Valsamakis, G.; Thomas, N.; Toulis, K.A.; Wang, J.; Daly, B.; Saravanan, P.; Sumilo, D.; et al. Increased risk of non-alcoholic fatty liver disease in women with gestational diabetes mellitus: A population-based cohort study, systematic review and meta-analysis. J. Diabetes Its Complicat. 2019, 33, 107401.
- De Souza, L.R.; Berger, H.; Retnakaran, R.; Vlachou, P.A.; Maguire, J.L.; Nathens, A.B.; Connelly, P.W.; Ray, J.G. Hepatic fat and abdominal adiposity in early pregnancy together predict impaired glucose homeostasis in mid-pregnancy. Nutr. Diabetes 2016, 6, e229.
- Lee, S.M.; Kwak, S.H.; Koo, J.N.; Oh, I.H.; Kwon, J.E.; Kim, B.J.; Kim, S.M.; Kim, S.Y.; Kim, G.M.; Joo, S.K.; et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia 2019, 62, 238–248.
- Lee, S.M.; Hwangbo, S.; Norwitz, E.R.; Koo, J.N.; Oh, I.H.; Choi, E.S.; Jung, Y.M.; Kim, S.M.; Kim, B.J.; Kim, S.Y.; et al. Nonalcoholic fatty liver disease and early prediction of gestational diabetes mellitus using machine learning methods. Clin. Mol. Hepatol. 2022, 28, 105–116.
- Ajmera, V.H.; Gunderson, E.P.; VanWagner, L.B.; Lewis, C.E.; Carr, J.J.; Terrault, N.A. Gestational Diabetes Mellitus Is Strongly Associated with Non-Alcoholic Fatty Liver Disease. Am. J. Gastroenterol. 2016, 111, 658–664.
- Agarwal, P.; Brar, N.; Morriseau, T.S.; Kereliuk, S.M.; Fonseca, M.A.; Cole, L.K.; Jha, A.; Xiang, B.; Hunt, K.L.; Seshadri, N.; et al. Gestational Diabetes Adversely Affects Pancreatic Islet Architecture and Function in the Male Rat Offspring. Endocrinology 2019, 160, 1907–1925.
- Lorenzo, P.I.; Martín-Montalvo, A.; Vuilleumier, N.C.; Gauthier, B.R. Molecular Modelling of Islet β-Cell Adaptation to Inflammation in Pregnancy and Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2019, 20, 6171.
- Nordmann, T.M.; Dror, E.; Schulze, F.; Traub, S.; Berishvili, E.; Barbieux, C.; Böni-Schnetzler, M.; Donath, M.Y. The Role of Inflammation in β-cell Dedifferentiation. Sci. Rep. 2017, 7, 6285.
- Yang, Y.; Liu, L.; Liu, B.; Li, Q.; Wang, Z.; Fan, S.; Wang, L. Functional Defects of Regulatory T Cell Through Interleukin 10 Mediated Mechanism in the Induction of Gestational Diabetes Mellitus. DNA Cell Biol. 2018, 37, 278–285.
- Boyle, K.E.; Newsom, S.; Janssen, R.C.; Lappas, M.; Friedman, J.E. Skeletal muscle MnSOD, mitochondrial complex II, and SIRT3 enzyme activities are decreased in maternal obesity during human pregnancy and gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 2013, 98, E1601–E1609.
- Liong, S.; Lappas, M. Endoplasmic reticulum stress regulates inflammation and insulin resistance in skeletal muscle from pregnant women. Mol. Cell. Endocrinol. 2016, 425, 11–25.
- Ehses, J.A.; Perren, A.; Eppler, E.; Ribaux, P.; Pospisilik, J.A.; Maor-Cahn, R.; Gueripel, X.; Ellingsgaard, H.; Schneider, M.K.J.; Biollaz, G.; et al. Increased Number of Islet-Associated Macrophages in Type 2 Diabetes. Diabetes 2007, 56, 2356–2370.
- Sharma, R.B.; Alonso, L.C. Lipotoxicity in the Pancreatic Beta Cell: Not Just Survival and Function, but Proliferation as Well? Curr. Diabetes Rep. 2014, 14, 492.
- Morán, A.; Zhang, H.J.; Olson, L.K.; Harmon, J.S.; Poitout, V.; Robertson, R.P. Differentiation of glucose toxicity from beta cell exhaustion during the evolution of defective insulin gene expression in the pancreatic islet cell line, HIT-T15. J. Clin. Investig. 1997, 99, 534–539.
- Unger, R.H. Lipotoxicity in the Pathogenesis of Obesity-Dependent NIDDM: Genetic and Clinical Implications. Diabetes 1995, 44, 863.
- Kitamura, Y.I.; Kitamura, T.; Kruse, J.-P.; Raum, J.C.; Stein, R.; Gu, W.; Accili, D. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005, 2, 153–163.
- Tumurbaatar, B.; Poole, A.; Olson, G.; Makhlouf, M.; Sallam, H.S.; Thukuntla, S.; Kankanala, S.; Ekhaese, O.; Gomez, G.; Chandalia, M.; et al. Adipose Tissue Insulin Resistance in Gestational Diabetes. Metab. Syndr. Relat. Disord. 2017, 15, 86–92.
- Sevillano, J.; De Castro, J.; Bocos, C.; Herrera, E.; Ramos-Alvarez, M.P. Role of Insulin Receptor Substrate-1 Serine 307 Phosphorylation and Adiponectin in Adipose Tissue Insulin Resistance in Late Pregnancy. Endocrinology 2007, 148, 5933–5942.
- Oliva, K.; Barker, G.; Rice, G.E.; Bailey, M.J.; Lappas, M. 2D-DIGE to identify proteins associated with gestational diabetes in omental adipose tissue. J. Endocrinol. 2013, 218, 165–178.
- Catalano, P.A.; Ehrenberg, H. The short-and long-term implications of maternal obesity on the mother and her offspring. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1126–1133.
- Jarvie, E.; Hauguel-De-Mouzon, S.; Nelson, S.; Sattar, N.; Catalano, P.M.; Freeman, D.J. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin. Sci. 2010, 119, 123–129.
- Chen, X.; Scholl, T.O. Oxidative stress: Changes in pregnancy and with gestational diabetes mellitus. Curr. Diabetes Rep. 2005, 5, 282–288.




