Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Babak Panahi | -- | 1839 | 2023-08-18 11:57:20 | | | |
| 2 | Jason Zhu | Meta information modification | 1839 | 2023-08-21 03:58:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ehmig, J.; Engel, G.; Lotz, J.; Lehmann, W.; Taheri, S.; Schilling, A.F.; Seif Amir Hosseini, A.; Panahi, B. Diagnosis of Osteoarthritis. Encyclopedia. Available online: https://encyclopedia.pub/entry/48216 (accessed on 07 February 2026).
Ehmig J, Engel G, Lotz J, Lehmann W, Taheri S, Schilling AF, et al. Diagnosis of Osteoarthritis. Encyclopedia. Available at: https://encyclopedia.pub/entry/48216. Accessed February 07, 2026.
Ehmig, Jonathan, Günther Engel, Joachim Lotz, Wolfgang Lehmann, Shahed Taheri, Arndt F. Schilling, Ali Seif Amir Hosseini, Babak Panahi. "Diagnosis of Osteoarthritis" Encyclopedia, https://encyclopedia.pub/entry/48216 (accessed February 07, 2026).
Ehmig, J., Engel, G., Lotz, J., Lehmann, W., Taheri, S., Schilling, A.F., Seif Amir Hosseini, A., & Panahi, B. (2023, August 18). Diagnosis of Osteoarthritis. In Encyclopedia. https://encyclopedia.pub/entry/48216
Ehmig, Jonathan, et al. "Diagnosis of Osteoarthritis." Encyclopedia. Web. 18 August, 2023.
Copy Citation
Osteoarthritis (OA) is a common degenerative joint disease that affects millions of people worldwide. Magnetic resonance imaging (MRI) has emerged as a powerful tool for the evaluation and monitoring of OA due to its ability to visualize soft tissues and bone with high resolution.
osteoarthritis
magnetic resonance imaging (MRI)
joint disease
degenerative disease
1. Introduction
Diagnosis of OA is primarily based on a thorough clinical examination of the joint, and imaging has always been important in detecting joint damage. Radiography has so far played an important role in the diagnostic process even though it is limited to the assessment of osseous structures. Additionally, patients with symptomatic OA show radiographic changes in only about half of the cases [1]. Earlier stages and potentially reversible changes of the joint can be detected by magnetic resonance imaging (MRI) which allows to assess soft tissues such as cartilage, synovia, menisci, and the surrounding muscles and ligaments [2]. However, so far MRI only plays a minor role in the primary diagnosis of OA in clinical routine, even though its sensitivity to detect structural changes in the joint has been confirmed in research settings [3].
2. Radiography
Radiography, which is still the most commonly used imaging technique for OA, is usually acquired in two planes, i.e., the lateral and anterior-posterior view. It is widely available and inexpensive. In addition, weight-bearing images can be obtained [4]. Depending on the clinical facility and the clinical patient history, additional views, such as the patella view or the Rosenberg view, can be obtained to evaluate specific regions of the joint. Introduced by Kellgren and Lawrence in 1957, the grading of OA is still conducted on a four-grade scale (Figure 1). Grade 0 indicates the absence of OA-specific changes in the joint, Grade I is defined as doubtful OA-changes, and Grades II and III refer to minimal and moderate changes, respectively, and can be distinguished by the presence or absence of subchondral sclerosis. Finally, Grade IV considers severe stages of OA associated with joint deformity and severely reduced joint space width (JSW) [5].
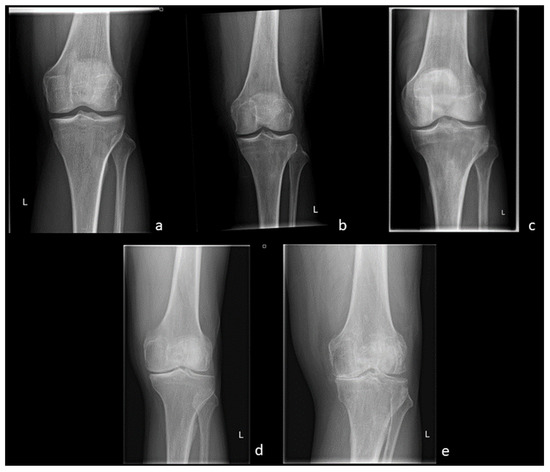
Figure 1. OA Stages (Kellgren and Lawrence, 1957)—(a) Grade 0: Physiological joint. (b) Grade I: Subtle JSN in the medial compartment with osteophytic lipping. (c) Grade II: Definite JSN in the medial compartment. (d) Grade III: Definite JSN in the medial compartment and sclerosis of the subchondral bone. (e) Grade IV: JSN with a bone-on-bone phenomenon and deformity of the medial tibial plateau as well as the medial femoral condyle.
OA grading on plain radiographs is based on the assessment of osseous tissues while cartilage thickness can only be evaluated indirectly as a measure of JSW [4]. However, MRI studies have shown that joint space narrowing (JSN) is not solely dependent on loss of cartilage thickness but can rather be considered as a composite of meniscal damage, meniscal extrusion, and cartilage damage [6].
3. MRI in Musculoskeletal Imaging
MRI is an established imaging technique available in most clinical institutions. Most available scanners have preset protocols. For the imaging of cartilage, 1.5 T and 3 T scanners do not differ in sensitivity for detecting pathologies. As imaging at higher field strengths results in a higher signal-to-noise ratio (SNR), spatial resolution, accuracy, and specificity are increased at 3 T [7]. Furthermore, acquisition time can be reduced at 3 T. However, it should be noted that if orthopedic hardware is implanted close to or in the imaged region, higher field strengths can lead to an increase in susceptibility artifacts caused by magnetic field inhomogeneities [8].
In addition to standard high-field MRI (HF-MRI) systems which typically operate at field strengths of 1.5 T and 3.0 T, low-field MRI (LF-MRI) systems have recently gained new attention. LF-MRI systems are available in two main configurations: standard large-bore machines and dedicated extremity scanners. Dedicated extremity scanners have demonstrated several advantages, including reduced noise and high patient comfort, making them an attractive option for focused joint examinations. In addition, these scanners have a more economical profile and offer a degree of portability, facilitating their use in different clinical settings [9][10][11].
Historically, LF-MRI has faced challenges in competing with HF-MRI regarding image resolution and contrast, limiting its diagnostic utility. However, innovative imaging protocols that exploit the unique characteristics of low-field strengths, such as shortened T1 times and longer T2 and T2* times, have significantly improved image quality. Low SNR can be addressed by applying multiple averaging which effectively increases the overall quality of LF-MRI images at the expense of longer acquisition times [10].
Despite these advances, few studies have directly compared contemporary LF-MRI systems to HF-MRI counterparts in the musculoskeletal domain. Early evidence suggests that LF-MRI performs comparably to HF-MRI in the examination of the ankle, foot, and knee. The results for shoulder imaging have been somewhat inconsistent, with certain studies reporting more management-changing results with HF-MRI. LF-MRI may be particularly suitable for acute injuries, but its sensitivity for smaller, chronic abnormalities may be limited due to lower resolution. A notable advantage of LF-MRI is its reduced susceptibility to artefacts from orthopedic hardware [10][11][12].
To ensure that patients receive accurate and high-quality diagnostic images for effective musculoskeletal management, decisions regarding the best-suited imaging modality should be made by experienced personnel. As more studies are warranted to comprehensively compare LF-MRI and HF-MRI, ongoing advancements in LF-MRI may shape the future of musculoskeletal imaging, contributing to improved outcomes.
In addition, image quality depends on the choice of the receiver coil. Lutterbey et al. demonstrated that even in a high-field-strength scanner the image quality can be impeded by a wrong choice of coil [13]. The use of a dedicated surface coil is recommended. Furthermore, multichannel coils allow for parallel imaging, which can be used to obtain better image quality or shorter acquisition time [14].
4. MR Acquisition Protocols—The Current Standard of Clinical Care
Despite the paradigm shift towards a more holistic view of OA, the current state of imaging protocols remains tailored to evaluate the internal structures of the knee, rather than the entire joint.
The current guidelines for MRI of the knee as published by the European Society of Skeletal Radiology (ESSR) suggest the acquisition of fat-saturated proton density-weighted (PDw FS) images in three standard planes as well as a T1-weighted (T1w) image in a sagittal or coronal orientation.
After correct placement of the patient and the receiver coil (Figure 2), the workflow starts with the acquisition of three-plane localizers. Axial images are planned parallel to the knee joint line, while sagittal planes are planned parallel to the medial facet of the lateral condyle. Coronal planes are arranged parallel to the posterior facets of the femoral condyles. A contrast-enhanced T1w FS image may be added as an option if inflammation, such as synovitis or osteitis, or malignancy is suspected. The ESSR (Table 1) recommends a slice thickness of 3 mm for all images. The field of view (FOV) for the PDw images is recommended to be 16 cm with a matrix of 288 × 384. T1w-images should be acquired at an FOV of 18 cm with a matrix of 358 × 512 [15][16].
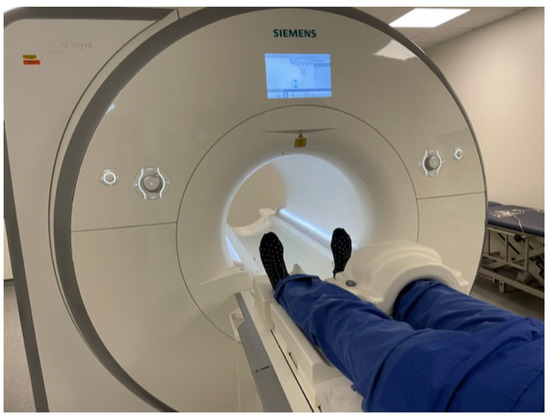
Figure 2. Patients enter the scanner feet first in a supine position. The coil is placed around the knee and the joints are immobilized with adequate padding.
Table 1. Standard knee protocol as recommended by the ESSR.
| Sequence | FOV | Slice Thickness | TR | TE | Matrix |
|---|---|---|---|---|---|
| Sag PD FS | 160 | 3 | 3570 | 39 | 288 × 384 |
| Cor PD FS | 160 | 3 | 3570 | 39 | 288 × 384 |
| Ax PD FS | 160 | 3 | 3570 | 39 | 288 × 384 |
| Cor/Sag T1 | 180 | 3 | 470 | 13 | 358 × 512 |
| Optional CE T1 FS | 180 | 3 | 470 | 13 | 358 × 512 |
The acquisition of three planes is very important to ensure each compartment can be adequately evaluated. Finally, articular cartilage is best assessed in an orthogonal image. For example, while evaluation of retropatellar cartilage is limited on coronal images due to slice thickness and partial volume effects, it is easily conducted on axial or sagittal images. PDw-images allow detailed assessment of intra-articular cartilage and internal cartilage composition. However, some institutions prefer intermediate-weighted images by selecting a slightly longer echo time (TE) to benefit from the advantages of T2-weighted (T2w) sequences. These are less susceptible to magic angle artifacts and improve delineation between cartilage and synovial fluid. The overall signal on intermediate-weighted images is increased compared to T2w images [17][18]. Additional T1w images are useful for evaluation of the general anatomy, bone marrow lesions (BML), and subchondral sclerosis, as well as the search for loose bodies, that may cause locking of the joint [19]. As the T1w-images are the only non-fat-suppressed images in a standard acquisition protocol, they are particularly useful for evaluation of bone marrow and muscles that are physiologically separated by fatty tissues.
5. Fat Suppression
Fat suppression can be useful to increase contrast at the osteochondral junction. Techniques include fat saturation (FS), inversion recovery (IR) imaging, and in- and opposed-phase imaging.
FS uses a fat-specific 90° pulse immediately before the imaging sequence, tipping the protons in fatty tissues out of plane and suppressing their signal. FS relies on a homogeneous magnetic field and is very sensitive to inhomogeneities such as those caused by metallic implants. Fat suppression also has limitations owing to the signal from water in adipose tissue, and from a minor portion of fatty acids that have a resonance frequency equal to that of water. Fat saturation pulses are administered for approximately 10 ms, which can lead to a substantial increase in acquisition time. At low field strength, the difference in resonance frequencies between water and fat is reduced, resulting in incorrect tissue discrimination. Since radiofrequency pulses are tissue-specific, they can be used universally even after administration of a contrast agent [20].
IR-imaging achieves fat suppression by inverting spins along the z-axis and imaging at a specific time point where the protons of fatty tissues have no longitudinal magnetization at all. This technique is fast and less prone to magnetic field inhomogeneities [19]. However, the overall SNR is reduced and tissues with similar T1 compared to fat are equally suppressed.
In- and opposed-phase imaging is based on the differing precession frequencies of fat and water protons. Immediately after excitation, protons are in-phase and start dephasing until they reach a 180° dephasation. Echo time can be adapted in order to either acquire in-phase images with signal from both fat and water or opposed-phase images that show a subtraction of the two [20]. The Dixon technique is based on acquisition of both types of images and results in a pure water or pure fat image achieved by addition and subtraction of in- and opposed-phase images. Although sensitive to small amounts of fat, this technique does not suppress the signal from pure fat and is sensitive to magnetic field inhomogeneites [21][22].
Most commercially available MRI scanners today have field strengths of either 1.5 or 3.0 T, placing them in the high-field range. This allows sufficient accuracy of fat-specific radiofrequency pulses and makes FS the most commonly used technique for MRI of the joint with preserved efficacy on contrast-enhanced scans. Given its sensitivity to magnetic field inhomogeneities, IR should be considered as an alternative in the presence of metallic implants. Moreover, IR can be chosen as an effective fat-suppression technique in low-field systems. The Dixon technique can be used for the detection of small amounts of fat as well as for fat quantification, which may be helpful in case of tumors or neuromuscular disorders. In this case, as explained above, two images must be acquired, which doubles the acquisition time.
References
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285.
- Guermazi, A.; Niu, J.; Hayashi, D.; Roemer, F.W.; Englund, M.; Neogi, T.; Aliabadi, P.; McLennan, C.E.; Felson, D.T. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study). BMJ 2012, 345, e5339.
- Roemer, F.W.; Guermazi, A.; Demehri, S.; Wirth, W.; Kijowski, R. Imaging in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 913–934.
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126.
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502.
- Crema, M.D.; Nevitt, M.; Guermazi, A.; Felson, D.; Wang, K.; Lynch, J.; Marra, M.D.; Torner, J.; Lewis, C.; Roemer, F. Progression of cartilage damage and meniscal pathology over 30 months is associated with an increase in radiographic tibiofemoral joint space narrowing in persons with knee OA—The MOST study. Osteoarthr. Cartil. 2014, 22, 1743–1747.
- Kijowski, R.; Blankenbaker, D.G.; Davis, K.W.; Shinki, K.; Kaplan, L.D.; De Smet, A.A. Comparison of 1.5- and 3.0-T MR Imaging for Evaluating the Articular Cartilage of the Knee Joint. Radiology 2009, 250, 839–848.
- Masi, J.N.; Sell, C.A.; Phan, C.; Han, E.; Newitt, D.; Steinbach, L.; Majumdar, S.; Link, T.M. Cartilage MR Imaging at 3.0 versus That at 1.5 T: Preliminary Results in a Porcine Model. Radiology 2005, 236, 140–150.
- Arnold, T.C.; Freeman, C.W.; Litt, B.; Stein, J.M. Low-field MRI: Clinical promise and challenges. J. Magn. Reson. Imaging 2023, 57, 25–44.
- Khodarahmi, I.M.; Keerthivasan, M.B.; Brinkmann, I.M.; Grodzki, D.; Fritz, J. Modern Low-Field MRI of the Musculoskeletal System. Investig. Radiol. 2023, 58, 76–87.
- Sutter, R.; Tresch, F.; Buck, F.M.; Pfirrmann, C.W.A. Is Dedicated Extremity 1.5-T MRI Equivalent to Standard Large-Bore 1.5-T MRI for Foot and Knee Examinations? Am. J. Roentgenol. 2014, 203, 1293–1302.
- Klein, H.-M. Low-Field Magnetic Resonance Imaging. RöFo-Fortschritte Geb. Röntgenstrahlen Bildgeb. Verfahr. 2020, 192, 537–548.
- Lutterbey, G.; Behrends, K.; Falkenhausen, M.V.; Wattjes, M.P.; Morakkabati, N.; Gieseke, J.; Schild, H. Is the body-coil at 3 Tesla feasible for the MRI evaluation of the painful knee? A comparative study. Eur. Radiol. 2007, 17, 503–508.
- Link, T.M. MR Imaging in Osteoarthritis: Hardware, Coils, and Sequences. Radiol. Clin. N. Am. 2009, 47, 617–632.
- Guglielmi, G.; Lennart, J.; Simoni, P.; Mascarenhas, V. Knee. MRI Protocols of the ESSR Arthritis Subcommittee. 2018. Available online: https://www.essr.org/content-essr/uploads/2018/05/Knee.pdf (accessed on 22 February 2023).
- Sudoł-Szopińska, I.; Jurik, A.G.; Eshed, I.; Lennart, J.; Grainger, A.; Østergaard, M.; Klauser, A.; Cotten, A.; Wick, M.C.; Maas, M.; et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin. Musculoskelet. Radiol. 2015, 19, 396–411.
- Freeman, D.M.; Bergman, G.; Glover, G. Short TE MR microscopy: Accurate measurement and zonal differentiation of normal hyaline cartilage. Magn. Reson. Med. 1997, 38, 72–81.
- Link, T.M.; Stahl, R.; Woertler, K. Cartilage imaging: Motivation, techniques, current and future significance. Eur. Radiol. 2007, 17, 1135–1146.
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. RadioGraphics 2011, 31, 37–61.
- Delfaut, E.M.; Beltran, J.; Johnson, G.; Rousseau, J.; Marchandise, X.; Cotten, A. Fat Suppression in MR Imaging: Techniques and Pitfalls. RadioGraphics 1999, 19, 373–382.
- Dixon, W.T. Simple proton spectroscopic imaging. Radiology 1984, 153, 189–194.
- Guerini, H.; Omoumi, P.; Guichoux, F.; Vuillemin, V.; Morvan, G.; Zins, M.; Thevenin, F.; Drape, J.L. Fat Suppression with Dixon Techniques in Musculoskeletal Magnetic Resonance Imaging: A Pictorial Review. Semin. Musculoskelet. Radiol. 2015, 19, 335–347.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
585
Revisions:
2 times
(View History)
Update Date:
21 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




