Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Corey Andrew Mealer | -- | 3129 | 2023-08-16 22:51:34 | | | |
| 2 | Catherine Yang | Meta information modification | 3129 | 2023-08-17 03:08:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mealer, C.; Konsek, H.; Travis, Z.; Suk, R.N.; Rajab, T.K. Physiology of Ischemia and Reperfusion Injury for Transplantation. Encyclopedia. Available online: https://encyclopedia.pub/entry/48143 (accessed on 07 February 2026).
Mealer C, Konsek H, Travis Z, Suk RN, Rajab TK. Physiology of Ischemia and Reperfusion Injury for Transplantation. Encyclopedia. Available at: https://encyclopedia.pub/entry/48143. Accessed February 07, 2026.
Mealer, Corey, Haley Konsek, Zachary Travis, Rebecca N. Suk, Taufiek Konrad Rajab. "Physiology of Ischemia and Reperfusion Injury for Transplantation" Encyclopedia, https://encyclopedia.pub/entry/48143 (accessed February 07, 2026).
Mealer, C., Konsek, H., Travis, Z., Suk, R.N., & Rajab, T.K. (2023, August 16). Physiology of Ischemia and Reperfusion Injury for Transplantation. In Encyclopedia. https://encyclopedia.pub/entry/48143
Mealer, Corey, et al. "Physiology of Ischemia and Reperfusion Injury for Transplantation." Encyclopedia. Web. 16 August, 2023.
Copy Citation
Cold preservation is a key component to organ procurement and transplantation. Cold preservation functions by slowing metabolic activity of procured organs and begins the period known as cold ischemic time (CIT). Reducing CIT and warm ischemic time (WIT) are paramount to minimizing donor organ damage from ischemia and the build-up of waste products and signals that drive reperfusion injury prior to transplantation into a matching recipient.
ischemia and reperfusion injury
congenital heart disease
warm ischemic time
cold ischemic time
transplantation
organ procurement
1. Metabolic and Biochemical Basis of CIT and WIT
Ischemia is a state of insufficient blood flow to an area of tissue due to obstruction, disrupted gas exchange, or hemostasis [1]. Ischemia can lead to injury through a multitude of immunological, metabolic, and reactive oxygen species forming mechanisms (Figure 1) [2]. Ischemia injuries are a major challenge for many fields of medicine as they can lead to irreversible, life-threatening damage to affected organs. Nationally, the definitive beginning of ischemic time varies. The time of cross clamp is widely accepted as the beginning of the ischemic time, while other centers use a definitive oxygen saturation percentage of 80% on room air or a specific systolic blood pressure range as the beginning of the ischemic time. The ischemic phase ends at the time of organ reperfusion following transplantation with sufficiently oxygenated circulation [3]. Following the donor asystole in a procurement, a cold cardioplegia and aortic flush are administered throughout the donor’s body and ice is placed directly on transplantable organs to begin cooling the body to slow cellular metabolism, since ischemia immediately results in a buildup of metabolic waste products, such as carbon dioxide, lactate, and hydrogen ions.
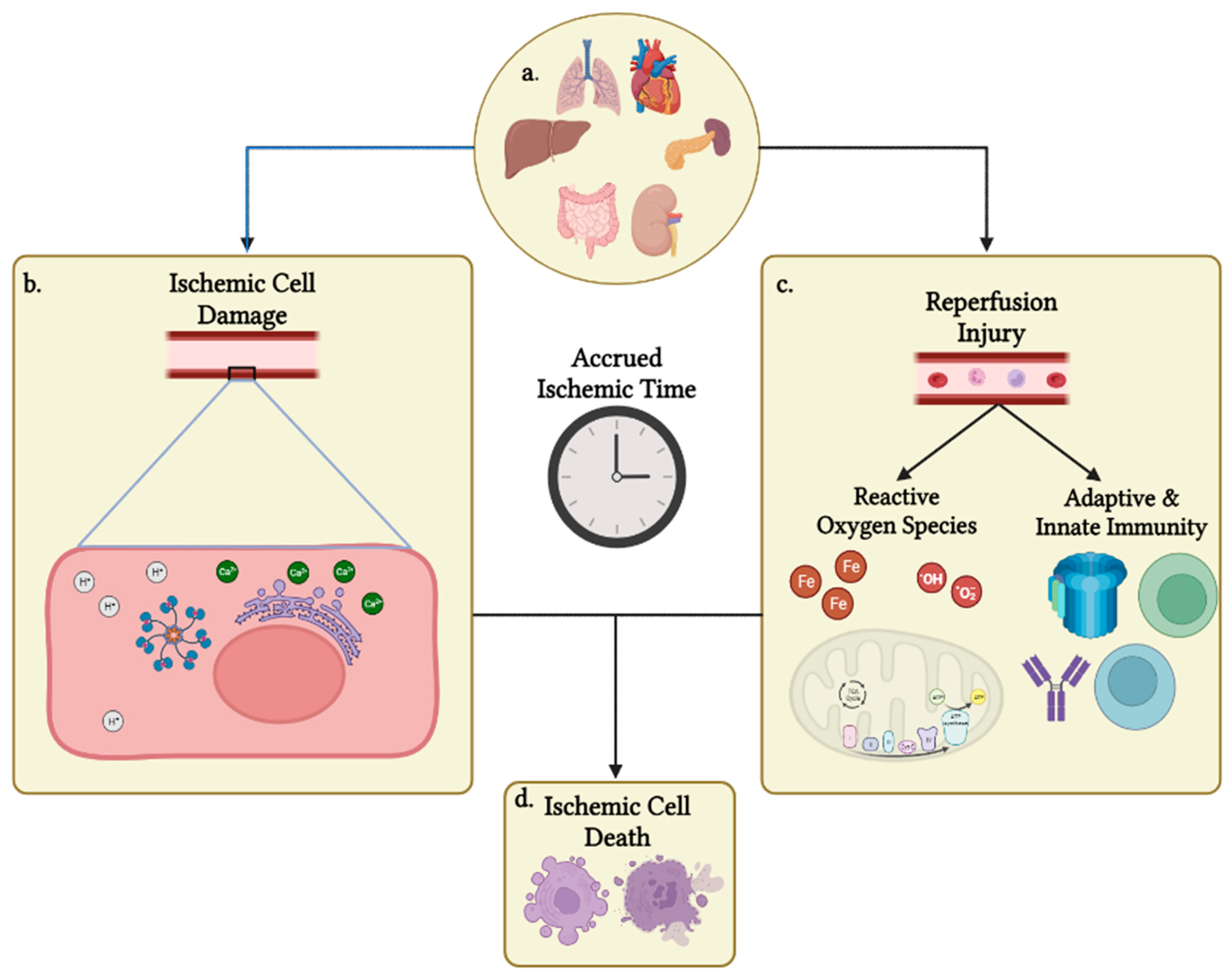
Figure 1. Overview of IRI affecting organs following procurement. (a) Solid organs that are currently able to be procured for transplantation. Each organ is unique in its susceptibility to cell ischemia based on a multitude of factors. (b) Organ ischemia begins a process of waste build up and dysregulation of cell homeostasis. Ultimately, the intracellular pH drops to deleterious levels, Ca++ dysregulation leads to calcium-induced apoptosis, and accumulation of cellular waste products and energy deficiency in the form of ATP depletion induces necrosis of cells. (c) Following transplantation, the donor organ is reperfused with oxygenated blood, which forms ROS and leads to the translocation of recipient-immune cells. Multiple mediators from both processes, such as T cells, B cells, complement proteins, iron ions, and the electron transport chain, further drive cellular and nuclear damage, as well as necrosis and apoptosis. (d) Processes listed in (a,b) both contribute to a reduction in donor organ viability via necrosis and apoptosis based on prior donor organ damage, the donation type conducted, CIT and WIT accrued, and recipient sensitization. Created with BioRender.com (accessed on 3 June 2023).
Initially following hypoxic exposure, ischemic cells begin to deplete intracellular ATP through normal biochemical activity, eventually halting the action of Na+/K+ ATPases, membrane Ca++ ATPases, and endoplasmic reticulum Ca++ ATPases. This results in increased levels of intracellular sodium and calcium, which causes cellular swelling and increased extracellular potassium. Increased hydrogen and sodium ions inside the cell lead to a decreased activity of Na+/H+ exchangers, trapping sodium and hydrogen inside of the cell. The increased level of H+ lowers the pH of the cell, potentially leading to impaired enzymatic activity, ribosomal dysfunction, and clumping of nuclear chromatin [4]. Additionally, the increased cytoplasmic sodium slows the activity of Na+/Ca++ exchangers, further exacerbating intracellular Ca++ levels [5][6]. High levels of intracellular Ca++ activate Ca++-dependent proteases, which damage intracellular structures, compromise membrane integrity, and increases the permeability of mitochondria, further impairing ATP production while releasing pro-apoptotic proteins [7]. Therefore, many cells are exposed to an acidic environment acutely, but prolonged ischemia accrued over the procurement process eventually leads to apoptosis, autophagy, or in severe damage, necrosis [8]. Apoptosis is a non-inflammatory, programmed-cellular death that is induced by a toxic accumulation of reactive oxygen species (ROS), cellular toxins, and metabolic waste products, as well as by dysregulated calcium within the cell and acute or chronic hypoxia. Necrosis is more common than apoptosis in IRI and is defined as cell death due to irreversible cell damage either by membrane disruption or from extreme organelle swelling, leading to decreased organ viability [9]. Necrosis is a major driving force of inflammation after reperfusion of the newly transplanted organ that stokes and further precipitates the immunological role of IRI [10].
2. Reactive Oxygen Species Generation and Damage
Hypoxia not only leads to organ damage through metabolic processes of ATP depletion, but states of hypoxia also lead to a buildup of intermediate products and a depletion of regulatory processes that can cause severe damage to the organ following reperfusion, known as reperfusion injury [11]. One major mechanism of damage in IRI is the production of ROS’. ROS’ are oxygen-derived products with extra, unpaired valence electrons that are highly reactive with surrounding cellular components. ROS’ are produced in stable, physiological processes for energy production, immune defense, and the degradation of cellular waste products like xanthine in the ischemic environment. ROS’ are usually cleared by antioxidants available in the normal environment like NADPH, glutathione, tetrahydrobiopterin, and enzymes, glutathione peroxidase, superoxide dismutase and catalase [12]. ROS’ cause damage in the body by the ROS’ unpaired electrons reacting with surrounding amino acids, lipids, nucleic acids, and nitric oxide, or, importantly, by producing more ROS’ in the presence of iron through the Haber–Weiss and Fenton reactions [13]. ROS stripping of electrons from surrounding structures disturb their physiological functions by disrupting membrane permeability, impeding enzymatic function, and by damaging DNA. There are a few major pathways which lead to the production of oxidative stress and damage, including the production of xanthine oxidase from AMP, the NADPH oxidase pathway, iron-dependent reactions, the electron transport chain (ETC), and the nitric oxide system all in the absence of antioxidant agents, such as reduced glutathione and tetrahydrobiopterin that are depleted prior to or following reperfusion [4][14].
2.1. Xanthine Oxidase Pathway in IRI
In the xanthine oxidase pathway, the ischemic environment causes energy depletion through the conversion of ATP to AMP. AMP is further converted to hypoxanthine by xanthine oxidase and produces superoxide as a byproduct. Xanthine oxidase can further break down hypoxanthine into uric acid as a waste product and an additional molecule of superoxide. The superoxide waste products made in the hypoxanthine metabolism can only be formed once the organ is reperfused with oxygenated blood and is not a direct consequence of WIT or CIT, but hypoxanthine does accumulate during these ischemic periods [15][16]. The superoxide formed from this process and other forms of ROS that form during ischemia and reperfusion that escape antioxidant fixation lead to a free radical attack, causing lipid peroxidation and nuclear damage due to reactive free radicals, further reducing organ viability post-transplant [15][16][17]. Hypoxanthine and xanthine oxidase’s role in IRI have been targets of therapy for many years and led to the addition of allopurinol and glutathione to most preservation solutions used during the procurement process [18].
2.2. NADPH Oxidase Pathway in IRI
The NADPH oxidase pathway has increased activity and expression in the environment of IRI and is a major producer of ROS. NADPH oxidase is composed of seven isoforms that differ in tissue localization, which consist of five NOX enzymes and two dual oxidases. Each NADPH oxidase uses oxygen as an electron acceptor and ultimately forms two molecules of superoxide per reaction [17]. The most well-described isoform of NADPH oxidase is found primarily in phagocytic cells and produces ROS to aid in the host’s defenses against bacteria, but NADPH oxidase enzymes also participate in differentiation, endocrine signaling, cell growth, proliferation, apoptosis, cytoskeleton regulation, and migration of tissues [19][20]. Since NADPH oxidase enzymes are integral to cell function and regulation and two molecules of superoxide are made per oxidase reaction, NADPH oxidase is an important driver of ROS production and IRI.
2.3. Iron-Dependent Mechanisms of ROS Production
It has become an increasingly important topic of interest to target iron-dependent mechanisms of ROS production. As mentioned earlier, free iron molecules are a large source of ROS via the Haber–Weiss and Fenton reactions, which are both reactions forming highly reactive hydroxyl radicals in the presence of iron and hydrogen peroxide [13]. While these reactions are important to free radical formation of superoxide and hydroxyl radicals, it is believed that iron-dependent IRI is due to intracellular redox-reactive mechanisms and is not due to increased concentrations of circulating ROS [21]. The mechanism driving this damage appears to be, in part, due to hypoxia-inducible factor proteins (HIF). HIFs are transcription factors that increase cell survivability or lead to programmed cell death in a state of hypoxia by regulating anaerobic metabolism, angiogenesis, and apoptosis. HIFs in the setting of IRI, however, appear to also overexpress transferrin receptor 1 (TfR1), causing a cellular iron overload and oxidative injury. Iron overload and intracellular damage in the setting of IRI is also mediated by aberrant function of intracellular iron-binding proteins, membrane transporters, and proteins involved in iron processing and metabolism [13].
The hypothesis of intracellular iron being the driver of IRI is currently supported by research into the addition of iron chelators in cold preservation storage solutions, such as membrane-impermeable deferoxamine and membrane-permeable LK-614, with membrane-permeable iron chelators alone showing a significant reduction in IRI while membrane-impermeable iron chelators alone do not show a significant reduction in IRI. There are controversial results on the benefits of using both membrane-permeable and membrane-impermeable iron-chelators, with Radovitis et al. showing that the combination of membrane-permeable and membrane-impermeable iron chelators had similar results to groups treated with the membrane-permeable iron chelator alone [21][22]. A phase III clinical trial from 2020 using Custodiol-N, a cold preservation solution variation of Custodiol that has alterations to the recipe to decrease IRI including iron chelators deferoxamine and LK-614, showed a significant improvement in hypoxic injury, reduction of cold-induced IRI, and alleviation of adverse events following a warm solution exposure when used for kidney, liver, and kidney–pancreas procurement [23]. Additionally, a more recent paper from 2022 showed an improved preservation efficacy when Custodiol-N was used versus the original Custodiol recipe in a canine model of an orthotopic heart transplant [24]. A substantial body of data has shown that regulating iron levels in the setting of preservation improves outcomes in multiple organ models by maintaining and ameliorating ROS damage, but few centers have adopted this novel method of preservation.
2.4. Reactive Nitrogen Species and the Electron Transport Chain in IRI
The presence and production of nitric oxide (NO) is protective against ischemic damage under normal circumstances. However, due to the accumulation of ROS from other metabolic processes during the preservation process, ROS’ have a greater opportunity to react with circulating NO, forming reactive nitrogen species, like peroxynitrite. Ultimately, these reactive nitrogen species can damage cellular components very similarly to the pathways used by ROS [6][25].
A final important producer of ROS following reperfusion is through the ETC. In the presence of oxygen, most cells produce most of their ATP to drive enzymatic and biochemical reactions through the ETC pathway. The ETC is a series of mitochondrial enzymes that are initiated by the acceptance of electrons from NADH and FADH2, which were formed from other catabolic processes. Those donated electrons are transferred across a series of proteins with an increasing reduction potential, driving hydrogen ions into the intermembrane space of the mitochondria and producing a proton motive force that drives ATP synthesis. The transfer of these electrons can only occur in the presence of oxygen, which understandably increases the likelihood of producing ROS. The ROS formed in this pathway shortly after exposure to oxygen primarily comes from premature leakage of electrons from Complexes I, II, and III, and Coenzyme Q [26]. Understandably, reperfusion of oxygenated blood after a sustained period of ischemia can lead to a substantial increase in energy production through the ETC and resulting production of ROS.
3. Innate Immunity and Complement in IRI
In addition to metabolic imbalances, the immune system is a large function of ischemic injury and post-transplant rejection. These immune-mediated responses are the basis for immunosuppression in patients post solid organ transplant, as early inflammatory signals drive important downstream processes such as the recognition of foreign-donor antigen. Local cell death following procurement in the warm and cold ischemic phase leads to an immunogenic, yet sterile environment. Damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) are highly expressed in the transplanted organ and recipient following the transplant procedure. As ischemic time continues, DAMPs and PAMPs continue to accumulate. Immediately following reperfusion, toll-like receptors (TLR) and other damage or pathogen recognizing receptors interact with present DAMPs and PAMPs to create an inflammatory environment through the nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways [1][12][27]. Since MAPK and NF-κB are major drivers of immunological processes in transplant and many other fields, they are common targets for pharmacological and biochemical research. Animal models using TLR-4 knockout mice and siRNA NF-kB inhibitors have been shown to have a protective role and can prolong kidney survival, respectively, but this result has not been tested in other solid organs. NF-κB and MAPK can signal for significant inflammation in the transplant recipient post-reperfusion and can lead to a highly immunogenic environment that promotes the migration of antigen-presenting cells to activate lymphocytes, followed by the translocation of macrophages, lymphocytes, and neutrophils to the donor organ. ICAM-1 and VCAM are upregulated on the apical side of endothelial cells by inflammatory signals, such as IL-1 and TNF-a, to promote the adhesion and release of TNF-a, IL-1, and IL-8 for proliferation of immune mediators and for translocation and chemotaxis to the inflamed tissue [28]. These events precipitate cell death, activate complement, drive thrombosis, and increase vascular permeability, ultimately further driving rejection.
4. Adaptive Immunity in the Physiology of IRI
The adaptive immune system plays a key role in organ rejection, and the creation of immunosuppressants that mitigate the adaptive immune system, such as cyclosporine, tacrolimus, prednisone, and other immunomodulators, were imperative to the success of organ transplantation. The adaptive immune system has some overlap with the innate immune system, but it is generally considered to be any immunological process or response that is modulated and improved with repeated exposure to foreign antigens. The main cell types of the adaptive Immune system are the lymphocytes, T cells, and B cells. T cells can be further described as cytotoxic T cells or helper T cells. Cytotoxic T cells, or CD8+ T cells, recognize host cells that have been invaded by foreign antigens—or, in the case of transplantation, recipient T cells recognize donor antigens on the transplanted organ’s cell surface. Helper T cells, or CD4+ T cells, are integral to organ rejection and primarily modulate the immune response by releasing cytokines and activating other immune mediators. CD4+ T cells have been shown to appear in the donor organ, respond to, activate, and produce IFN-γ within 12 h of transplantation in mice models of liver, kidney, lung, and intestine transplantation [29].
4.1. T Cells in Reperfusion Injury following CIT
T cells play a major role in rejection following donor organ transplantation through antigen-dependent and antigen-independent activation [30]. CD4+ T cells have repeatedly been shown in animal studies to play an integral role in facilitating donor organ inflammation and damage following transplantation through cytokine secretion and a CD154 and CD40-mediated process [31][32]. CD4+ T cell activation leads to differentiation into Th1, Th2, Th17, and Treg cell lineages, which each play unique roles in the immune response [33][34]. Th1 and Th17 have been shown to cause a pro-inflammatory environment in the post-transplant milieu promoting tissue damage and rejection. On the opposite end of the spectrum, Th2 cells have been associated with inflammation inhibition, at least in part by blocking the action and differentiation of Th1 cells [35]. Increased differentiation into the Treg cell (Foxp3 expressing T cells) lineage has shown evidence of improved graft survival rates. Additionally, when Tregs were recovered from normal syngeneic mice and implanted into T cell-deficient mice following an organ allograft transplantation, animal survival was significantly improved [36][37][38].
In addition to the CD4+ T cell lineages, CD8+ T cells contribute to IRI by the recognition of alloantigens on MHC class I of donor cells and initiates subsequent cytotoxic killing. While CD8+ T cells have been shown to be important to early donor organ damage and rejection, cell-mediated donor damage is limited by CD4+ T cell activation, and CD8+ T cell tolerance appears to form over time. While CD8+ T cells play a role in IRI, evidence of CD8+ T cell cytotoxicity in late-stage damage of donor allografts is still lacking evidence. In addition, cytotoxic T cell depletion by anti-CD8 antibodies in multiple animal models have not been protective against acute rejection [39][40]. While the information surrounding T cells and their effects in organ preservation and transplantation is promising, as well as the foundation of immunosuppression therapy in transplant, it comes at the cost of universal suppression of the patient’s immune system, making them susceptible to severe infection and sepsis. To improve immunotherapy in transplanted patients, a more thorough understanding of pertinent cytokines’ effects, crosstalk between cells, and the role of co-stimulation between immune mediators will need to be provoked before there will be a meaningful impact on recipient outcomes and livelihood.
4.2. B Cells in Reperfusion Injury following CIT
B cells are heavily involved in the immunological response following ischemia and reperfusion by producing donor-specific antibodies or alloantibodies following organ reperfusion. In the context of transplant, if T cells are absent or if the T cell costimulation of B cells is inhibited, donor-specific antibodies are not able to be formed [41][42][43]. The humoral mechanisms proven to drive acute organ rejection are anti-donor immunoglobulin-binding with subsequent complement-mediated cell lysis, antigen-dependent cell-mediated toxicity by natural killer cells, leukocyte recruitment, and vascular thrombosis due to the inflammatory activation of coagulation intermediates and immune-complex deposition [41]. While acute damage is a contributing factor to total organ rejection, the chronic effects of B cell activation are proposed to be the main drivers of late-stage allograft rejection as T cell mediated rejection and damage starts to dwindle within a year of transplant, while anti-donor antibodies continue to be formed by plasma cells following T cell tolerance [44]. CD4+ T cells, CD8+ T cells, Treg cells, and B cells have all been shown to play key roles in the progression of donor organ damage following transplantation, leading to the universal use of immunosuppressants, such as tacrolimus and cyclosporine.
The hypoxic organ environment created during procurement, metabolism of donor tissues, production of ROS, and the immune reaction of the two environments brought together, work in tandem to cause IRI. It is a primary interest to modulate these circumstances to expand viable organ ischemic times and to produce an environment that reduces CIT/WIT damage and the subsequent effects of reperfusion injury. Advances to minimize the effects of CIT/WIT and reperfusion injury are essential for reducing recipient complications and ensuring that the sparse availability of transplantable organs are not squandered.
References
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401.
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42.
- Kalisvaart, M.; Croome, K.P.; Hernandez-Alejandro, R.; Pirenne, J.; Cortes-Cerisuelo, M.; Miñambres, E.; Abt, P.L. Donor Warm Ischemia Time in DCD Liver Transplantation—Working Group Report from the ILTS DCD, Liver Preservation, and Machine Perfusion Consensus Conference. Transplantation 2021, 105, 1156–1164.
- Wu, M.-Y.; Yiang, G.-T.; Liao, W.-T.; Tsai, A.P.-Y.; Cheng, Y.-L.; Cheng, P.-W.; Li, C.-Y.; Li, C.-J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667.
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170.
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034.
- Orrenius, S.; Burkitt, M.J.; Kass, G.E.N.; Dypbukt, J.M.; Nicotera, P. Calcium ions and oxidative cell injury. Ann. Neurol. 1992, 32, S33–S42.
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301.
- McCully, J.D.; Wakiyama, H.; Hsieh, Y.J.; Jones, M.; Levitsky, S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1923–H1935.
- Gottlieb, R.A. Cell death pathways in acute ischemia/reperfusion injury. J. Cardiovasc. Pharm. 2011, 16, 233–238.
- Fernández, A.R.; Sánchez-Tarjuelo, R.; Cravedi, P.; Ochando, J.; López-Hoyos, M. Review: Ischemia Reperfusion Injury—A Translational Perspective in Organ Transplantation. Int. J. Mol. Sci. 2020, 21, 8549.
- Tang, S.P.; Mao, X.L.; Chen, Y.H.; Yan, L.L.; Ye, L.P.; Li, S.W. Reactive Oxygen Species Induce Fatty Liver and Ischemia-Reperfusion Injury by Promoting Inflammation and Cell Death. Front. Immunol. 2022, 29, 870239.
- Li, J.Y.; Liu, S.Q.; Yao, R.Q.; Tian, Y.P.; Yao, Y.M. A Novel Insight Into the Fate of Cardiomyocytes in Ischemia-Reperfusion Injury: From Iron Metabolism to Ferroptosis. Front. Cell. Dev. Biol. 2021, 9, 799499.
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.A.; Zweier, J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 2014, 53, 3679–3688.
- Yapca, O.E.; Borekci, B.; Suleyman, H. Ischemia-reperfusion damage. Eurasian J. Med. 2013, 45, 126–127.
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551.
- Chung, H.Y.; Baek, B.S.; Song, S.H.; Kim, M.S.; Huh, J.I.; Shim, K.H.; Kim, K.W.; Lee, K.H. Xanthine dehydrogenase/xanthine oxidase and oxidative stress. Age 1997, 20, 127–140.
- Petrenko, A.; Carnevale, M.; Somov, A.; Osorio, J.; Rodríguez, J.; Guibert, E.; Fuller, B.; Froghi, F. Organ Preservation into the 2020s: The Era of Dynamic Intervention. Transfus. Med. Hemother 2019, 46, 151–172.
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313.
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 17, w13659.
- Radovits, T.; Lin, L.N.; Zotkina, J.; Koch, A.; Rauen, U.; Köhler, G.; Karck, M.; Szabó, G. Endothelial dysfunction after long-term cold storage in HTK organ preservation solutions: Effects of iron chelators and N-alpha-acetyl-L-histidine. J. Heart Lung Transplant. 2008, 27, 208–216.
- Schaefer, B.; Effenberger, M.; Zoller, H. Iron metabolism in transplantation. Transpl. Int. 2014, 27, 1109–1117.
- Kniepeiss, D.; Houben, P.; Stiegler, P.; Berghold, A.; Riedl, R.; Kahn, J.; Schemmer, P. A prospective, randomized, single-blind, multicentre, phase III study on organ preservation with Custodiol-N solution compared with Custodiol® solution in organ transplantation (kidney, liver and pancreas). Trials 2020, 10, 62.
- Szabó, G.; Loganathan, S.; Korkmaz-Icöz, S.; Balogh, Á.; Papp, Z.; Brlecic, P.; Hegedüs, P.; Radovits, T.; Karck, M.; Merkely, B.; et al. Improvement of Left Ventricular Graft Function Using an Iron-Chelator-Supplemented Bretschneider Solution in a Canine Model of Orthotopic Heart Transplantation. Int. J. Mol. Sci. 2022, 5, 7453.
- Bice, J.S.; Jones, B.R.; Chamberlain, G.R.; Baxter, G.F. Nitric oxide treatments as adjuncts to reperfusion in acute myocardial infarction: A systematic review of experimental and clinical studies. Basic Res. Cardiol. 2016, 111, 23.
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674.
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837.
- Perry, B.C.; Soltys, D.; Toledo, A.H.; Toledo-Pereyra, L.H. Tumor Necrosis Factor-α in Liver Ischemia/Reperfusion Injury. J. Investig. Surg. 2011, 24, 177–188.
- de Perrot, M.; Young, K.; Imai, Y.; Waddell, T.K.; Fischer, S.; Zhang, L.; Keshavjee, S. Recipient T Cells Mediate Reperfusion Injury after Lung Transplantation in the Rat. J. Immunol. 2003, 171, 4995–5002.
- Satpute, S.R.; Park, J.M.; Jang, H.R.; Agreda, P.; Liu, M.; Gandolfo, M.T.; Racusen, L.; Rabb, H. The role for T cell repertoire/antigen-specific interactions in experimental kidney ischemia reperfusion injury. J. Immunol. 2009, 183, 984–992.
- Shen, X.; Wang, Y.; Gao, F.; Ren, F.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Zhai, Y. CD4 T cells promote tissue inflammation via CD40 signaling without de novo activation in a murine model of liver ischemia/reperfusion injury. Hepatology 2009, 50, 1537–1546.
- Ke, B.; Shen, X.D.; Gao, F.; Tsuchihashi, S.; Farmer, D.G.; Briscoe, D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation 2005, 79, 1078–1083.
- Loverre, A.; Divella, C.; Castellano, G.; Tataranni, T.; Zaza, G.; Rossini, M.; Ditonno, P.; Battaglia, M.; Palazzo, S.; Gigante, M.; et al. T helper 1, 2 and 17 cell subsets in renal transplant patients with delayed graft function. Transpl. Int. 2011, 24, 233–242.
- Tang, Q.; Dong, C.; Sun, Q. Immune response associated with ischemia and reperfusion injury during organ transplantation. Inflamm. Res. 2022, 71, 1463–1476.
- Rao, J.; Lu, L.; Zhai, Y. T cells in organ ischemia reperfusion injury. Curr. Opin. Organ. Transplant. 2014, 19, 115–120.
- Shan, J.; Guo, Y.; Luo, L.; Lu, J.; Li, C.; Zhang, C.; Huang, Y.; Feng, L.; Wu, W.; Long, D.; et al. Do CD4+Foxp3+ Treg cells correlate with transplant outcomes: A systematic review on recipients of solid organ transplantation. Cell. Immunol. 2011, 270, 5–12.
- Eiji, N.; Toshiko, S.; Ruka, S.; Koichi, T.; Shimon, S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+ CD25+ CD4+ regulatory T cells. Int. Immunol. 2004, 16, 1189–1201.
- Monteiro, R.M.; Camara, N.O.; Rodrigues, M.M.; Tzelepis, F.; Damiao, M.J.; Cenedeze, M.A.; Teixeira Vde, P.; dos Reis, M.A.; Pacheco-Silva, A. A role for regulatory T cells in renal acute kidney injury. Transpl. Immunol. 2009, 21, 50–55.
- Siu, J.H.Y.; Surendrakumar, V.; Richards, J.A.; Pettigrew, G.J. T cell allorecognition pathways in solid organ transplantation. Front. Immunol. 2018, 9, 2548.
- Haudebourg, T.; Poirier, N.; Vanhove, B. Depleting T-cell subpopulations in organ transplantation. Transpl. Int. 2008, 22, 509–592.
- Chong, A.S. Mechanisms of organ transplant injury mediated by B cells and antibodies: Implications for antibody-mediated rejection. Am. J. Transplant. 2020, 20, 23–32.
- Li, Y.; Ma, L.; Yin, D.; Shen, J.; Chong, A.S. Long-term control of alloreactive B cell responses by the suppression of T cell help. J. Immunol. 2008, 180, 6077–6084.
- Rabant, M.; Gorbacheva, V.; Fan, R.; Yu, H.; Valujskikh, A. CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity. Am. J. Transpl. 2013, 13, 2831–2841.
- Halloran, P.F.; Chang, J.; Famulski, K.; Hidalgo, L.G.; Salazar, I.D.R.; Lopez, M.M.; Matas, A.; Picton, M.; De Freitas, D.; Bromberg, J.; et al. Disappearance of T cell-mediated rejection despite continued antibody-mediated rejection in late kidney transplant recipients. J. Am. Soc. Nephrol. 2015, 26, 1711–1720.
More
Information
Subjects:
Transplantation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
441
Revisions:
2 times
(View History)
Update Date:
17 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




