Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mauro Maniscalco | -- | 1709 | 2023-08-16 17:24:23 | | | |
| 2 | Dean Liu | -1 word(s) | 1708 | 2023-08-17 02:33:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marcuccio, G.; Ambrosino, P.; Merola, C.; Manzo, F.; Motta, A.; Rea, G.; Cantone, E.; Maniscalco, M. Applications of Nasal Nitric Oxide in Allergic Rhinitis. Encyclopedia. Available online: https://encyclopedia.pub/entry/48135 (accessed on 07 February 2026).
Marcuccio G, Ambrosino P, Merola C, Manzo F, Motta A, Rea G, et al. Applications of Nasal Nitric Oxide in Allergic Rhinitis. Encyclopedia. Available at: https://encyclopedia.pub/entry/48135. Accessed February 07, 2026.
Marcuccio, Giuseppina, Pasquale Ambrosino, Claudia Merola, Fabio Manzo, Andrea Motta, Gaetano Rea, Elena Cantone, Mauro Maniscalco. "Applications of Nasal Nitric Oxide in Allergic Rhinitis" Encyclopedia, https://encyclopedia.pub/entry/48135 (accessed February 07, 2026).
Marcuccio, G., Ambrosino, P., Merola, C., Manzo, F., Motta, A., Rea, G., Cantone, E., & Maniscalco, M. (2023, August 16). Applications of Nasal Nitric Oxide in Allergic Rhinitis. In Encyclopedia. https://encyclopedia.pub/entry/48135
Marcuccio, Giuseppina, et al. "Applications of Nasal Nitric Oxide in Allergic Rhinitis." Encyclopedia. Web. 16 August, 2023.
Copy Citation
Allergic rhinitis, a common allergic disease affecting a significant number of individuals worldwide, is observed in 25% of children and 40% of adults, with its highest occurrence between the ages of 20 and 40. Its pathogenesis, like other allergic diseases, involves innate and adaptive immune responses, characterized by immunologic hypersensitivity to environmental substances.
allergy
rhinitis
chronic respiratory disease
rehabilitation
exercise
1. Introduction
Allergic rhinitis (AR) is the most common allergic disease worldwide, impacting approximately 400 million people [1][2][3]. Over the past 30 years, its prevalence has significantly increased, affecting 25% of children and 40% of adults [2][3][4]. This rise can be attributed to the effects of urbanization and heightened levels of pollutants, which exacerbate pollen sensitization [2][3][4]. Symptoms of AR typically manifest in childhood, adolescence, and early adulthood, peaking between the ages of 20 and 40 [3][4]. In preschool-age children, AR has a notable incidence of 17.9%, with a higher prevalence among males [4][5][6].
The pathogenesis of AR, like other allergic diseases, involves innate and adaptive immune responses, characterized by immunologic hypersensitivity to environmental substances [2][3][4]. This response is mediated by type 2 immunity, which involves T-helper 2 (Th2) cells, eosinophils, mast cells, and M2 macrophages [7][8]. According to the Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines [2][9], the clinical diagnosis of AR is based on positive skin-prick testing for allergens or serum immunoglobulin E (IgE) tests. AR symptoms, including sneezing, nasal obstruction, itching, and rhinorrhea triggered by allergen exposure, can also be associated with other conditions such as asthma, rhinosinusitis, otitis media, and conjunctivitis, leading to clinical complexities in management and treatment [2]. These complications also contribute to a decreased quality of life (QoL) and substantial healthcare costs, amounting to billions of dollars in the United States [10][11].
In the era of precision medicine, certain molecules have been identified as key biomarkers in the pathogenesis of AR, providing crucial information for precise diagnosis and treatment monitoring [7]. These biomarkers may help identify disease subtypes (endotypes) and clusters, guiding targeted interventions and monitoring treatment effectiveness [7]. In this regard, nitric oxide (NO) has been proposed as the most relevant biomarker of type 2 allergic diseases, including AR [7]. NO is an inflammatory mediator and, therefore, it has been extensively studied in various clinical conditions. Thus, measurement of fractional exhaled NO (FeNO) has become a useful tool for monitoring inflammatory diseases of lower airways, such as bronchial asthma [12]. Similarly, nasal NO (nNO), which plays a significant role in physiological and pathological processes like neuro-transmission, immunity, inflammation, and mucociliary regulation [13], has been proposed as an objective measure for monitoring upper airway inflammation [14][15]. However, the relationship between AR and nNO remains controversial, with conflicting findings in the scientific literature [16][17].
2. Nitric Oxide and Allergic Rhinitis: Clinical and Functional Mechanisms
nNO has been studied in different clinical diseases of the upper airways, being a potential tool in diagnosis and monitoring AR in both adults and children [18][19].
In AR, as with FeNO in asthma, nNO appears to be related to the degree of eosinophilic inflammation [20], as it comes from a Th2 inflammatory cascade and its production depends on allergen exposure [20]. After intranasal allergen exposure, nNO decreases in the first 20 min, later increasing after about 7 h and peaking after 24 h [21].
Using the same analyzer (Niox® Mino, Aerocrine AB, Solna, Sweden), the same flow rate (0.3 L/min) and the same method (breath hold), two authors reported similar cut-off values (169.4 and 161.4 nL/min) with good specificity and sensitivity for nNO in AR [1][22][23]. Using other analyzers (Nano Coulomb® Breath Analyzer, Sunvou-CA2122, Wuxi, China) instead, other authors reported cut-off values in AR and in healthy control subjects of 684.2 and 355.4 ppb, respectively [24].
According to the studies available in the literature, nNO levels have been found in individuals with RA to be higher than in non-RA controls. This was confirmed by a recent meta-analysis from the group, which consistently indicated that AR is associated with increased nNO levels when measured by both aspiration and expiration methods for perennial and seasonal disease [25]. In this meta-analysis, patients with seasonal AR exhibited increased levels of nNO as compared to controls only during the exposure to the allergens. This can be considered indirect evidence that the production of nNO in the nasal mucosa of RA patients is triggered by allergen exposure and subsequent inflammation, with an increased expression of iNOS in epithelial cells [26][27]. Furthermore, AR patients present an elevated nNOS immune reactivity around mucosal glands [28], as well as an overexpression of eNOS in the mucosal epithelium [29]. Therefore, a relationship between the increased expression of the different isoforms of NO synthase and the anatomical damage of the nasal mucosa in AR has been hypothesized [30].
Further investigation is still warranted to explore the relationships between various NOS isoforms and the extent of mucosal damage in AR [31]. To date, high levels of nNO in AR appear to be related to nasal mucosal damage, such as lack of vibrating cilia and basement membrane alterations, including absence of tight junctions with increased intercellular space [30]. Among all inflammatory molecules, NO modulates leukotriene B4 (LTB4)-induced neutrophil recruitment by changing rhinorrhea, thus indicating both a clinical manifestation of RA and a defensive mechanism [32]. nNO levels in AR patients seem to link even with symptoms severity because NO has effects on nasal mucosa [33], sneezing, and nasal leakage, even if some authors did not find this association statistically significant [34].
However, the increase in nNO in AR as compared to healthy controls is evident when there is no prominent obstruction of the paranasal sinus ostia, as the occlusion or blockage of the sinus ostia can impact the distribution of NO to the nasal cavity [35][36]. This variation in nNO distribution helps to explain the conflicting findings of certain studies that have suggested no significant difference in nNO levels between individuals with AR and healthy individuals [35][36]. Certain authors have examined nNO levels in relation to the opacification of the paranasal sinuses [20]. Their findings have shown a positive association between nNO and paranasal sinus opacification in patients with AR, particularly in cases without significant signs of chronic rhinosinusitis (CRS) according to the Lund-Mackay radiological staging system [20]. Therefore, the association between nNO and the inflammatory cascade in AR has become a matter of controversy in the literature. This is because the presence of nasal mucosa edema, which can hinder the patency of the paranasal sinuses, is a significant risk factor for CRS [20]. This is particularly relevant in cases of persistent AR, where nasal congestion persists for longer periods compared to intermittent AR [1][37]. Furthermore, when nasal obstruction at Visual Analogic Scale (VAS) score is lower than 7, or Nasal Airway Resistance (NAR) to airflow is lower than 0.65 Pa/cm3/s at anterior rhinomanometry, nNO could be considered as a real biomarker for AR and, for this reason, it may reflect nasal eosinophilic inflammation in patients only affected by AR with mild to moderate nasal obstruction [38]. On the other hand, in AR with severe nasal obstruction, identified by a VAS score higher than 7 or NAR higher than 0.65 Pa/cm3/s, nNO is not different from healthy controls [38]. In keeping with this, it is noteworthy that in cases where both the osteo–meatal complex and spheno-ethmoidal recess are obstructed, the inflammation and infection associated with CRS with (CRSwNP) or without nasal polyposis (CRSsNP) can lead to a decrease in the release of nNO from paranasal sinuses (Figure 1). This reduction in nNO release is significant as the paranasal sinuses serve as a reservoir of NO [20][33]. When comparing patients with AR and CRSwNP to patients with AR and CRSsNP, it has been observed that the former group tends to have lower nNO levels compared to the latter group, with a rapid increase in nNO observed after endoscopic sinus surgery [37][39]. Even if CRSwNP adult patients have high levels of iNOS in the nasal mucosa, it has been observed that nNO levels are decreased compared to those of non-complicated AR patients [40].
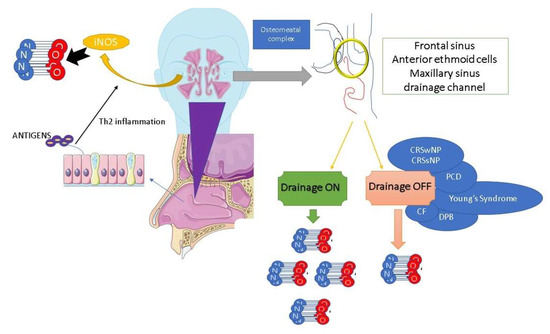
Figure 1. Nasal nitric oxide production and osteo-metal complex patency. NO: nitric oxide, iNOS: inducible nitric oxide synthase, CRSwNP: chronic rhinosinusitis with nasal polyps, CRSsNP: chronic rhinosinusitis without nasal polyps, PCD: primary ciliary dyskinesia, CF: cystic fibrosis, DPB: diffuse panbronchiolitis.
The evidence that measurements of nNO during humming is correlated with ostial function [41][42] has led to its potential use as test for osteo–meatal patency in AR, where humming does not cause any increase in nNO (humming non-responder). This method has been suggested as a suitable noninvasive test to assess the ostium patency and the effect of therapy in AR and in nasal polyposis [15][43][44].
3. Drug-Induced nNO Levels in Allergic Rhinitis
The topical application of L-NAME, a NOS inhibitor, has been found to decrease nNO production and prevent the increase in nasal airways resistance (NAR) induced by bradykinin, while partially inhibiting plasma extravasation mediated by platelet-activating factor (PAF), all mechanisms involved in AR [43][45].
Significant clinical evidence has emerged from the analysis of nNO levels after the administration of intranasal steroids (INS) and/or antihistamines (ATH) [46]. In particular, it has been observed that nNO levels may significantly decrease after topical treatment with these medications [46]. This decrease in nNO levels primarily reflects the effects of INS in reducing the expression of iNOS, thus highlighting the impact of INS on the regulation of NO production in the nasal mucosa [46]. In these patients, nNO was detected in the area of the inferior turbinate; in this part of nasal cavity, the metabolism of NO seems to be similar to that of bronchial mucosa in asthma [47]. It has been reported that nNO levels, blood eosinophils count, and severity of obstructive sleep apnea are higher in patients with persistent AR than in controls, and the administration of INS gives better results than ATH or leukotriene receptor antagonist (LRA) [48].
The levels of nNO in children with AR are influenced by their age, showing a positive association, which is likely explained by the increased development and pneumatization of the paranasal sinuses as children grow older [49]. In children with moderate-to-severe AR, higher nNO levels are associated with more severe nasal symptoms, as measured by VAS scores, and indicate greater severity of the disease, with a consequent decreased QoL for both patients and their caregivers [50]. In contrast, when these patients are treated with INS or ATH, a significant reduction in nNO levels and VAS scores for nasal symptoms should be expected, along with an improvement in QoL [50].
However, it is worth noting that several studies utilizing nasal sprays might encounter a potential limitation due to the presence of substances that could influence the levels of nNO [51].
References
- Wang, B.; Wu, Z.; Wang, F.; Yin, Z.; Shi, L.; Liu, Y. Nasal nitric oxide testing for allergic rhinitis patients: Systematic review and meta-analysis. Immun. Inflamm. Dis. 2021, 9, 635–648.
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; van Weel, C.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008, 63 (Suppl. S86), 8–160.
- Nur Husna, S.M.; Tan, H.T.; Md Shukri, N.; Mohd Ashari, N.S.; Wong, K.K. Allergic Rhinitis: A Clinical and Pathophysiological Overview. Front. Med. 2022, 9, 874114.
- Wang, I.J.; Tung, T.H.; Tang, C.S.; Zhao, Z.H. Allergens, air pollutants, and childhood allergic diseases. Int. J. Hyg. Environ. Health 2016, 219, 66–71.
- Hill, D.A.; Grundmeier, R.W.; Ram, G.; Spergel, J.M. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: A retrospective cohort study. BMC Pediatr. 2016, 16, 133.
- Testa, D.; Bari, M.D.; Nunziata, M.; Cristofaro, G.; Massaro, G.; Marcuccio, G.; Motta, G. Allergic rhinitis and asthma assessment of risk factors in pediatric patients: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2020, 129, 109759.
- Ogulur, I.; Pat, Y.; Ardicli, O.; Barletta, E.; Cevhertas, L.; Fernandez-Santamaria, R.; Huang, M.; Bel Imam, M.; Koch, J.; Ma, S.; et al. Advances and highlights in biomarkers of allergic diseases. Allergy 2021, 76, 3659–3686.
- Agache, I.; Akdis, C.A. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J. Clin. Investig. 2019, 129, 1493–1503.
- Wise, S.K.; Damask, C.; Roland, L.T.; Ebert, C.; Levy, J.M.; Lin, S.; Luong, A.; Rodriguez, K.; Sedaghat, A.R.; Toskala, E.; et al. International consensus statement on allergy and rhinology: Allergic rhinitis—2023. Int. Forum. Allergy Rhinol. 2023, 13, 293–859.
- Meltzer, E.O. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol. Allergy Clin. N. Am. 2016, 36, 235–248.
- Pawankar, R. Allergic diseases and asthma: A global public health concern and a call to action. World Allergy Organ. J. 2014, 7, 12.
- Heffler, E.; Carpagnano, G.E.; Favero, E.; Guida, G.; Maniscalco, M.; Motta, A.; Paoletti, G.; Rolla, G.; Baraldi, E.; Pezzella, V.; et al. Fractional Exhaled Nitric Oxide (FENO) in the management of asthma: A position paper of the Italian Respiratory Society (SIP/IRS) and Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). Multidiscip. Respir. Med. 2020, 15, 36.
- Maniscalco, M.; Sofia, M.; Pelaia, G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res 2007, 56, 58–69.
- Wu, Y.; Zhang, H.; Wang, J.; Han, Y.; Shi, Y.; Zhang, Q.; Shen, L.; Jiang, H.; Jia, C.; Yu, Y.; et al. Nasal nitric oxide in healthy Chinese children aged 6-18 years. Front. Pediatr. 2023, 11, 990510.
- Maniscalco, M.; Sofia, M.; Weitzberg, E.; De Laurentiis, G.; Stanziola, A.; Rossillo, V.; Lundberg, J.O. Humming-induced release of nasal nitric oxide for assessment of sinus obstruction in allergic rhinitis: Pilot study. Eur. J. Clin. Investig. 2004, 34, 555–560.
- Zhao, C.; Qin, M.; Jin, L.; Lai, J.; Wang, Y.; Liu, S.; Yu, S. Significance of Exhaled Nitric Oxide and Carbon Monoxide in Auxiliary Diagnosis and Evaluation of Allergic Rhinitis. Mediat. Inflamm. 2022, 2022, 2083057.
- Abdullah Alwi, A.H.; Zahedi, F.D.; Husain, S.; Wan Hamizan, A.K.; Abdullah, B. Diagnostic Value and Clinical Application of Nasal Fractional Exhaled Nitric Oxide in Subjects with Allergic Rhinitis. Am. J. Rhinol. Allergy 2023, 37, 307–312.
- Benedict, J.J.; Lelegren, M.; Han, J.K.; Lam, K. Nasal Nitric Oxide as a Biomarker in the Diagnosis and Treatment of Sinonasal Inflammatory Diseases: A Review of the Literature. Ann. Otol. Rhinol. Laryngol. 2023, 132, 460–469.
- Beydon, N.; Kouis, P.; Marthin, J.K.; Latzin, P.; Colas, M.; Davis, S.D.; Haarman, E.; Harris, A.L.; Hogg, C.; Kilbride, E.; et al. Nasal nitric oxide measurement in children for the diagnosis of primary ciliary dyskinesia: European Respiratory Society technical standard. Eur. Respir. J. 2023, 61, 2202031.
- Suojalehto, H.; Vehmas, T.; Lindstrom, I.; Kennedy, D.W.; Kilpelainen, M.; Plosila, T.; Savukoski, S.; Sipila, J.; Varpula, M.; Wolff, H.; et al. Nasal nitric oxide is dependent on sinus obstruction in allergic rhinitis. Laryngoscope 2014, 124, E213–E218.
- Boot, J.D.; de Kam, M.L.; Mascelli, M.A.; Miller, B.; van Wijk, R.G.; de Groot, H.; Cohen, A.F.; Diamant, Z. Nasal nitric oxide: Longitudinal reproducibility and the effects of a nasal allergen challenge in patients with allergic rhinitis. Allergy 2007, 62, 378–384.
- Nesic, V.S.; Djordjevic, V.Z.; Tomic-Spiric, V.; Dudvarski, Z.R.; Soldatovic, I.A.; Arsovic, N.A. Measuring nasal nitric oxide in allergic rhinitis patients. J. Laryngol. Otol. 2016, 130, 1064–1071.
- Wen, Y.S.; Lin, C.Y.; Yang, K.D.; Hung, C.H.; Chang, Y.J.; Tsai, Y.G. Nasal nitric oxide is a useful biomarker for acute unilateral maxillary sinusitis in pediatric allergic rhinitis: A prospective observational cohort study. World Allergy Organ. J. 2019, 12, 100027.
- Wen, S.; Cheng, S.; Xie, S.; Zhang, H.; Zhang, J.; Wang, F.; Xie, S.; Xie, Z.; Jiang, W. Predictive Value of Nasal Nitric Oxide and Serum NOS2 Levels in the Efficacy of Subcutaneous Immunotherapy in Pediatric Patients with Allergic Rhinitis. Mediat. Inflamm. 2022, 2022, 1679536.
- Ambrosino, P.; Parrella, P.; Formisano, R.; Papa, A.; Spedicato, G.A.; Di Minno, M.N.D.; Motta, A.; Maniscalco, M. Clinical application of nasal nitric oxide measurement in allergic rhinitis: A systematic review and meta-analysis. Ann. Allergy Asthma Immunol. 2020, 125, 447–459.e5.
- Yuksel, H.; Kirmaz, C.; Yilmaz, O.; Pinar, E.; Vatansever, S.; Degirmenci, P.B.; Ozbilgin, K. Nasal mucosal expression of nitric oxide synthases in patients with allergic rhinitis and its relation to asthma. Ann. Allergy Asthma Immunol. 2008, 100, 12–16.
- Kang, B.H.; Chen, S.S.; Jou, L.S.; Weng, P.K.; Wang, H.W. Immunolocalization of inducible nitric oxide synthase and 3-nitrotyrosine in the nasal mucosa of patients with rhinitis. Eur. Arch. Otorhinolaryngol. 2000, 257, 242–246.
- Olthoff, A.; Rohrbach, S.; Faber, M.; Gotz, W.; Laskawi, R. Neuronal nitric oxide synthase immunoreactivity in the nasal mucosa of patients with idiopathic and allergic rhinitis. ORL J. Otorhinolaryngol. Relat. Spec. 2002, 64, 180–185.
- Takeno, S.; Osada, R.; Furukido, K.; Chen, J.H.; Yajin, K. Increased nitric oxide production in nasal epithelial cells from allergic patients--RT-PCR analysis and direct imaging by a fluorescence indicator: DAF-2 DA. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2001, 31, 881–888.
- Giannessi, F.; Fattori, B.; Ursino, F.; Giambelluca, M.A.; Soldani, P.; Scavuzzo, M.C.; Ruffoli, R. Ultrastructural and ultracytochemical study of the human nasal respiratory epithelium in vasomotor rhinitis. Acta Otolaryngol. 2003, 123, 943–949.
- Sadek, A.A.; Abdelwahab, S.; Eid, S.Y.; Almaimani, R.A.; Althubiti, M.A.; El-Readi, M.Z. Overexpression of Inducible Nitric Oxide Synthase in Allergic and Nonallergic Nasal Polyp. Oxid. Med. Cell Longev. 2019, 2019, 7506103.
- Cardell, L.O.; Agusti, C.; Nadel, J.A. Nitric oxide-dependent neutrophil recruitment: Role in nasal secretion. Clin. Exp. Allergy 2000, 30, 1799–1803.
- Maniscalco, M.; Bianco, A.; Mazzarella, G.; Motta, A. Recent Advances on Nitric Oxide in the Upper Airways. Curr. Med. Chem. 2016, 23, 2736–2745.
- Lee, K.J.; Cho, S.H.; Lee, S.H.; Tae, K.; Yoon, H.J.; Kim, S.H.; Jeong, J.H. Nasal and exhaled nitric oxide in allergic rhinitis. Clin. Exp. Otorhinolaryngol. 2012, 5, 228–233.
- Moody, A.; Fergusson, W.; Wells, A.; Bartley, J.; Kolbe, J. Nasal levels of nitric oxide as an outcome variable in allergic upper respiratory tract disease: Influence of atopy and hayfever on nNO. Am. J. Rhinol. 2006, 20, 425–429.
- Henriksen, A.H.; Sue-Chu, M.; Holmen, T.L.; Langhammer, A.; Bjermer, L. Exhaled and nasal NO levels in allergic rhinitis: Relation to sensitization, pollen season and bronchial hyperresponsiveness. Eur. Respir. J. 1999, 13, 301–306.
- Williamson, P.A.; Vaidyanathan, S.; Clearie, K.; Stewart, M.; Lipworth, B.J. Relationship between fractional exhaled nitric oxide and nasal nitric oxide in airways disease. Ann. Allergy Asthma Immunol. 2010, 105, 162–167.
- Hou, J.; Lou, H.; Wang, Y.; He, F.; Cao, F.; Wang, C.; Zhang, L. Nasal ventilation is an important factor in evaluating the diagnostic value of nasal nitric oxide in allergic rhinitis. Int. Forum. Allergy Rhinol. 2018, 8, 686–694.
- Ambrosino, P.; Molino, A.; Spedicato, G.A.; Parrella, P.; Formisano, R.; Motta, A.; Di Minno, M.N.D.; Maniscalco, M. Nasal Nitric Oxide in Chronic Rhinosinusitis with or without Nasal Polyps: A Systematic Review with Meta-Analysis. J. Clin. Med. 2020, 9, 200.
- Colantonio, D.; Brouillette, L.; Parikh, A.; Scadding, G.K. Paradoxical low nasal nitric oxide in nasal polyposis. Clin. Exp. Allergy 2002, 32, 698–701.
- Weitzberg, E.; Lundberg, J.O. Humming greatly increases nasal nitric oxide. Am. J. Respir. Crit. Care Med. 2002, 166, 144–145.
- Maniscalco, M.; Weitzberg, E.; Sundberg, J.; Sofia, M.; Lundberg, J.O. Assessment of nasal and sinus nitric oxide output using single-breath humming exhalations. Eur. Respir. J. 2003, 22, 323–329.
- Maniscalco, M.; Pelaia, G.; Sofia, M. Exhaled nasal nitric oxide during humming: Potential clinical tool in sinonasal disease? Biomark. Med. 2013, 7, 261–266.
- Lundberg, J.O.; Maniscalco, M.; Sofia, M.; Lundblad, L.; Weitzberg, E. Humming, nitric oxide, and paranasal sinus obstruction. JAMA 2003, 289, 302–303.
- Kiss, H.; Orlos, Z.; Gellert, A.; Megyesfalvi, Z.; Mikaczo, A.; Sarkozi, A.; Vasko, A.; Miklos, Z.; Horvath, I. Exhaled Biomarkers for Point-of-Care Diagnosis: Recent Advances and New Challenges in Breathomics. Micromachines 2023, 14, 391.
- Takahara, D.; Kono, T.; Takeno, S.; Ishino, T.; Hamamoto, T.; Kubota, K.; Ueda, T. Nasal nitric oxide in the inferior turbinate surface decreases with intranasal steroids in allergic rhinitis: A prospective study. Auris Nasus Larynx 2019, 46, 507–512.
- Struben, V.M.; Wieringa, M.H.; Feenstra, L.; de Jongste, J.C. Nasal nitric oxide and nasal allergy. Allergy 2006, 61, 665–670.
- Vo-Thi-Kim, A.; Van-Quang, T.; Nguyen-Thanh, B.; Dao-Van, D.; Duong-Quy, S. The effect of medical treatment on nasal exhaled nitric oxide (NO) in patients with persistent allergic rhinitis: A randomized control study. Adv. Med. Sci. 2020, 65, 182–188.
- Wald, E.R.; Applegate, K.E.; Bordley, C.; Darrow, D.H.; Glode, M.P.; Marcy, S.M.; Nelson, C.E.; Rosenfeld, R.M.; Shaikh, N.; Smith, M.J.; et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics 2013, 132, e262–e280.
- Wang, P.P.; Wang, G.X.; Ge, W.T.; Tang, L.X.; Zhang, J.; Ni, X. Nasal nitric oxide in allergic rhinitis in children and its relationship to severity and treatment. Allergy Asthma Clin. Immunol. 2017, 13, 20.
- Costache, A.; Berghi, O.N.; Cergan, R.; Dumitru, M.; Neagos, A.; Popa, L.G.; Giurcaneanu, C.; Vrinceanu, D. Respiratory allergies: Salicaceae sensitization (Review). Exp. Ther. Med. 2021, 21, 609.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
478
Revisions:
2 times
(View History)
Update Date:
17 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




