Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nimeet Desai | -- | 1727 | 2023-08-15 16:54:40 | | | |
| 2 | Wendy Huang | Meta information modification | 1727 | 2023-08-16 03:17:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rana, D.; Desai, N.; Salave, S.; Karunakaran, B.; Giri, J.; Benival, D.; Gorantla, S.; Kommineni, N. Types of Collagens. Encyclopedia. Available online: https://encyclopedia.pub/entry/48096 (accessed on 02 March 2026).
Rana D, Desai N, Salave S, Karunakaran B, Giri J, Benival D, et al. Types of Collagens. Encyclopedia. Available at: https://encyclopedia.pub/entry/48096. Accessed March 02, 2026.
Rana, Dhwani, Nimeet Desai, Sagar Salave, Bharathi Karunakaran, Jyotsnendu Giri, Derajram Benival, Srividya Gorantla, Nagavendra Kommineni. "Types of Collagens" Encyclopedia, https://encyclopedia.pub/entry/48096 (accessed March 02, 2026).
Rana, D., Desai, N., Salave, S., Karunakaran, B., Giri, J., Benival, D., Gorantla, S., & Kommineni, N. (2023, August 15). Types of Collagens. In Encyclopedia. https://encyclopedia.pub/entry/48096
Rana, Dhwani, et al. "Types of Collagens." Encyclopedia. Web. 15 August, 2023.
Copy Citation
Collagen, a widely recognized extracellular matrix protein, has found extensive use in medical, pharmaceutical, and cosmetic applications. This is due to its crucial role in tissue and organ formation, and its involvement in various cellular functions. Additionally, collagen serves as an effective surface-active agent and displays its capacity to permeate lipid-free interfaces. In comparison to other natural polymers like albumin and gelatin, collagen showcases exceptional biodegradability, minimal antigenicity, and remarkable biocompatibility.
collagen
biomedical applications
recombinant collagen
marine collagen
animal collagen
1. Introduction
Collagen plays a crucial role in the structural integrity of various tissues in living organisms. It is widely distributed throughout the body, spanning from the skin to the cornea of the eye. Collagen serves as a predominant component in several tissues, including skin, bone, tendon, cartilage, blood vessels, and teeth. The diverse functions of collagen in these tissues necessitate the presence of different collagen types, each exhibiting unique interactions with other collagen molecules and the surrounding tissue components. While over 29 types of collagen have been identified, collagen types I to V are the most abundant and extensively studied for their biomedical applications [1]. Type I collagen, also the most abundant type of collagen in the human body, is primarily derived from bovine or porcine sources, specifically from skin, tendons, and bones. Type II collagen, on the other hand, is predominantly located in cartilage, particularly within joint tissues. Type III collagen is commonly found in the skin, blood vessels, and internal organs. As for Type IV collagen, it serves as a key constituent of basement membranes and can be obtained from the human placenta. Finally, Type V collagen, which is mainly utilized in the context of corneal treatment, is found in structures such as hair, cell surfaces, and the placenta [2].
Collagen types exhibit significant variations in their amino acid compositions, leading to distinct chemical and physical properties. These variations impact parameters that govern their biomedical utilization, like thermal stability, solution viscosity, and cross-linking density [3][4]. Within collagen, specific amino acid sequences play a crucial role as substrates for integrins, a class of transmembrane receptors facilitating cell–extracellular matrix (ECM) adhesion. Integrin receptors consist of α and β subunits that form transmembrane heterodimers. The binding specificity demonstrated by various receptors implies that structural differences in collagens derived from different sources will result in functional changes in their interactions [5]. In the past, animal collagen was the primary choice for various biological applications. However, as the field has advanced, other sources like marine-derived and synthetically produced recombinant collagen have gained significant attention (Figure 1).
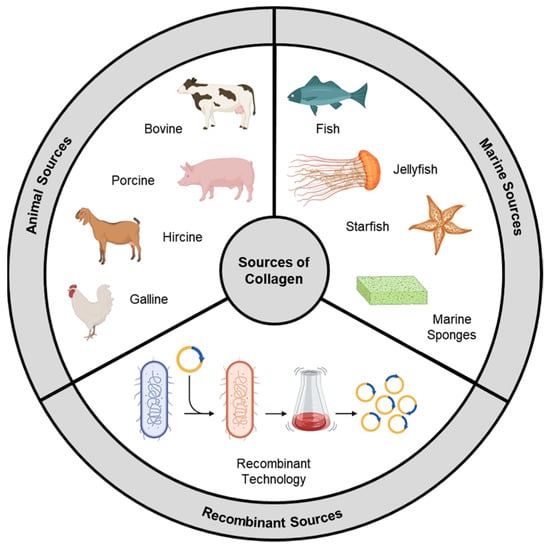
Figure 1. Sources of collagen.
2. Animal Source
Animal collagen is a conventional and predominant source of collagen for biomedical applications. It is derived from various tissues and organs of vertebrate animals, with a primary emphasis on mammals. This source offers several advantages due to its abundance, similarity to human collagen, and suitability for a diverse range of in vivo applications. The livestock industry has been thriving for many decades, and when animals are processed for meat, not all parts are fit for human consumption. Hides and other non-edible parts, including bones, tendons, ligaments, and connective tissues, contain significant amounts of collagen. These collagen-rich byproducts can be processed to extract a significant amount of collagen [6].
Approximately one-third of the protein mass of bovine (beef) species consists of collagen, with tendons containing up to 60–85% collagen [7]. Bovine collagen primarily falls into the category of type I collagen and is characterized by its elongated fibrillar structure, which imparts tensile strength and structural integrity to a variety of tissues. It is composed of two α1 chains and one α2 chain, forming a triple helix conformation. Apart from tendons, type I collagen is abundant in ligaments, bones, and other connective tissues [8]. Type III collagen, on the other hand, is exclusively found in the skin. The yield of collagen during the isolation process varies depending on the age of the bovine tissue, with younger tissues generally yielding higher amounts of collagen. Additionally, the distribution of proteoglycans and post-translational modifications also varies with age, potentially influencing the thermal stability and fibrous self-assembly [9]. Bovine collagen exhibits favorable characteristics of biocompatibility and low immunogenicity. It is generally well-tolerated in vivo and does not induce an immune response in the majority of individuals, except in cases of significant collagen allergies [10]. Porcine (pork) collagen, derived from pigskin, bones, and intestines, can be extracted with a high yield (mainly type I). It exhibits exceptional tensile strength, cell adhesion, and proliferation-promoting properties, rendering it highly suitable as a biomaterial for implantation or reconstructive surgeries [11].
Bovine and porcine collagen are extensively researched and widely used sources of collagen. However, their applicability may be limited in certain regions or among specific populations due to religious beliefs and dietary practices. Other animal-derived collagen sources include ovine (lamb), hircine (goat), equine (horse), and galline (chicken) [12]. While these animal sources can be obtained on an industrial scale and are suitable for commercial applications, among researchers in academia, rat-tail tendon collagen is the most commonly utilized source of type I collagen. The preference for rat-tail tendon collagen in academic research can be attributed to the widespread use of rats as animal models in scientific investigations. The tails of rats are easily accessible and can be isolated after the termination of studies. Rat-tail tendons are particularly advantageous because they contain a high concentration of type I collagen, ranging from 90 to 95% by weight. This characteristic makes rat-tail tendon collagen a convenient, cost-effective, and high-yield source for researchers [13].
3. Marine Source
The use of mammal-derived collagen is constrained by potential risks associated with immune reactions and the transmission of dangerous zoonotic diseases like bovine spongiform encephalopathies. In recent years, there has been a growing interest in marine organisms as alternative and safe sources of collagen [14]. Biomass derivatives from fish-processing industries and fisheries, including fish and sea urchin waste, undersized fish, and by-catch organisms like jellyfish, starfish, and sponges, hold potential as valuable but currently underutilized sources of collagen [15]. The global per capita consumption of fish has significantly increased (from 9.0 kg in 1961 to 20.5 kg in 2019), resulting in a substantial amount of marine waste, comprising discarded fish parts such as skin, scales, and skeletons (including heads, tails, and fins), as well as low-quality whole fish and non-edible marine species like echinoderms. With approximately 70–85% of the total catch weight being generated as waste or low-value byproducts, there is a compelling incentive to extract valuable bioactive compounds, including collagen, from marine debris to enhance the environmental and social sustainability of the fishing industry [16]. The utilization of fish byproducts as collagen sources not only promotes eco-friendliness but also offers attractive prospects in terms of profitability and cost-effectiveness.
Skin and scales obtained from bighead carp, catla carp (Indian carp), globefish, paddlefish, and Japanese sea bass are preferred sources for marine collagen extraction due to their naturally high collagen content [17][18]. By employing suitable isolation techniques, the yield of collagen extracted from marine sources can reach up to 50% of the dry mass [19]. Marine collagen exhibits comparable biocompatibility and functionality to mammalian collagen, as the genetic sequence of collagen is generally conserved and similar across species. Moreover, marine collagen poses a lower immunological risk to individuals allergic to mammalian products, as marine tissues lack mammalian antigens such as alpha-gal.
Numerous studies have demonstrated that fish collagen, as well as the collagen of most jellyfish species, is characterized by the typical repetitive sequence (Glycine-X-Y)n, where X and Y are imino acid residues. Fish collagen exhibits high concentrations of glycine (approximately 30%) and hydroxyproline (8–10%), while levels of histidine, a precursor to histamines associated with allergic responses, are relatively low [20]. The presence of imino acids plays a crucial role in maintaining the structural integrity of collagen. The pyrrolidine rings in imino acids restrict the flexibility of the polypeptide chain, reinforcing the triple helix structure and influencing the thermal stability of the collagen molecule. As a result, the denaturation temperature of collagen increases with the imino acid content [21]. Cold-water fish species typically possess lower imino acid content, resulting in collagen with a lower denaturation/melting temperature (approximately 15–20 °C) compared to warm-water species (approximately 30–35 °C). To enhance the thermal stability of marine collagen, suitable cross-linking treatments can be employed, such as those based on carbodiimide or glutaraldehyde. These treatments help to improve the collagen’s resistance to thermal denaturation [22].
4. Recombinant Collagen
Recombinant collagen is produced through genetic engineering techniques using recombinant DNA technology. Unlike animal-derived or marine-derived collagen, recombinant collagen is synthesized in a laboratory setting, offering several advantages and opportunities for customization in biomedical applications. Its production involves the introduction of specific collagen genes or gene fragments into host cells [23]. These host cells are modified to express and produce the desired collagen protein. The introduced genes are often derived from human or animal collagen sequences to ensure the production of collagen that closely resembles native collagen found in living organisms. Uninterrupted large-scale production and precise control over the product’s composition/properties are key advantages [24].
Through genetic engineering techniques, specific modifications can be made to the collagen sequence, allowing for the incorporation of desirable functionalities or targeting specific applications. For example, the introduction of specific amino acid sequences or motifs can promote cell adhesion, enhance tissue regeneration, or improve the stability of the collagen scaffold [25].
The assembly of recombinant collagen into higher-order structures is an essential aspect of its structural properties. Native collagen forms fibrils and networks that contribute to the strength and stability of tissues. Recombinant collagen can also self-assemble into fibrils, mimicking the natural behavior of collagen [26]. The structural properties of recombinant collagen can be further modulated by various post-translational modifications, such as glycosylation or hydroxylation. These modifications, which occur naturally in collagen biosynthesis, can be introduced during the recombinant production process to enhance the stability, functionality, and bioactivity of the collagen protein [27].
The characterization and quality control of recombinant collagen are crucial in its production. Techniques such as SDS-PAGE, mass spectrometry, and circular dichroism spectroscopy are employed to assess the molecular weight, purity, and secondary structure of the recombinant collagen [28]. These analyses ensure that the synthesized collagen meets the desired specifications for its intended application. While recombinant collagen offers numerous advantages, there are also challenges associated with its production. Achieving the correct folding and assembly of the recombinant collagen protein to form the native triple helical structure can be complex. Various strategies, such as the co-expression of molecular chaperones or the use of specialized expression systems, are employed to enhance the proper folding and assembly of recombinant collagen [29].
References
- Noorzai, S.; Verbeek, C.J.R.; Noorzai, S.; Verbeek, C.J.R. Collagen: From Waste to Gold. In Biotechnological Applications of Biomass; InTech Open: London, UK, 2020.
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868.
- Sorushanova, A.; Skoufos, I.; Tzora, A.; Mullen, A.M.; Zeugolis, D.I. The Influence of Animal Species, Gender and Tissue on the Structural, Biophysical, Biochemical and Biological Properties of Collagen Sponges. J. Mater. Sci. Mater. Med. 2021, 32, 1–12.
- Ali, S.M.; Patrawalla, N.Y.; Kajave, N.S.; Brown, A.B.; Kishore, V. Species-Based Differences in Mechanical Properties, Cytocompatibility, and Printability of Methacrylated Collagen Hydrogels. Biomacromolecules 2022, 23, 5137–5147.
- Zeltz, C.; Gullberg, D. The Integrin-Collagen Connection—A Glue for Tissue Repair? J. Cell Sci. 2016, 129, 653–664.
- Lutfee, T.; Alwan, N.F.; Alsaffar, M.A.; Ghany, M.A.R.A.; Mageed, A.K.; AbdulRazak, A.A. An Overview of the Prospects of Extracting Collagens from Waste Sources and Its Applications. Chem. Pap. 2021, 75, 6025–6033.
- Thorpe, C.T.; Birch, H.L.; Clegg, P.D.; Screen, H.R.C. The Role of the Non-Collagenous Matrix in Tendon Function. Int. J. Exp. Pathol. 2013, 94, 248–259.
- Mokrejs, P.; Langmaier, F.; Mladek, M.; Janacova, D.; Kolomaznik, K.; Vasek, V. Extraction of Collagen and Gelatine from Meat Industry By-Products for Food and Non Food Uses. Waste Manag. Res. 2009, 27, 31–37.
- Ferraro, V.; Gaillard-Martinie, B.; Sayd, T.; Chambon, C.; Anton, M.; Santé-Lhoutellier, V. Collagen Type I from Bovine Bone. Effect of Animal Age, Bone Anatomy and Drying Methodology on Extraction Yield, Self-Assembly, Thermal Behaviour and Electrokinetic Potential. Int. J. Biol. Macromol. 2017, 97, 55–66.
- Sheehy, E.J.; Cunniffe, G.M.; O’Brien, F.J. Collagen-Based Biomaterials for Tissue Regeneration and Repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier: Amsterdam, The Netherlands, 2018; pp. 127–150.
- Lin, Z.; Nica, C.; Sculean, A.; Asparuhova, M.B. Enhanced Wound Healing Potential of Primary Human Oral Fibroblasts and Periodontal Ligament Cells Cultured on Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 3819.
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-Based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999.
- Rittié, L. Type I Collagen Purification from Rat Tail Tendons. Methods Mol. Biol. 2017, 1627, 287–308.
- Eser, B.E.; Gozde, K.I. Marine Collagen. Stud. Nat. Prod. Chem. 2021, 71, 121–139.
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214.
- Pal, G.K.; Suresh, P.V. Sustainable Valorisation of Seafood By-Products: Recovery of Collagen and Development of Collagen-Based Novel Functional Food Ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215.
- Jia, Y.; Wang, H.; Wang, H.; Li, Y.; Wang, M.; Zhou, J. Biochemical Properties of Skin Collagens Isolated from Black Carp (Mylopharyngodon Piceus). Food Sci. Biotechnol. 2012, 21, 1585–1592.
- Pal, G.K.; Nidheesh, T.; Suresh, P.V. Comparative Study on Characteristics and in Vitro Fibril Formation Ability of Acid and Pepsin Soluble Collagen from the Skin of Catla (Catla Catla) and Rohu (Labeo Rohita). Food Res. Int. 2015, 76, 804–812.
- Krishnan, S.; Sekar, S.; Katheem, M.F.; Krishnakumar, S.; Sastry, T.P. Fish Scale Collagen—A Novel Material for Corneal Tissue Engineering. Artif. Organs 2012, 36, 829–835.
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine Collagen and Its Derivatives: Versatile and Sustainable Bio-Resources for Healthcare. Mater. Sci. Eng. C 2020, 113, 110963.
- Bao, Z.; Sun, Y.; Rai, K.; Peng, X.; Wang, S.; Nian, R.; Xian, M. The Promising Indicators of the Thermal and Mechanical Properties of Collagen from Bass and Tilapia: Synergistic Effects of Hydroxyproline and Cysteine. Biomater. Sci. 2018, 6, 3042–3052.
- Liu, S.; Lau, C.S.; Liang, K.; Wen, F.; Teoh, S.H. Marine Collagen Scaffolds in Tissue Engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103.
- Wang, T.; Lew, J.; Premkumar, J.; Poh, C.L.; Naing, M.W. Production of Recombinant Collagen: State of the Art and Challenges. Eng. Biol. 2017, 1, 18–23.
- Ramshaw, J.A.M.; Werkmeister, J.A.; Glattauer, V. Recent Progress with Recombinant Collagens Produced in Escherichia Coli. Curr. Opin. Biomed. Eng. 2019, 10, 149–155.
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155.
- Ilamaran, M.; Janeena, A.; Valappil, S.; Ramudu, K.N.; Shanmugam, G.; Niraikulam, A. A Self-Assembly and Higher Order Structure Forming Triple Helical Protein as a Novel Biomaterial for Cell Proliferation. Biomater. Sci. 2019, 7, 2191–2199.
- Beygmoradi, A.; Homaei, A.; Hemmati, R.; Fernandes, P. Recombinant Protein Expression: Challenges in Production and Folding Related Matters. Int. J. Biol. Macromol. 2023, 233, 123407.
- Gibney, R.; Patterson, J.; Ferraris, E. High-Resolution Bioprinting of Recombinant Human Collagen Type Iii. Polymers 2021, 13, 2973.
- Liu, W.; Lin, H.; Zhao, P.; Xing, L.; Li, J.; Wang, Z.; Ju, S.; Shi, X.L.; Liu, Y.; Deng, G.; et al. A Regulatory Perspective on Recombinant Collagen-Based Medical Devices. Bioact. Mater. 2022, 12, 198–202.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
16 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





