Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Laura Carucci | -- | 1284 | 2023-08-15 08:26:14 | | | |
| 2 | Conner Chen | Meta information modification | 1284 | 2023-08-15 09:36:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Coppola, S.; Carucci, L.; Oglio, F.; Di Sarra, C.; Ozen, G.; Berni Canani, R. Cow’s Milk Allergy Preventive Nutritional Strategies. Encyclopedia. Available online: https://encyclopedia.pub/entry/48063 (accessed on 08 February 2026).
Coppola S, Carucci L, Oglio F, Di Sarra C, Ozen G, Berni Canani R. Cow’s Milk Allergy Preventive Nutritional Strategies. Encyclopedia. Available at: https://encyclopedia.pub/entry/48063. Accessed February 08, 2026.
Coppola, Serena, Laura Carucci, Franca Oglio, Claudia Di Sarra, Gulsum Ozen, Roberto Berni Canani. "Cow’s Milk Allergy Preventive Nutritional Strategies" Encyclopedia, https://encyclopedia.pub/entry/48063 (accessed February 08, 2026).
Coppola, S., Carucci, L., Oglio, F., Di Sarra, C., Ozen, G., & Berni Canani, R. (2023, August 15). Cow’s Milk Allergy Preventive Nutritional Strategies. In Encyclopedia. https://encyclopedia.pub/entry/48063
Coppola, Serena, et al. "Cow’s Milk Allergy Preventive Nutritional Strategies." Encyclopedia. Web. 15 August, 2023.
Copy Citation
Cow’s milk allergy (CMA) is one of the most common pediatric food allergies. The prevalence and severity of CMA have increased dramatically in the last decades, under the pressure of environmental factors in genetically predisposed individuals. Among the environmental influences, nutritional factors play a crucial role. Diet is the most modifiable factor, representing a potential target for the prevention and treatment of CMA.
food allergy
gut microbiome
immunonutrition
1. Introduction
Cow’s milk allergy (CMA) is one of the most common pediatric food allergies (FAs), affecting up to 3% of the children population. CMA derives from a breakdown of immune tolerance against cow’s milk proteins (α-lactalbumin, β-lactoglobulin, serum albumin, caseins, bovine serum albumins and others) that generally occurs in the first years of life [1]. Commercial milk is subjected to a heat treatment process to make it sterile, which through the Maillard reaction can induce the formation of deleterious compounds, such as the advanced glycation end products Nε-(carboxyethyl) lysine, Nε-(carboxymethyl) lysine, pentosidine, pyrraline, methylglyoxal-lysine dimer, glyoxal-lysine dimer and argpyrimidine, that may play a role in the pathogenesis of allergies [2]. CMA may present different phenotypes based on the immune mechanisms: IgE mediated, non-IgE mediated or mixed [1]. Infants with IgE-CMA may present from gastrointestinal (i.e., vomiting and diarrhea), cutaneous (i.e., erythema, urticarial and angioedema), respiratory and/or systemic symptoms up to the most severe reaction anaphylaxis, occurring within 2 h after cow’s milk exposure. Non-IgE-CMA is characterized by subacute or chronic gastrointestinal symptoms and affected infants may present bloody stools, delayed vomiting, crying and chronic diarrhea with the risk of malnutrition and failure to thrive [1]. The diagnosis of CMA requires a positive oral food challenge to cow’s milk protein; that is the gold standard test to confirm CMA [3][4]. The type and the severity of CMA symptoms dramatically worsened in the last years, as well the epidemiology picture showed an increase in CMA prevalence and persistence [5][6]. Several hypotheses have been postulated to explain the spread of CMA in the last years and the most likely one seems related to an impaired gene-environment interaction [6][7]. Some dietary habits (i.e., the Western diet), infections, cesarean delivery, formula consumption in the first week of life and the massive use of drugs in the first stage of life have been proposed as the main environmental factors responsible for the occurrence of FAs, including CMA [8][9][10]. Indeed, operating on an unfavorable genetic background, these factors impair the gut microbiome (GM), with consequent alterations of the GM-immune system axis. The perturbation of this axis could lead to a breakdown of immune tolerance and to CMA occurrence [9][10]. On the other hand, the Mediterranean diet, vaginal delivery and breastfeeding could positively modulate the axis and may represent an innovative approach to prevent and treat GM-immune system-derived diseases, such as CMA [9][10][11]. Among the environmental factors, diet represents one of the main modifiable ones. Since dietary habits have a role in eliciting potentially negative or positive effects in CMA occurrence, nutritional modulation could be considered an effective target for the prevention and management of CMA. The potential to influence the immune system functionality of selected dietary habits has been described with the term “immunonutrition” that, in the FA field, is based on a proactive approach focused on the prevention and the acquisition of immune tolerance in allergy treatment [12].
2. Cow’s Milk Allergy Preventive Nutritional Strategies
The alarming increasing rate of CMA prevalence advocates the necessity for effective preventive nutritional strategies against the disease burden.
The CMA’s primary prevention should start from the prenatal period, focusing on a maternal healthy lifestyle and food diversity during pregnancy [13]. Maternal diet during pregnancy has been considered a potential target for allergy prevention. Maternal diet may affect, through direct or indirect mechanisms, infant GM, which is associated with a range of allergy outcomes [13][14][15]. High adherence to the Mediterranean diet has been reported to increase GM diversity [16]. Thus, a maternal diet rich in Mediterranean diet-based foods, including vegetables and yogurt, was associated with protective effects for offspring allergies [17]. On the contrary, dietary intake of Western heat-processed foods high in advanced glycation end products (e.g., fried foods, red and processed meat and fruit juice), has been associated with a reduced diversity of GM and the occurrence of pediatric allergies [2]. In addition, the maternal avoidance of allergenic solids foods during pregnancy and lactation have been considered ineffective for CMA prevention and no recommendation by international guidelines have been provided [8]. Indeed, the maternal intake of allergenic solids foods during pregnancy could ensure the placental transmission of inhibitory IgG- allergen immune complexes, reducing the risk of pediatric allergy occurrence [18][19]. Furthermore, no international recommendations for or against the use of prebiotics, probiotics or synbiotics during breastfeeding and lactation alone or in combination with other approaches to prevent pediatric allergies have been formulated [8].
Through a positive modulation of the GM and the proper development of the immune system during infancy, breastfeeding is the first nutritional postnatal factor able to protect against allergy occurrence [20]. Several protective mechanisms of breastfeeding have been proposed. Breast milk has anti-allergic immune properties and contains a large amount of biologically active compounds, including lysozyme, lactoferrin, immunoglobulins (Ig)A, IgM, cytokines, nucleotides, microRNAs and hormones that provide passive immunity and could induce oral tolerance to food antigens [21][22][23]. Among the most abundant protective component of breast milk, are the human milk oligosaccharides (HMOs) and prebiotics, resulting in the production of sub-products such as lactate and short-chain fatty acids (SCFAs) and metabolites, able to modulate the immune system function [24]. In particular, the SCFA butyrate enhances the suppressive capacity of regulatory T cells (Treg), suppressing the allergic response and sustaining immune tolerance to allergens in the offspring [25][26]. Butyrate in human milk modulates the mechanisms of immune tolerance, including the increase in biomarkers of gut barrier integrity and tolerogenic cytokines in concentrations able to protect against allergy occurrence [27]. Nevertheless, due to the low certainty of evidence, no recommendations for or against using breastfeeding to prevent food allergy or CMA have been provided at the European level [8]. Thus, considering the multiple benefits for infants and mothers, breastfeeding should be encouraged wherever possible, as stated by most scientific society guidelines [28]. Notably, guidelines from the European Academy of Allergy and Clinical Immunology (EAACI) recommend avoiding supplementation with cow’s milk formula in the first week of life. Other possible temporary supplementary options for breastfed infants could include donor breast milk, amino acid or hydrolyzed formula [8]. Regarding the introduction of complementary foods in infants’ diets for allergy prevention, the European guidelines recommend the importance of not avoiding the intake of potentially allergenic foods during weaning, emphasizing that there is no reason for delaying their introduction after 12 months nor for an early introduction <4 months [29]. Evidence supports the role of early exposure to potential allergens in the development of immune tolerance [30][31]; indeed, the regular ingestion of food allergens between 4 and 6 months of life can lead to immune tolerance and alter the immunological responses to food antigens; conversely, the skin passage of food antigens in the condition of inflammation, before the achievement of immune tolerance, can lead to sensitization to food allergens [32]. In addition, the infants’ diet, influencing the GM composition and function, could have a pivotal role in protecting against the occurrence of food allergy. Evidence has shown that a high diet diversity and the introduction in the first year of life of fruits, vegetables, yogurt and fish, through a fecal increase in the tolerogenic metabolite butyrate, is associated with protection against the development of allergies, even in later stages of life [33][34]. Figure 1 shows a summary of the protective and risk nutritional factors in CMA occurrence.
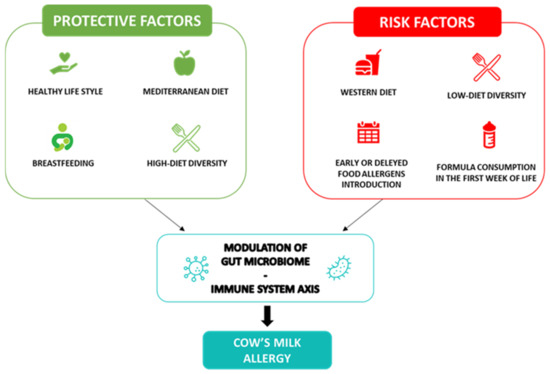
Figure 1. Cow’s milk allergy protective and risk nutritional factors. Legend: The figure depicts the nutritional protective and risk factors in CMA.
References
- Jensen, S.A.; Fiocchi, A.; Baars, T.; Jordakieva, G.; Nowak-Wegrzyn, A.; Pali-Schöll, I.; Passanisi, S.; Pranger, C.L.; Roth-Walter, F.; Takkinen, K.; et al. Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines up-date—III—Cow’s milk allergens and mechanisms triggering immune activation. World Allergy Organ. J. 2022, 15, 100668.
- Smith, P.K.; Masilamani, M.; Li, X.M.; Sampson, H.A. The false alarm hypothesis: Food allergy is associated with high dietary advanced glycation end-products and proglycating dietary sugars that mimic alarmins. J. Allergy Clin. Immunol. 2017, 139, 429–437.
- Bird, J.A.; Leonard, S.; Groetch, M.; Assa’ad, A.; Cianferoni, A.; Clark, A.; Crain, M.; Fausnight, T.; Fleischer, D.; Green, T.; et al. Conducting an Oral Food Challenge: An Update to the 2009 Adverse Reactions to Foods Committee Work Group Report. J. Allergy Clin. Immunol. Pract. 2020, 8, 75–90.e17.
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025.
- Nocerino, R.; Leone, L.; Cosenza, L.; Berni Canani, R. Increasing rate of hospitalizations for food-induced anaphylaxis in Italian children: An analysis of the Italian ministry of health database. J. Allergy Clin. Immunol. 2015, 135, 833–835.e3.
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk. Allergy Nutr. 2019, 11, 1051.
- Neeland, M.R.; Martino, D.J.; Allen, K.J. The role of gene-environment interactions in the development of food allergy. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 1371–1378.
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858.
- Aitoro, R.; Paparo, L.; Amoroso, A.; Di Costanzo, M.; Cosenza, L.; Granata, V.; Di Scala, C.; Nocerino, R.; Trinchese, G.; Montella, M.; et al. Gut Microbiota as a Target for Preventive and Therapeutic Intervention against Food Allergy. Nutrients 2017, 9, 672.
- Devonshire, A.; Gautam, Y.; Johansson, E.; Mersha, T.B. Multi-omics profiling approach in food allergy. World Allergy Organ. J. 2023, 16, 100777.
- Lee, K.H.; Song, Y.; Wu, W.; Yu, K.; Zhang, G. The gut microbiota, environmental factors, and links to the development of food allergy. Clin. Mol. Allergy 2020, 18, 5.
- Carucci, L.; Coppola, S.; Luzzetti, A.; Voto, L.; Giglio, V.; Paparo, L.; Nocerino, R.; Berni Canani, R. Immunonutrition for Pediatric Patients with Cow’s Milk Allergy: How Early Interventions Could Impact Long-Term Outcomes. Front. Allergy 2021, 2, 676200.
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912.
- Selma-Royo, M.; García-Mantrana, I.; Calatayud, M.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Maternal diet during pregnancy and intestinal markers are associated with early gut microbiota. Eur. J. Nutr. 2021, 60, 1429–1442.
- García-Mantrana, I.; Selma-Royo, M.; González, S.; Parra-Llorca, A.; Martínez-Costa, C.; Collado, M.C. Distinct maternal microbiota clusters are associated with diet during pregnancy: Impact on neonatal microbiota and infant growth during the first 18 months of life. Gut Microbes 2020, 11, 962–978.
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821.
- Venter, C.; Palumbo, M.P.; Glueck, D.H.; Sauder, K.A.; O’Mahony, L.; Fleischer, D.M.; Ben-Abdallah, M.; Ringham, B.M.; Dabelea, D. The maternal diet index in pregnancy is associated with offspring allergic diseases: The Healthy Start study. Allergy 2022, 77, 162–172.
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A., Jr.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J. Allergy Clin. Immunol. 2014, 133, 1373–1382.
- Uthoff, H.; Spenner, A.; Reckelkamm, W.; Ahrens, B.; Wölk, G.; Hackler, R.; Hardung, F.; Schaefer, J.; Scheffold, A.; Renz, H.; et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J. Immunol. 2003, 171, 3485–3492.
- Järvinen, K.M.; Martin, H.; Oyoshi, M.K. Immunomodulatory effects of breast milk on food allergy. Ann. Allergy Asthma Immunol. 2019, 123, 133–143.
- Yeruva, L.; Munblit, D.; Collado, M.C. Editorial: Impact of Early Life Nutrition on Immune System Development and Related Health Outcomes in Later Life. Front. Immunol. 2021, 25, 668569.
- Adel-Patient, K.; Bernard, H.; Fenaille, F.; Hazebrouck, S.; Junot, C.; Verhasselt, V. Prevention of Allergy to a Major Cow’s Milk Allergen by Breastfeeding in Mice Depends on Maternal Immune Status and Oral Exposure During Lactation. Front. Immunol. 2020, 21, 1545.
- Ohsaki, A.; Venturelli, N.; Buccigrosso, T.M.; Osganian, S.K.; Lee, J.; Blumberg, R.S.; Oyoshi, M.K. Maternal IgG immune complexes induce food allergen-specific tolerance in offspring. J. Exp. Med. 2018, 215, 91–113.
- Plaza-Díaz, J.; Fontana, L.; Gil, A. Human Milk Oligosaccharides and Immune System Development. Nutrients 2018, 10, 1038.
- Vonk, M.M.; Blokhuis, B.R.J.; Diks, M.A.P.; Wagenaar, L.; Smit, J.J.; Pieters, R.H.H.; Garssen, J.; Knippels, L.M.J.; van Esch, B.C.A.M. Butyrate Enhances Desensitization Induced by Oral Immunotherapy in Cow’s Milk Allergic Mice. Mediat. Inflamm. 2019, 16, 9062537.
- Folkerts, J.; Redegeld, F.; Folkerts, G.; Blokhuis, B.; van den Berg, M.P.M.; de Bruijn, M.J.W.; van IJcken, W.F.J.; Junt, T.; Tam, S.Y.; Galli, S.J.; et al. Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling. Allergy 2020, 75, 1966–1978.
- Paparo, L.; Nocerino, R.; Ciaglia, E.; Di Scala, C.; De Caro, C.; Russo, R.; Trinchese, G.; Aitoro, R.; Amoroso, A.; Bruno, C.; et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy 2021, 76, 1398–1415.
- WHO. Maternal, Infant and Young Child Nutrition. Available online: https://apps.who.int/iris/handle/10665/250636 (accessed on 26 June 2023).
- Muraro, A.; Halken, S.; Arshad, S.H.; Beyer, K.; Dubois, A.E.; Du Toit, G.; Eigenmann, P.A.; Grimshaw, K.E.; Hoest, A.; Lack, G.; et al. EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 2014, 69, 590–601.
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. LEAP Study Team. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813.
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Plaut, M.; Bahnson, H.T.; Mitchell, H.; Radulovic, S.; Chan, S.; Fox, A.; Turcanu, V.; et al. Identifying infants at high risk of peanut allergy: The Learning Early About Peanut Allergy (LEAP) screening study. J. Allergy Clin. Immunol. 2013, 131, 135–143.e12.
- Lack, G. Update on risk factors for food allergy. J. Allergy Clin. Immunol. 2012, 129, 1187–1197.
- Roduit, C.; Frei, R.; Depner, M.; Schaub, B.; Loss, G.; Genuneit, J.; Pfefferle, P.; Hyvärinen, A.; Karvonen, A.M.; Riedler, J.; et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J. Allergy Clin. Immunol. 2014, 133, 1056–1064.
- Roduit, C.; Frei, R.; Ferstl, R.; Loeliger, S.; Westermann, P.; Rhyner, C.; Schiavi, E.; Barcik, W.; Rodriguez-Perez, N.; Wawrzyniak, M.; et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy 2019, 74, 799–809.
More
Information
Subjects:
Allergy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
537
Revisions:
2 times
(View History)
Update Date:
15 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




