Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katharina N. Muth | -- | 2567 | 2023-08-11 09:53:46 | | | |
| 2 | Jessie Wu | Meta information modification | 2567 | 2023-08-11 10:59:40 | | | | |
| 3 | Jessie Wu | Meta information modification | 2567 | 2023-08-11 11:00:35 | | | | |
| 4 | Jessie Wu | + 4 word(s) | 2571 | 2023-08-14 06:01:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Muth, K.N.; Rech, J.; Losch, F.O.; Hoerning, A. Tumor Necrosis Factor-α Inhibitors. Encyclopedia. Available online: https://encyclopedia.pub/entry/47940 (accessed on 08 February 2026).
Muth KN, Rech J, Losch FO, Hoerning A. Tumor Necrosis Factor-α Inhibitors. Encyclopedia. Available at: https://encyclopedia.pub/entry/47940. Accessed February 08, 2026.
Muth, Katharina N., Juergen Rech, Florian O. Losch, André Hoerning. "Tumor Necrosis Factor-α Inhibitors" Encyclopedia, https://encyclopedia.pub/entry/47940 (accessed February 08, 2026).
Muth, K.N., Rech, J., Losch, F.O., & Hoerning, A. (2023, August 11). Tumor Necrosis Factor-α Inhibitors. In Encyclopedia. https://encyclopedia.pub/entry/47940
Muth, Katharina N., et al. "Tumor Necrosis Factor-α Inhibitors." Encyclopedia. Web. 11 August, 2023.
Copy Citation
Immune-mediated inflammatory diseases, such as rheumatoid arthritis, psoriatic arthritis, peripheral and/or axial spondyloarthritis, Crohn’s disease, and ulcerative colitis, are characterized by molecular and cellular changes in the immune system. Due to the systemic nature of these diseases, organs such as the liver or cardiovascular system are often affected by the inflammatory process. Tumor necrosis factor-α inhibitor therapy reduces the activation of pro-inflammatory signaling cascades, mitigates the chronic inflammatory process by restoring cellular balance, and alleviates clinical consequences, such as pain and tissue damage.
cytokines
immune-mediated inflammatory diseases
inflammation
rheumatoid arthritis
crohn's disease
colitis ulcerosa
tumor-necrosis alpha inhibitor
1. Tumor Necrosis Factor-α Inhibitor Therapy Restores Cytokine Balance and Normalizes Organ Function
TNF-α inhibitors can counteract the entire disease process in patients with chronic inflammatory diseases (e.g., RA, psoriatic arthritis (PsA), CD, and ulcerative colitis (UC)) by fundamentally modulating the molecular and cellular changes of several inflammatory networks of the innate immune system [1][2][3][4][5][6][7]. The focus is on the reduction of TNF-α due to TNF-α inhibitors and the subsequent pro-inflammatory cytokines, such as IL-1, IL-6, or IFN-γ [3][5][6][7], and, thus, the restoration of cellular homeostasis. The decrease in these pro-inflammatory cytokines leads to the normalized differentiation of T cell subtypes, which increases the number of anti-inflammatory cells (e.g., Treg cells and anti-inflammatory TH17 cells) and reduces the number of pathogenic TH17.1 cells [3][8][9]. Treg cells can now exert their suppressing function by releasing the anti-inflammatory cytokines IL-10 and TGF-β, which subsequently influence cytokine release by effector T cells (Figure 1). In IBD patients, it has been shown that therapy with a TNF-α inhibitor can also exert a modulating effect on specific subtypes of B lymphocytes (CD24hiCD38hi-B cells). The inhibition of TNF-α leads not only to a numeric reconstitution of B cellular subset distribution, but also achieves a normalization of IL-10 production. This observation reflects at least a reconstitution of the immune system [10]. Further studies have shown that a restoration of B cell numbers correlated with the biological response to the TNF-α inhibitor therapy [11][12]. Regarding the reconstituting effect, earlier studies have provided evidence that infliximab exerts its anti-inflammatory effects by modifying the IBD-characteristic cytokine networks. Ringheanu and colleagues found that infliximab downregulated the production of inflammatory cytokines by showing that monocytes produced less TNF-α, IL-1β, IL-6, and IL-8 mRNA, as measured at the RNA and protein level [13]. In addition, activated T cells isolated from colonic biopsies of patients with CD cultured in the presence of infliximab were found to decrease their expression of IFN-γ [14].
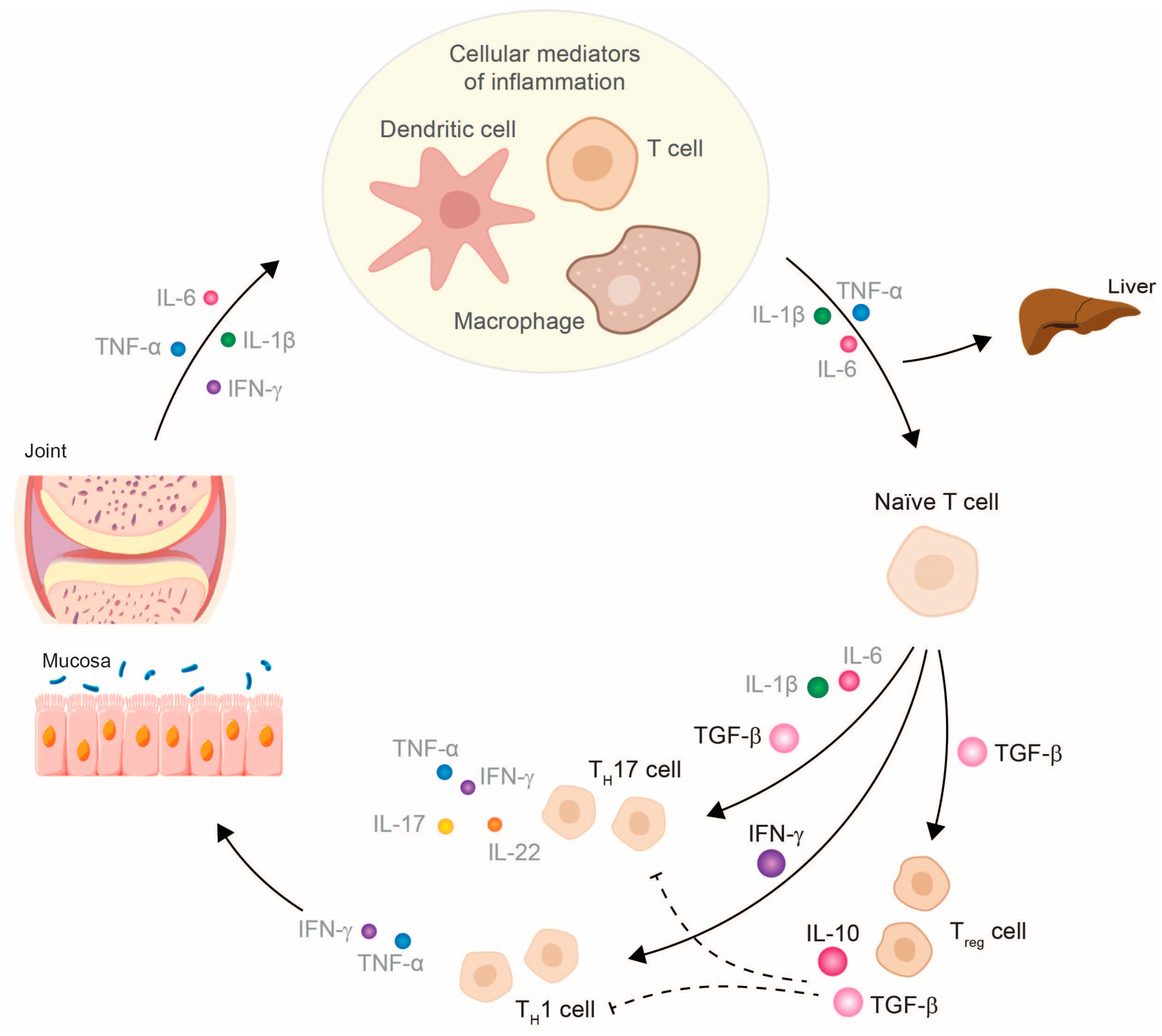
Figure 1. Therapy with a TNF-α inhibitor restores the cytokine balance in chronic inflammatory diseases and, thus, also the cellular balance. Abbreviations: IFN, interferon; IL, interleukin; TGF, tumor necrosis growth factor; TH, T helper cell; TNF, tumor necrosis factor; Treg, regulatory T cell. The size of the cytokines depicted schematically represents their released amount.
Further findings indicate that in IBD, TNF-α blockage has been shown to result in reduced intestinal permeability through decreased endothelial cell apoptosis and decreased permeability of tight junctions, increased Treg cell activity, reduced activity of various inflammatory mediators and T cells, and a reduction in inflammation-mediated mucosal angiogenesis by preventing the production of vascular endothelial growth factor A from intestinal fibroblasts [15][16].
Following the treatment of RA patients with infliximab, a restored population of Treg cells expressing low levels of CD62L was identified that mediates the suppression of T effector cells via TGF-β and IL-10, resulting in reduced IFN-γ production [17]. Patients clinically responding to the fully humanized anti-TNF-α antibody adalimumab also showed an increased percentage of FoxP3+ cells with restored regulatory function, as these cells suppress and resist conversion to TH17 cells [18].
Studies investigating the effects of anti-TNF-α therapy for B cell subsets in RA are scarce and yielded, in part, similar results when compared to the IBD setting. The frequency and the absolute number of mature/memory (CD24hiCD27+) cells, of immature (CD24hiCD38hi) transitional B cells, and of IL-10-producing Breg cells have been shown to be decreased in RA versus healthy controls, and increased after therapy with MTX together with the anti-TNF-α inhibitors, adalimumab or etanercept [19][20][21][22].
Consequently, the inhibition of TNF-α attenuates inflammatory reactions and allows anti-inflammatory signaling pathways to prevail again, giving the chance for a restoration of the homeostatic immunological balance [3][5][6][8][9][23]. TNF-α inhibitors also reduce the expression of molecules involved in chemotaxis, invasion, and the adhesion of immune cells at the site of inflammation, thereby reducing local inflammatory processes [6][8][24].
Therapy with TNF-α inhibitors can thus be used to break the vicious cycle of chronic inflammation early and effectively [25][26], since TNF-α blockade weakens several inflammatory networks and promotes anti-inflammatory processes [5][6][7][8][9][10][27]. Consequently, the blockade of TNF-α has a systemic effect and is not restricted to a specific inflammatory disease, which is why the development of further organic manifestations can be prevented and chronic comorbidities can be treated efficiently [1][2][28][29][30][31]. In contrast, the therapeutic inhibition of other pro-inflammatory cytokines, such as IL-1, IL-6, or IL-17/23, only affects downstream networks and, therefore, has a narrowly defined mode of action in the inflammatory process [2].
In the case of the liver, therapy with a TNF-α inhibitor can thus be associated with the normalization of liver function and lead to an improvement in hepatic cytochrome activity, since lower concentrations of inhibitory cytokines, such as IL-6 and IL-1Ra, are present [6][32][33][34][35][36][37][38]. In addition, TNF-α inhibitors cause a reduction in acute-phase proteins, such as fibrinogen, haptoglobin, hepcidin, and serum amyloid A in the liver [30][39][40].
Therapy with TNF-α inhibitors can reduce the risk of overall cardiovascular events, myocardial infarction, and serious venous thrombotic events (VTE) in RA patients [41]. The normalization of fibrinogen and thrombocytosis and the reduction of the expression of vascular endothelial growth factor, as well as of oxidative stress and endothelial dysfunction, by TNF-α inhibitors may reduce the relevant risk factors for atherosclerosis and cardiovascular events in patients with chronic inflammatory disease and increased cardiovascular risk, such as in RA [23][39][41][42][43]. Patients with IBD are at increased risk of thrombotic events. Thus, the epidemiological aspects and drug-related risks of venous (VTE) and arterial thrombotic events (ATE) were summarized in the Evidence-Based Guideline in order to provide recommendations for the optimized medical treatment of IBD patients. The Evidence-Based Guideline concluded that TNF-α inhibitors are associated with a decreased risk of VTE and arterial events, implicating a potentially protective role of TNF-α inhibitors against VTE in IBD patients [44]. In this regard, csDMARDs and JAK-inhibitors indicated for the treatment of chronic inflammatory diseases are associated with increased risks of cardiovascular events and VTE in comparison to TNF-α inhibitors [45][46].
CD is also associated with an increased risk of osteoporosis. A retrospective analysis suspected that TNF-α inhibitor therapy in post-menopausal/estrogen-deficient patients could prevent osteoporosis but further studies are needed for clarification of the impact of TNF-α inhibitors [47]. Therefore, organ functions and the general clinical condition of the patient improve due to the balance of pro-inflammatory and anti-inflammatory factors restored by therapy with a TNF-α inhibitor [35][36][39][40]. Notably, the comorbidities of patients with chronic inflammatory diseases need to be considered in treatment decision-making. Expert recommendations for the detection and monitoring of comorbidities in the treatment of psoriasis, PsA, and RA patients were developed to provide further guidance on achieving improved disease outcomes [48][49].
2. Tumor Necrosis Factor-α Inhibitor Therapy and Its Clinical Impact
With infliximab as the first biologic agent approved for the therapy of CD in 1998, TNF-α inhibitors have a long history regarding the treatment of chronic inflammatory diseases. By gaining approval in increasing numbers of indications, TNF-α inhibitors soon became an attractive treatment option for numerous chronic inflammatory diseases. TNF-α is involved at each stage of the pathogenesis of autoinflammation through its control of multiple cytokine networks and their activity. Hence, the blockade of TNF-α crucially results in the omission of subsequent pro-inflammatory signaling pathways regulated by subordinate cytokines. TNF-α inhibitors interrupt the cycle of inflammation early and effectively, thus, preventing the formation of comorbidities. For example, the development of extra-intestinal manifestations in pediatric CD patients could be significantly reduced, or even prevented, by the early use of infliximab [29][30][50].
Currently, five different TNF-α inhibitors of chronic inflammatory diseases are available: infliximab, etanercept, adalimumab, certolizumab pegol, and golimumab. Although all TNF-α inhibitors are designed to bind TNF-α, they have different molecular structures, which translate into different pharmacokinetic properties, binding affinities, as well as dosage regimens (Supplementary Table S1) [51]. Precisely, infliximab, adalimumab, and golimumab are anti-TNF-α monoclonal antibodies (mAbs), whereas certolizumab pegol is a pegylated single Fab’ antibody fragment and etanercept is a fusion protein, in which TNF receptor 2 is fused to the human IgG1-Fc domain. Adalimumab has the broadest spectrum of therapeutic approvals, ranging from uveitis and IBD to hidradenitis suppurativa; thus, patients with chronic inflammation manifested in various organ systems, such as PsA patients, could be treated with only one drug. Here, adalimumab demonstrates efficacy in several domains, i.e. peripheral arthritis, axial involvement, enthesitis, and dactylitis, as well as extra-articular manifestations [52].
Because TNF-α inhibitors have been on the market for a long time, their safety profiles for vulnerable patient populations, such as pediatric patients, can also be extensively established. Importantly, as chronic diseases mainly last for the whole lifetime, the effect of TNF-α treatment on the further course of the disease, and also with regard to the total health of pediatric patients, is of great relevance. It is therefore worth mentioning that TNF-α inhibitors are currently the only biologic drug approved for the anti-inflammatory therapy of pediatric patients suffering from IBD.
Furthermore, the early application of biologic agents significantly prevents treatment failure in pediatric IBD [29]. In 2020, a randomized trial directly comparing first-line infliximab with exclusive enteral nutrition or corticosteroids as a first-line treatment in pediatric patients with moderate-to-severe CD was provided by Jongsma and colleagues [25]. Of the patients treated with first-line anti-TNF-α therapy, a significantly higher percentage achieved clinical and endoscopic remission. In addition, first-line TNF-α-blocker therapy needed less dose escalation while achieving mucosal healing [25]. In addition, the probability of continued clinical remission at week 52 with azathioprine monotherapy was higher in children who received infliximab as a first-line remission-inducing therapy. Comparable results were shown for adalimumab [26].
TNF-α inhibitors have long been established for the treatment of JIA, and data for treatment duration of more than 10 years is available confirming its effectiveness and tolerability in children and adolescents [53]. This understanding and practical experience, especially for the treatment of children, is missing for other biological treatments that have recently been developed and approved, such as Janus kinase (JAK) inhibitors, where data collection on efficacy and safety is still ongoing.
Therapeutic strategies such as “hit hard and early” and “treat-to-target”, especially with TNF-α inhibitors, can modulate the course of the disease enormously, as they have been shown to lead to drug-free remission, lower disease activity, and higher functional status, which ultimately leads to an improvement in patients’ quality of life [54][55][56].
In CD, the early and effective use of TNF-α inhibitors has also been shown to prevent the development of disease complications, for example, strictures or penetrating ulcerations and disease progression, which led to a lower risk of surgery during disease progression and significantly reduced the risk of penetrating complications, but not stenosing complications [50].
The current research data provide evidence that treating patients with inflammatory diseases, such as CD or juvenile idiopathic arthritis (JIA), early after diagnosis with TNF-α inhibitors prevents not only disease progression, but also the loss of structural functioning and other disease complications [50][57]. Especially in young patients, an effective therapy with rapid onset is necessary to prevent growth retardation and structural damage. For pediatric patients with IBD, data from the RISK study demonstrates that patients treated early with TNF-α blockers regain weight and body growth is facilitated [31]. TNF-α inhibitors were shown to induce disease remission within three months, thus, being superior to other immunomodulators [58].
This concept of a “window of opportunity”, meaning that early treatment with TNF-α inhibitors can impact the further disease course, is also established in adult patients suffering from RA, PsA, and CD [59]. In PsA, TNF-α inhibitor treatment can decrease the rate of radiographic progression, thus, reducing structural damage, such as erosion and joint space narrowing [56]. The preservation of structural functions and, consequently, of physical activities is associated with a better quality of life of patients treated with TNF-α inhibitors, as reported by real-world data from PsA patients [55]. In this non-interventional study, TNF-α inhibitors resulted in significant improvements in disease activity indicators, i.e., DAS28-CRP and DAPSA28; in patient-reported outcomes; as well as in the improved working ability of patients [55]. This effect on structural preservation was also shown for etanercept in early RA, where it not only slowed the rate, but even halted the radiographic progression of the disease in most of the patients [55]. The prevention of the further functional disability of the patients allows them to participate in social life and to work, leading to improved well-being and quality of life. Consistently, early therapeutical intervention with biologics, i.e., TNF-α inhibitors, is supported by national and international guidelines for the treatment of patients with moderate-to-severe disabling CD. In a retrospective study, this therapy regimen was shown to positively affect the disease course as more patients achieved endoscopic remission while observing a reduced number of structural behavior issues and surgeries [57]. Thus, TNF-α inhibitors also indirectly contribute to cost savings, as fewer complications and lower hospitalization rates also reduce the burden on the healthcare system [60]. Currently, therapeutic drug monitoring (TDM) is being debated in terms of the management of TNF-α inhibitor therapy for inflammatory diseases, such as RA or IBD. It enables the physician to proactively adapt the therapy algorithm if the patient experiences no clinical response. This precise and individual therapy adjustment can also lead to cost savings in the long term, as the exact amount of biologics can be determined with the avoidance of overtreatment [61].
At present, research is being performed in order to find the most suitable treatment options for the individual patient in clinical practice. As direct head-to-head studies are scarce, several network meta-analyses have compared the various agents approved for their therapeutic utility in treating several inflammatory diseases, i.e., IBD, ankylosing spondylitis (AS), RA, and PsA.
TNF-α inhibitors have proved that they continue to have an enormous relevance in the therapeutic landscape and are still established as therapeutics to control chronic inflammatory diseases despite, the era of newly developed types of treatment. This becomes evident when the different mechanisms of action are examined and compared with regard to their safety profiles and effectiveness.
The observational cohort JAK-pot study analyzed the real world data of four classes (JAK-inhibitors, TNF-α inhibitors, IL-6 inhibitor, abatacept) of second-line treatment approved for RA [62]. Their effectiveness was compared by evaluating the time of and reasoning for drug discontinuation, as well as Clinical Disease Activity Index (CDAI) response rates at 1 year. The key finding was the comparable clinical effectiveness among those four therapeutic agent groups. However, there were differences in the reasons for drug discontinuation, as JAK inhibitors were mainly stopped for safety reasons and less commonly as a result of their ineffectiveness.
As such, a recent study highlighted the importance of TNF-α inhibitors in AS therapy, showing that TNF-α inhibitors were superior to IL-6, -17, -23, and JAK inhibitors regarding efficacy and safety and should be used as the preferred replacement for conventional drug therapy in patients suffering from a rapid disease progression and physical functional limitations [63]. For the treatment of radiographic axSpA (r-axSpA), a direct comparison of the TNF-α blocker adalimumab with the IL-17 inhibitor secukinumab revealed that both biologic agents were comparably efficient in reducing radiographic progression over 2 years [64].
References
- Szekanecz, Z.; McInnes, I.B.; Schett, G.; Szamosi, S.; Benkő, S.; Szűcs, G. Autoinflammation and autoimmunity across rheumatic and musculoskeletal diseases. Nat. Rev. Rheumatol. 2021, 17, 585–595.
- Schett, G.; Elewaut, D.; McInnes, I.B.; Dayer, J.M.; Neurath, M.F. How cytokine networks fuel inflammation: Toward a cytokine-based disease taxonomy. Nat. Med. 2013, 19, 822–824.
- Bjarnadóttir, U.; Einarsdóttir, H.K.; Stefánsdóttir, E.; Helgason, E.A.; Jónasdóttir, D.; Gudmundsson, S.; Gudbjornsson, B.; Ludviksson, B.R. Resolution of Th/Tc17-driven inflammation during anti-TNFα treatment of rheumatoid arthritis reveals a unique immune biomarker profiling pattern. Scand. J. Immunol. 2022, 95, e13116.
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288.
- Bystrom, J.; Clanchy, F.I.; Taher, T.E.; Mangat, P.; Jawad, A.S.; Williams, R.O.; Mageed, R.A. TNFα in the regulation of Treg and Th17 cells in rheumatoid arthritis and other autoimmune inflammatory diseases. Cytokine 2018, 101, 4–13.
- Sauzullo, I.; Scrivo, R.; Sessa, P.; Mengoni, F.; Vullo, V.; Valesini, G.; Mastroianni, C.M. Changes in T cell effector functions over an 8-year period with TNF antagonists in patients with chronic inflammatory rheumatic diseases. Sci. Rep. 2018, 8, 7881.
- Wen, H.; Chen, D.; Lu, J.; Jiao, Z.; Chen, B.; Zhang, B.; Ye, C.; Liu, L. Probable drug interaction between etanercept and cyclosporine resulting in clinically unexpected low trough concentrations: First case report. Front. Pharmacol. 2020, 11, 939.
- van Hamburg, J.P.; Tas, S.W. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 2018, 87, 69–81.
- Pesce, B.; Ribeiro, C.H.; Larrondo, M.; Ramos, V.; Soto, L.; Catalán, D.; Aguillón, J.C. TNF-α affects signature cytokines of Th1 and Th17 T cell subsets through differential actions on TNFR1 and TNFR2. Int. J. Mol. Sci. 2022, 23, 9306.
- Schnell, A.; Schwarz, B.; Wahlbuhl, M.; Allabauer, I.; Hess, M.; Weber, S.; Werner, F.; Schmidt, H.; Rechenauer, T.; Siebenlist, G.; et al. Distribution and cytokine profile of peripheral B cell subsets is perturbed in pediatric IBD and partially restored during a successful IFX therapy. Inflamm. Bowel Dis. 2021, 27, 224–235.
- Defendenti, C.; Atzeni, F.; Malandrin, S.; Ardizzone, S.; Almasio, P.L.; Saibeni, S.; Bezzio, C.; Bollani, S.; Salerno, R.; Declich, P.; et al. Anti-tumour necrosis factor-α antibodies and B cell homeostasis in human inflammatory bowel diseases. Int. Immunopharmacol. 2018, 54, 329–335.
- Li, Z.; Vermeire, S.; Bullens, D.; Ferrante, M.; Van Steen, K.; Noman, M.; Bossuyt, X.; Rutgeerts, P.; Ceuppens, J.L.; Van Assche, G. Anti-tumor necrosis factor therapy restores peripheral blood B-cell subsets and CD40 expression in inflammatory bowel diseases. Inflamm. Bowel Dis. 2015, 21, 2787–2796.
- Ringheanu, M.; Daum, F.; Markowitz, J.; Levine, J.; Katz, S.; Lin, X.; Silver, J. Effects of infliximab on apoptosis and reverse signaling of monocytes from healthy individuals and patients with Crohn’s disease. Inflamm. Bowel Dis. 2004, 10, 801–810.
- Agnholt, J.; Kaltoft, K. Infliximab downregulates interferon-gamma production in activated gut T-lymphocytes from patients with Crohn’s disease. Cytokine 2001, 15, 212–222.
- Holleran, G.; Lopetuso, L.; Petito, V.; Graziani, C.; Ianiro, G.; McNamara, D.; Gasbarrini, A.; Scaldaferri, F. The innate and adaptive immune system as targets for biologic therapies in inflammatory bowel disease. Int. J. Mol. Sci. 2017, 18, 2020.
- Karsulovic, C.; Tempio, F.; Lopez, M.; Guerrero, J.; Goecke, A. In vitro phenotype induction of circulating monocytes: CD16 and CD163 analysis. J. Inflamm. Res. 2021, 14, 191–198.
- Nadkarni, S.; Mauri, C.; Ehrenstein, M.R. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J. Exp. Med. 2007, 204, 33–39.
- McGovern, J.L.; Nguyen, D.X.; Notley, C.A.; Mauri, C.; Isenberg, D.A.; Ehrenstein, M.R. Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum. 2012, 64, 3129–3138.
- Bankó, Z.; Pozsgay, J.; Gáti, T.; Rojkovich, B.; Ujfalussy, I.; Sármay, G. Regulatory B cells in rheumatoid arthritis: Alterations in patients receiving anti-TNF therapy. Clin. Immunol. 2017, 184, 63–69.
- Flores-Borja, F.; Bosma, A.; Ng, D.; Reddy, V.; Ehrenstein, M.R.; Isenberg, D.A.; Mauri, C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl. Med. 2013, 5, 173ra123.
- Ma, L.; Liu, B.; Jiang, Z.; Jiang, Y. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin. Rheumatol. 2014, 33, 187–195.
- Salomon, S.; Guignant, C.; Morel, P.; Flahaut, G.; Brault, C.; Gourguechon, C.; Fardellone, P.; Marolleau, J.P.; Gubler, B.; Goeb, V. Th17 and CD24(hi)CD27(+) regulatory B lymphocytes are biomarkers of response to biologics in rheumatoid arthritis. Arthritis. Res. Ther. 2017, 19, 33.
- Menegatti, S.; Guillemot, V.; Latis, E.; Yahia-Cherbal, H.; Mittermüller, D.; Rouilly, V.; Mascia, E.; Rosine, N.; Koturan, S.; Millot, G.A.; et al. Immune response profiling of patients with spondyloarthritis reveals signalling networks mediating TNF-blocker function in vivo. Ann. Rheum. Dis. 2021, 80, 475–486.
- Ohkura, N.; Kitagawa, Y.; Sakaguchi, S. Development and maintenance of regulatory T cells. Immunity 2013, 38, 414–423.
- Jongsma, M.M.E.; Aardoom, M.A.; Cozijnsen, M.A.; van Pieterson, M.; de Meij, T.; Groeneweg, M.; Norbruis, O.F.; Wolters, V.M.; van Wering, H.M.; Hojsak, I.; et al. First-line treatment with infliximab versus conventional treatment in children with newly diagnosed moderate-to-severe Crohn’s disease: An open-label multicentre randomised controlled trial. Gut 2022, 71, 34–42.
- Payen, E.; Neuraz, A.; Zenzeri, L.; Talbotec, C.; Abi Nader, E.; Chatenoud, L.; Chhun, S.; Goulet, O.; Ruemmele, F.M.; Pigneur, B. Adalimumab therapy in pediatric Crohn disease: A 2-year follow-up comparing “top-down” and “step-up” strategies. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 166–173.
- Jongsma, M.M.E.; Costes, L.M.M.; Tindemans, I.; Cozijnsen, M.A.; Raatgreep, R.H.C.; van Pieterson, M.; Li, Y.; Escher, J.C.; de Ridder, L.; Samsom, J.N. Serum immune profiling in pediatric Crohn’s disease demonstrates stronger immune modulation with first-line infliximab than conventional therapy and pre-treatment profiles predict clinical response to both treatments. J. Crohns Colitis 2023, jjad049.
- Schett, G.; McInnes, I.B.; Neurath, M.F. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N. Engl. J. Med. 2021, 385, 628–639.
- Claßen, M.; de Laffolie, J.; Claßen, M.; Schnell, A.; Sohrabi, K.; Hoerning, A. Significant advantages for first line treatment with TNF-alpha inhibitors in pediatric patients with inflammatory bowel disease—Data from the multicenter CEDATA-GPGE registry study. Front. Pediatr. 2022, 10, 903677.
- de Laffolie, J.; Zimmer, K.P.; Sohrabi, K.; Hauer, A.C. Early immune suppression in children and adolescents with Crohn’s disease—Data from the CEDATA GPGE registry. Dtsch. Arztebl. Int. 2021, 118, 421–422.
- Walters, T.D.; Kim, M.O.; Denson, L.A.; Griffiths, A.M.; Dubinsky, M.; Markowitz, J.; Baldassano, R.; Crandall, W.; Rosh, J.; Pfefferkorn, M.; et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn’s disease. Gastroenterology 2014, 146, 383–391.
- de Jong, L.M.; Jiskoot, W.; Swen, J.J.; Manson, M.L. Distinct effects of inflammation on cytochrome P450 regulation and drug metabolism: Lessons from experimental models and a potential role for pharmacogenetics. Genes 2020, 11, 1509.
- Lenoir, C.; Rollason, V.; Desmeules, J.A.; Samer, C.F. Influence of inflammation on cytochromes P450 activity in adults: A systematic review of the literature. Front. Pharmacol. 2021, 12, 733935.
- Stanke-Labesque, F.; Gautier-Veyret, E.; Chhun, S.; Guilhaumou, R.; French Society of Pharmacology and Therapeutics. Inflammation is a major regulator of drug metabolizing enzymes and transporters: Consequences for the personalization of drug treatment. Pharmacol. Ther. 2020, 215, 107627.
- Wollmann, B.M.; Syversen, S.W.; Vistnes, M.; Lie, E.; Mehus, L.L.; Molden, E. Associations between cytokine levels and CYP3A4 phenotype in patients with rheumatoid arthritis. Drug Metab. Dispos. 2018, 46, 1384–1389.
- Tiegs, G.; Horst, A.K. TNF in the liver: Targeting a central player in inflammation. Semin. Immunopathol. 2022, 44, 445–459.
- Parlati, L.; Régnier, M.; Guillou, H.; Postic, C. New targets for NAFLD. JHEP Rep. 2021, 3, 100346.
- Tomita, K.; Tamiya, G.; Ando, S.; Ohsumi, K.; Chiyo, T.; Mizutani, A.; Kitamura, N.; Toda, K.; Kaneko, T.; Horie, Y.; et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut 2006, 55, 415–424.
- Charles, P.; Elliott, M.J.; Davis, D.; Potter, A.; Kalden, J.R.; Antoni, C.; Breedveld, F.C.; Smolen, J.S.; Eberl, G.; deWoody, K.; et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J. Immunol. 1999, 163, 1521–1528.
- Shu, W.; Pang, Z.; Xu, C.; Lin, J.; Li, G.; Wu, W.; Sun, S.; Li, J.; Li, X.; Liu, Z. Anti-TNF-α monoclonal antibody therapy improves anemia through downregulating hepatocyte hepcidin expression in inflammatory bowel disease. Mediat. Inflamm. 2019, 2019, 4038619.
- England, B.R.; Thiele, G.M.; Anderson, D.R.; Mikuls, T.R. Increased cardiovascular risk in rheumatoid arthritis: Mechanisms and implications. BMJ 2018, 361, k1036.
- He, B.; Li, Y.; Luo, W.W.; Cheng, X.; Xiang, H.R.; Zhang, Q.Z.; He, J.; Peng, W.X. The risk of adverse effects of TNF-α inhibitors in patients with rheumatoid arthritis: A network meta-analysis. Front. Immunol. 2022, 13, 814429.
- Conti, F.; Atzeni, F.; Massaro, L.; Gerardi, M.C.; Gremese, E.; Passiu, G.; Carletto, A.; Malavolta, N.; Foti, R.; Ramonda, R.; et al. The influence of comorbidities on the efficacy of tumour necrosis factor inhibitors, and the effect of tumour necrosis factor inhibitors on comorbidities in rheumatoid arthritis: Report from a National Consensus Conference. Rheumatology 2018, 57, vii11–vii22.
- Olivera, P.A.; Zuily, S.; Kotze, P.G.; Regnault, V.; Al Awadhi, S.; Bossuyt, P.; Gearry, R.B.; Ghosh, S.; Kobayashi, T.; Lacolley, P.; et al. International consensus on the prevention of venous and arterial thrombotic events in patients with inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 857–873.
- Yuan, S.; Carter, P.; Bruzelius, M.; Vithayathil, M.; Kar, S.; Mason, A.M.; Lin, A.; Burgess, S.; Larsson, S.C. Effects of tumour necrosis factor on cardiovascular disease and cancer: A two-sample Mendelian randomization study. EBiomedicine 2020, 59, 102956.
- Bundesinstitut für Arzneimittel und Medizinprodukte. Januskinase-Inhibitoren: Behandlung von Entzündungskrankheiten. Available online: https://www.bfarm.de/SharedDocs/Risikoinformationen/Pharmakovigilanz/DE/RV_STP/g-l/januskinase.html;jsessionid=BD9413EADD5BFB59861897542E2169D0.internet281?nn=471274 (accessed on 15 May 2023).
- Hakimiana, S.; Kheder, J.; Arum, S.; Cave, D.R.; Hyatt, B. Re-evaluating osteoporosis and fracture risk in Crohn’s disease patients in the era of TNF-alpha inhibitors. Scand. J. Gastroenterol. 2018, 53, 168–172.
- Torre-Alonso, J.C.; Carmona, L.; Moreno, M.; Galíndez, E.; Babío, J.; Zarco, P.; Linares, L.; Collantes-Estevez, E.; Barrial, M.F.; Hermosa, J.C.; et al. Identification and management of comorbidity in psoriatic arthritis: Evidence- and expert-based recommendations from a multidisciplinary panel from Spain. Rheumatol. Int. 2017, 37, 1239–1248.
- Loza, E.; Lajas, C.; Andreu, J.L.; Balsa, A.; González-Álvaro, I.; Illera, O.; Jover, J.; Mateo, I.; Orte, J.; Rivera, J.; et al. Consensus statement on a framework for the management of comorbidity and extra-articular manifestations in rheumatoid arthritis. Rheumatol. Int. 2015, 35, 445–458.
- Claßen, M.; Hoerning, A. Current role of monoclonal antibody therapy in pediatric IBD: A special focus on therapeutic drug monitoring and treat-to-target strategies. Children 2023, 10, 634.
- Leone, G.M.; Mangano, K.; Petralia, M.C.; Nicoletti, F.; Fagone, P. Past, Present and (Foreseeable) Future of Biological Anti-TNF Alpha Therapy. J. Clin. Med. 2023, 12, 1630.
- Luchetti, M.M.; Benfaremo, D.; Gabrielli, A. Biologics in Inflammatory and Immunomediated Arthritis. Curr. Pharm. Biotechnol. 2017, 18, 989–1007.
- Armaroli, G.; Klein, A.; Ganser, G.; Ruehlmann, M.J.; Dressler, F.; Hospach, A.; Minden, K.; Trauzeddel, R.; Foeldvari, I.; Kuemmerle-Deschner, J.; et al. Long-term safety and effectiveness of etanercept in JIA: An 18-year experience from the BiKeR registry. Arthritis Res. Ther. 2020, 22, 258.
- Filippini, M.; Bazzani, C.; Atzeni, F.; Sarzi Puttini, P.; Marchesoni, A.; Favalli, E.G.; Caporali, R.; Cavagna, L.; Gorla, R. Effects of anti-TNF alpha drugs on disability in patients with rheumatoid arthritis: Long-term real-life data from the Lorhen Registry. BioMed Res. Int. 2014, 2014, 416892.
- Karadag, O.; Dalkilic, E.; Ayan, G.; Kucuksahin, O.; Kasifoglu, T.; Yilmaz, N.; Koca, S.S.; Yazisiz, V.; Erten, P.T.; Sayarlioglu, M.; et al. Real-world data on change in work productivity, activity impairment, and quality of life in patients with psoriatic arthritis under anti-TNF therapy: A postmarketing, noninterventional, observational study. Clin. Rheumatol. 2022, 41, 85–94.
- van der Heijde, D.; Gladman, D.D.; Kavanaugh, A.; Mease, P.J. Assessing structural damage progression in psoriatic arthritis and its role as an outcome in research. Arthritis Res. Ther. 2020, 22, 18.
- Schnitzler, F.; Seitz, T.; Tillack-Schreiber, C.; Lange, S.; Waggershauser, C.; Ochsenkuhn, T. Early start of infliximab in Crohn’s Disease increases rates of endoscopic remission and decreases stenosis formation: Experiences from a single center cohort. Crohns Colitis 360 2021, 3, otab060.
- Peyrin-Biroulet, L.; Arkkila, P.; Armuzzi, A.; Danese, S.; Guardiola, J.; Jahnsen, J.; Lees, C.; Louis, E.; Lukáš, M.; Reinisch, W.; et al. Comparative efficacy and safety of infliximab and vedolizumab therapy in patients with inflammatory bowel disease: A systematic review and meta-analysis. BMC Gastroenterol. 2022, 22, 291.
- Genovese, M.C.; Bathon, J.M.; Fleischmann, R.M.; Moreland, L.W.; Martin, R.W.; Whitmore, J.B.; Tsuji, W.H.; Leff, J.A. Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J. Rheumatol. 2005, 32, 1232–1242.
- Marquez-Megias, S.; Nalda-Molina, R.; Sanz-Valero, J.; Más-Serrano, P.; Diaz-Gonzalez, M.; Candela-Boix, M.R.; Ramon-Lopez, A. Cost-Effectiveness of Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease: A Systematic Review. Pharmaceutics 2022, 14, 1009.
- Martelli, L.; Olivera, P.; Roblin, X.; Attar, A.; Peyrin-Biroulet, L. Cost-effectiveness of drug monitoring of anti-TNF therapy in inflammatory bowel disease and rheumatoid arthritis: A systematic review. J. Gastroenterol. 2017.
- Lauper, K.; Iudici, M.; Mongin, D.; Bergstra, S.A.; Choquette, D.; Codreanu, C.; Cordtz, R.; De Cock, D.; Dreyer, L.; Elkayam, O.; et al. Effectiveness of TNF-inhibitors, abatacept, IL6-inhibitors and JAK-inhibitors in 31,846 patients with rheumatoid arthritis in 19 registers from the ‘JAK-pot’ collaboration. Ann. Rheum. Dis. 2022, 81, 1358–1366.
- Tian, C.; Shu, J.; Shao, W.; Zhou, Z.; Guo, H.; Wang, J. Efficacy and safety of IL inhibitors, TNF-α inhibitors, and JAK inhibitors in patients with ankylosing spondylitis: A systematic review and Bayesian network meta-analysis. Ann. Transl. Med. 2023, 11, 178.
- Baraliakos, X.; Ostergaard, M.; Poddubnyy, D.; van der Heijde, D.; Deodhar, A.; Machado, P.M.; Navarro-Compán, V.; Hermann, K.G.; Kishimoto, M.; Lee, E.Y.; et al. Effect of secukinumab versus adalimumab biosimilar on radiographic progression in patients with radiographic axial spondyloarthritis: A randomized phase IIIb study. In Proceedings of the American College of Rheumatology Convergence 2022, Philadelphia, PA, USA, 10–14 November 2022.
More
Information
Subjects:
Rheumatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
659
Revisions:
4 times
(View History)
Update Date:
14 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




