Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | LEONIE ASFORA SARUBBO | -- | 1483 | 2023-08-07 18:22:13 | | | |
| 2 | Dean Liu | Meta information modification | 1483 | 2023-08-08 02:20:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
De Souza, T.C.; Costa, A.F.D.S.; Vinhas, G.M.; Sarubbo, L.A. Iron Oxides in Bacterial Cellulose Applications. Encyclopedia. Available online: https://encyclopedia.pub/entry/47751 (accessed on 08 February 2026).
De Souza TC, Costa AFDS, Vinhas GM, Sarubbo LA. Iron Oxides in Bacterial Cellulose Applications. Encyclopedia. Available at: https://encyclopedia.pub/entry/47751. Accessed February 08, 2026.
De Souza, Thaís Cavalcante, Andréa Fernanda De Santana Costa, Gloria Maria Vinhas, Leonie Asfora Sarubbo. "Iron Oxides in Bacterial Cellulose Applications" Encyclopedia, https://encyclopedia.pub/entry/47751 (accessed February 08, 2026).
De Souza, T.C., Costa, A.F.D.S., Vinhas, G.M., & Sarubbo, L.A. (2023, August 07). Iron Oxides in Bacterial Cellulose Applications. In Encyclopedia. https://encyclopedia.pub/entry/47751
De Souza, Thaís Cavalcante, et al. "Iron Oxides in Bacterial Cellulose Applications." Encyclopedia. Web. 07 August, 2023.
Copy Citation
Iron oxide nanoparticles have been investigated due to their suitable characteristics for diverse applications in the fields of biomedicine, electronics, water or wastewater treatment and sensors. Maghemite, magnetite and hematite are the most widely studied iron oxide particles and have ferrimagnetic characteristics.
iron oxides
synthesis
SPIONs
magnetism
bacterial cellulose
1. Introduction
Magnetism is an important property in numerous materials and devices found in daily living, such as electronics. Iron oxides are a widely investigated group of magnetic materials and include materials with specific magnetic properties (ferromagnetic and paramagnetic) that are useful in particular biotechnological applications [1][2].
Iron oxide nanoparticles have a large surface area in relation to their volume, can be used as dopants in the fabrication of composites and generally form magnetic monodomains, which has attracted the attention of a growing number of researchers [3]. Iron oxide nanoparticles have applications in the biomedical field, drug-release systems, the treatment of hyperthermia, magnetic resonance and tissue engineering as well as in sensors, biosensors, water or wastewater treatment and the fabrication of electronic components [4].
The different routes for the obtainment of iron oxide nanoparticles include mechanisms of coprecipitation, thermal decomposition, microemulsion, sol–gel, polyol and hydrothermal synthesis [5]. It is common for authors to state that each specific technique produces larger or smaller particles with a broad or narrow distribution of sizes. However, several studies have shown that these characteristics can be controlled by adjusting the reaction variables, such as the temperature, time, pH, reagents and proportions [6].
Such control is fundamental in certain applications, such as the production of superparamagnetic iron oxide nanoparticles (SPIONs), which are commonly used in the medical field [7][8]. In such applications, the control of the size distribution is essential, as very large particles can interfere with the action of SPIONs. In contrast, nanoparticles can have larger sizes in applications that require materials with ferromagnetic properties, in which the control over the size uniformity is less rigorous [9].
In many applications, iron oxide nanoparticles are incorporated into bacterial cellulose, which is a sustainable, biotechnological compound for the production of novel biotechnological materials that meet the needs of different fields and are used in the fabrication of various devices, such as contrasts for magnetic resonance, sensors and electronics [10].
2. Main Effects of Size Distribution in Different Applications
Among the applications and studies aimed at the synthesis of iron oxide nanoparticles, researchers generally seek materials that result in the formation of monodomains. The particle size exerts a considerable influence on the magnetic properties, as seen in the studies cited above. In some applications, however, there is greater concern with the size distribution and greater uniformity is desirable, especially if the material of interest is a SPION [7][11].
In applications that do not require greater rigour in the control of the size distribution, the objectives are to produce particles with ferrimagnetic characteristics and high saturation magnetisation or like Fenton’s reagent. Jędrzak et al. [12] synthesised magnetite nanoparticles via coprecipitation for the production of a blood glucose sensor and demonstrated efficiency in the quantification of the compound. The particles had an average size range of 8 to 12 nm, which was satisfactory for the application. The use of magnetic particles as transducers is common in sensors. In the case of blood glucose biosensors, however, magnetite can be used as a synthetic enzyme and as a Fenton reagent due to the presence of Fe2+, which can mimic the action of the enzyme peroxidase (which is present in the reactive strips of the sensor) [9].
In another study in which the authors obtained a broad distribution, Hwang et al. [13] produced a sensor for detecting phenylhydrazine. Iron oxide nanoparticles were synthesised and used to fabricate an electrode. The nanoparticles had a mean size of 40 ± 10 nm, with a somewhat large variation range that was acceptable for the application. Xu et al. [14] conducted a literature review to investigate the application of iron nanoparticles in supercapacitators and reported several studies in which the particle size distribution was broad but did not exert a negative impact on the applications.
In the case of SPIONs, the size distribution is crucial, as one larger particle synthesised with others could surpass the diameter limit and not have the desired superparamagnetic characteristics. Such characteristics in SPIONs are important in biomedical applications, as the nanoparticles are guided to the location of interest by the application of an external magnetic field to be used as necrophages or to increase the temperature around cancer cells to eliminate them [15].
Nanoparticles larger than SPIONs have ferrimagnetic behaviour, which hampers the action of the superparamagnetic nanoparticles. For instance, if the synthesised nanoparticles do not have uniform sizes, such as a mixture of SPIONs and larger particles, all particles will magnetise with the application of a magnetic field, although the SPIONs will return to their original state with the removal of the field, whereas the larger particles will maintain their magnetic alignment [3][16]. Taking this into consideration in a hypothetical situation, the action of a drug delivery system that has iron oxide particles of various sizes will be compromised, as the larger particles would maintain their magnetic action even after the removal of the magnetic field that guided them and would cause the clustering of SPIONs around them, leading to imprecision in the system [5][7].
3. Application in Bacterial Cellulose and Effect of Size Distribution
Iron oxide nanoparticles can be used as dopants to produce novel composites and magnetic materials. The matrices of these materials could be polymers, for example [17][18][19]. Bacterial cellulose (BC) is a biomaterial with sustainable production through the fermentation of bacteria that has unique properties, such as biocompatibility, biodegradability and high water absorption capacity [17][18][19][20]. The incorporation of iron oxide nanoparticles in BC can occur in situ, when the production process of the additives is performed within the BC, or ex situ, when the iron oxide is synthesised separately and subsequently inserted into the BC [10]. The graph presented in Figure 1 shows the frequency rates of iron oxide incorporation processes in bacterial cellulose films according to data extracted from the study by Souza et al. [10].
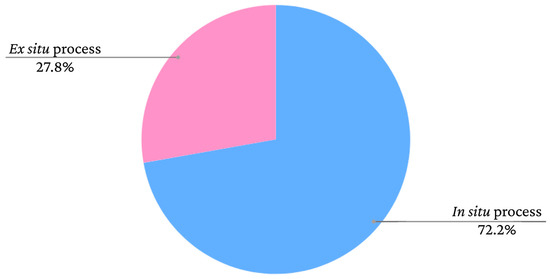
Figure 1. Frequency rates of iron oxide incorporation methods in bacterial cellulose in studies found in the literature.
As seen in the graph, there is a greater tendency to adopt in situ methods. However, the nanoparticles adhere to the BC fibres similarly in both cases. According to Mira-Cuenca et al. [19], iron nanoparticles adhere to BC fibres through hydrogen bridges. The fibres have nanometric thicknesses, which facilitates the adherence of the nanoparticles. Marins et al. [21] and Salidkul et al. [22] used coprecipitation to obtain iron oxide particles, although used different incorporation methods. Marins et al. [21] performed coprecipitation in situ, whereas Salidkul et al. [22] performed the method ex situ. However, the formed nanoparticles had a similar arrangement in the BC films, adhering throughout the fibres in a uniform manner.
As mentioned above, the applications vary depending on characteristics such as the particle size and distribution. In the studies presented above, the authors suggest distinct applications for BC composites with iron oxide. In the study by Salidkul et al. [22], the particles obtained a mean size of 63.2 ± 6.2 nm and the authors suggested applications in the fields of magnetic shielding, data storage and electromagnetic absorption. Marins et al. (2013) synthesised iron oxide with different variations in time. In the two subsamples obtained, the nanoparticles had mean sizes of 10 ± 1 to 13.4 ± 1 (smaller size and narrower distribution). Thus, the authors also suggested medical applications for the material obtained.
The study by Mira-Cuenca [19] exemplifies the application of a material composed of BC and iron oxide that required greater control in the size distribution of the final particles. The researchers developed a device for the contrast agent in magnetic resonance with SPIONs synthesised through in situ thermal decomposition in triturated BC membranes. Five grams of triturated BC was mixed into 40 mL of benzyl alcohol with 1100 mg of the precursor tris(acetylacetonate)iron (III) (Fe(acac)3). The mixture was first heated at 60 °C for 5 min and then at 210 °C for 10 min in a microwave oven. The mean SPION size was 13 ± 5 nm. The material was used a magnetic paint and was deposited on dry BC films using the screen-printing technique. The authors tested the device in pieces of pork loin in a magnetic resonance machine with a T2 relaxation time (spin-spin) and the device proved to be a good contrast agent. As a result, the authors suggested the application of the material in internal bone implants, enabling better monitoring of the implants through magnetic resonance images and avoiding the need for additional surgeries.
In this application, the control of the size distribution is fundamental, as superparamagnetic particles have a longer relaxation time, which is an important factor in contrasts for magnetic resonance.
As demonstrated in some articles, there are various applications for iron oxide nanoparticles. However, each requires a larger or smaller variations in size distribution and specific characteristics. Thus, BC-derived materials with the addition of iron oxide nanoparticles undergo the same selectivity depending on the additive material and can have applications in different fields.
References
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009.
- Bustamante-Torres, M.; Romero-Fierro, D.; Estrella-Nuñez, J.; Arcentales-Vera, B.; Chichande-Proaño, E.; Bucio, E. Polymeric Composite of Magnetite Iron Oxide Nanoparticles and Their Application in Biomedicine: A Review. Polymers 2022, 14, 752.
- Samrot, A.V.; Sahithy, C.S.; Selvarani, J.; Purayi, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042.
- Al-Anazi, A. Iron-based magnetic nanomaterials in environmental and energy applications: A short review. Curr. Opin. Chem. Eng. 2022, 36, 100794.
- Niculescu, A.G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2021, 199, 16–27.
- Yusoff, A.H.M.; Salimi, M.N.; Jamlos, M.F. A review: Synthetic strategy control of magnetite nanoparticles production. Adv. Nano Res. 2017, 6, 1–19.
- Akhtar, N.; Mohammed, H.A.; Yusuf, M.; Al-Subaiyel, A.; Sulaiman, G.M.; Khan, R.A. SPIONs Conjugate Supported Anticancer Drug Doxorubicin’s Delivery: Current Status, Challenges, and Prospects. Nanomaterials 2022, 12, 3686.
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236.
- Dudchenko, N.; Pawar, S.; Perelshtein, I.; Fixler, D. Magnetite-Based Biosensors and Molecular Logic Gates: From Magnetite Synthesis to Application. Biosensors 2023, 13, 304.
- Souza, T.C.; Amorim, J.D.P.d.; Silva Junior, C.J.G.d.; de Medeiros, A.D.M.; Santana Costa, A.F.d.; Vinhas, G.M.; Sarubbo, L.A. Magnetic Bacterial Cellulose Biopolymers: Production and Potential Applications in the Electronics Sector. Polymers 2023, 15, 853.
- Dheyab, M.A.; Aziz, A.A.; Jameel, M.S.; Noqta, O.A.; Mehrdel, B. Synthesis and coating methods of biocompatible iron oxide/gold nanoparticle and nanocomposite for biomedical applications. Chin. J. Phys. 2020, 64, 305–325.
- Jędrzak, A.; Rębiś, T.; Kuznowicz, M.; Jesionowski, T. Bio-inspired magnetite/lignin/polydopamine-glucose oxidase biosensing nanoplatform. From synthesis, via sensing assays to comparison with others glucose testing techniques. Int. J. Biol. Macromol. 2019, 127, 677–682.
- Hwang, S.W.; Umar, A.; Dar, G.N.; Kim, S.H.; Badran, R.I. Synthesis and Characterization of Iron Oxide Nanoparticles for Phenyl Hydrazine Sensor Applications. Sens. Lett. 2014, 12, 97–101.
- Xu, B.; Zheng, M.; Tang, H.; Chen, Z.; Chi, Y.; Wang, L.; Zhang, L.; Chen, Y.; Pang, H. Iron oxide-based nanomaterials for supercapacitors. Nanotechnology 2019, 30, 204002.
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68.
- Tanaka, S.; Kaneti, Y.V.; Septiani, N.L.W.; Dou, S.X.; Bando, Y.; Hossain, M.S.A.; Kim, J.; Yamauchi, Y. A Review on Iron Oxide-Based Nanoarchitectures for Biomedical, Energy Storage, and Environmental Applications. Small Methods 2019, 3, 1800512.
- Chaabane, L.; Chahdoura, H.; Mehdaoui, R.; Snoussi, M.; Beyou, E.; Lahcini, M.; Baouab, M.H.V. Functionalization of developed bacterial cellulose with magnetite nanoparticles for nanobiotechnology and nanomedicine applications. Carbohydr. Polym. 2020, 247, 116707.
- da Rosa Salles, T.; da Silva Bruckamann, F.; Viana, A.R.; Krause, L.M.F.; Mortari, S.R.; Rhoden, C.R.B. Magnetic nanocrystalline cellulose: Azithromycin adsorption and in vitro biological activity against melanoma cells. J. Polym. Environ. 2022, 30, 2695–2713.
- Mira-Cuenca, C.; Meslier, T.; Roig-Sanchez, S.; Laromaine, A.; Roig, A. Patterning Bacterial Cellulose Films with Iron Oxide Nanoparticles and Magnetic Resonance Imaging Monitoring. ACS Appl. Polym. Mater. 2021, 3, 4959–4965.
- Amorim, J.D.P.; Souza, K.C.; Duarte, C.R.; Duarte, I.S.; Ribeiro, F.A.S.; Silva, G.S.; Farias, P.M.A.; Sting, A.; Costa, A.F.S.; Vinhas, G.M.; et al. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869.
- Marins, J.A.; Soares, B.G.; Barud, H.S.; Ribeiro, S.J.L. Flexible magnetic membranes based on bacterial cellulose and its evaluation as electromagnetic interference shielding material. Mater. Sci. Eng. C 2013, 33, 3994–4001.
- Salidkul, N.; Mongkolthanaruk, W.; Faungnawakij, K.; Pinitsoontorn, S. Hard magnetic membrane based on bacterial cellulose–barium ferrite nanocomposites. Carbohydr. Polym. 2021, 264, 118016.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
488
Revisions:
2 times
(View History)
Update Date:
08 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




