Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | jasna klen | -- | 3130 | 2023-08-03 15:34:39 | | | |
| 2 | Sirius Huang | Meta information modification | 3130 | 2023-08-04 08:46:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Klen, J.; Dolžan, V. SGLT2 Inhibitors in the Treatment of DKD. Encyclopedia. Available online: https://encyclopedia.pub/entry/47624 (accessed on 08 February 2026).
Klen J, Dolžan V. SGLT2 Inhibitors in the Treatment of DKD. Encyclopedia. Available at: https://encyclopedia.pub/entry/47624. Accessed February 08, 2026.
Klen, Jasna, Vita Dolžan. "SGLT2 Inhibitors in the Treatment of DKD" Encyclopedia, https://encyclopedia.pub/entry/47624 (accessed February 08, 2026).
Klen, J., & Dolžan, V. (2023, August 03). SGLT2 Inhibitors in the Treatment of DKD. In Encyclopedia. https://encyclopedia.pub/entry/47624
Klen, Jasna and Vita Dolžan. "SGLT2 Inhibitors in the Treatment of DKD." Encyclopedia. Web. 03 August, 2023.
Copy Citation
Diabetic kidney disease (DKD) is a severe and common complication and affects a quarter of patients with type 2 diabetes mellitus (T2DM). Oxidative stress and inflammation related to hyperglycemia are interlinked and contribute to the occurrence of DKD. It was shown that sodium–glucose cotransporter-2 (SGLT2) inhibitors, a novel yet already widely used therapy, may prevent the development of DKD and alter its natural progression.
diabetes mellitus
type 2
diabetic nephropathies
sodium–glucose contransporter-2 inhibitors
1. Introduction
The prevalence of diabetes mellitus has reached epidemic proportions worldwide [1]. At the moment, 537 million adults from 20 to 79 years are living with diabetes of any type. The projection shows us that we can expect 783 million patients with diabetes in 2045. It is known that 541 million adults have impaired glucose tolerance, which could lead to type 2 diabetes mellitus (T2DM). A substantial portion of patients with diabetes mellitus remain undiagnosed. Moreover, late complications of diabetes mellitus could be the first sign of the disease. Hyperglycemia is causally related to microvascular complications, while dyslipidemia, hypertension, smoking, and genetics also play an important role. Patients with T2DM have a high prevalence of microvascular complications, such as diabetic retinopathy, neuropathy, and diabetic kidney disease (DKD). A diagnosis of chronic kidney disease (CKD) is characterized by persistence of elevated urinary albumin excretion (albuminuria), low estimated glomerular filtration rate (eGFR), or associated with other symptoms of kidney damage, such as hematuria and morphological pathological abnormalities persisting for a duration of three months or longer [2][3]. DKD is a severe and frequent complication of diabetes mellitus and has an incidence of 25% in T2DM patients [4]. It is the leading cause of end-stage kidney disease (ESKD), which is rising steeply and leads to significant morbidity and cardiovascular mortality [5]. According to recent estimates, the number of patients undergoing kidney replacement therapy (KRT) worldwide reached 3.9 million, with high-income countries having the highest prevalence [6]. The main factor contributing to the onset of DKD is hyperglycemia; therefore, aiming to maintain HbA1c < 6.5% should be the major goal of primary interventions [7]. Importantly, it has been reported that stringent glycemic control decreased the incidence of albuminuria by 50%, and this favorable effect was maintained for more than ten years after the trial’s conclusion [8]. Similar results were observed in patients with T2DM, with each 1% decline in HbA1c decreasing the risk of microvascular complications by 37%, especially the likelihood of microalbuminuria [9]. Although normalization of glycemia may slow progression of DKD, it cannot halt it entirely [1]. One of the important factors for the onset and progression of CKD is also hypertension [10]. Normotensive patients with advanced CKD have a slower progression of kidney disease than hypertensive patients [1]. Due to conflicting study results, it is still unclear which blood pressure values could be recommended for patients with diabetes mellitus, with the generally recommended levels of blood pressure being below 140/90 mmHg [10]. According to the ESC/EASD guidelines, patients with diabetes who have several risk factors or even just one type of organ damage are in the same very high cardiovascular risk group as patients taking secondary prevention with developed cardiovascular disease. The Nephropathy In Diabetes type 2 (NID-2) study, which was multi-center, cluster-randomized, open-label, and focused on primary cardiovascular prevention, demonstrated in T2DM patients with albuminuria and diabetic retinopathy that multifactorial intensive treatment may have lower major adverse cardiovascular events and overall mortality. Based on a post hoc analysis, they found that a higher number of risk factors achieving the target level is associated with better cardiovascular-free survival in T2DM patients [11].
Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which are RAAS blockers, demonstrated benefits in preventing CKD progression and are, therefore, considered as first-line antihypertensive drugs in patients with diabetes mellitus, hypertension, eGFR 60 mL/min/1.73 m2, and urine albumin-to-creatinine ratio (UACR) 300 mg/g Cr. Their good renoprotective effect may be due to the decrease in elevated intraglomerular pressure. They moderately lower the risk of albuminuria and reduce progression from CKD to ESRD [1][10][12]. Even with rigorous blood pressure control with ACE inhibitors or ARBs and strict glycemia control, only marginal success was achieved in lowering the risk for CKD, but the progression of CKD to ESKD and related mortality could be not halted [1]. On the other hand, it was found that adding non-steroidal mineralocorticoid receptor antagonists to the aforementioned therapy reduces proteinuria. Nevertheless, we must be very careful when prescribing non-steroidal mineralocorticoid receptors antagonists because of side effects, especially hyperkalemia. It has been shown that aldosterone has the ability to increase fibrosis and inflammation in addition to its role in controlling sodium balance via activating mineralocorticoid receptors [1].
The conventional treatments to maintain blood glucose levels do not always prevent DKD [13]. On the other hand, it was reported that sodium–glucose cotransporter-2 (SGLT2) inhibitors, which are quite novel but already a widely used therapy, may prevent the development and alter the natural progression of DKD by inducing systemic and glomerular hemodynamic changes, providing metabolic advantages, and diminishing inflammatory and oxidative stress pathways [1][14][15][16][17], which is shown in Figure 1.
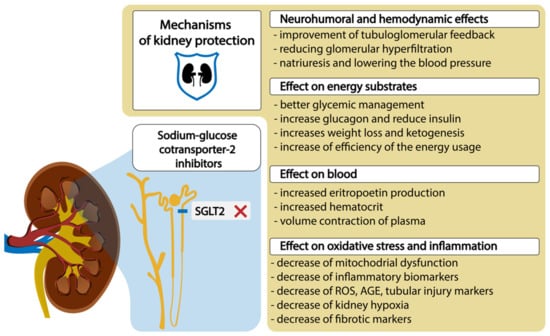
Figure 1. SGLT2 inhibitors and mechanisms of diabetic kidney protection. Abbreviations: ROS, reactive oxygen species; AGE, advanced glycolytic end product.
2. SGLT2 Transporters and SGLT2 Inhibitors in the Treatment of Diabetic Kidney Disease
Sodium-driven glucose symporters (SGLTs) are solute carriers facilitating glucose transport. Among the six SGLTs transporters, SGLT2 is responsible for 90% of glucose reabsorption and also for the majority of sodium reabsorption in kidneys. It has a low affinity and high capacity for glucose and is expressed in the brush border membrane of the S1 segment in the kidney cortex [18]. Many pathophysiological abnormalities are associated with increased SGLT2 activity in the proximal tubule. SGLT2 inhibitors reverse many of these abnormalities and significantly slow down DKD progression [1]. The administration of SGLT2 inhibitors is associated with proximal tubular natriuresis, which helps to restore distal sodium delivery and tubulo-glomerular feedback. This, in turn, leads to afferent arteriolar vasoconstriction, thereby reducing renal hyperfiltration and glomerular pressure, which lowers albuminuria levels. In T2DM patients, long-term treatment with SGLT2 inhibitors as opposed to placebo resulted in a slower decrease in GFR and a 30–50% reduction in albuminuria. The levels of albuminuria are acknowledged as a surrogate indicator of the advancement of kidney disease. The randomized double-blind RED trial found that SGLT2 inhibitors lower the measured GFR and filtration fraction and do not increase kidney vascular resistance [1][14][15][16][17]. The effects of SGLT2 inhibition on systemic hemodynamics may further contribute to kidney protection through changes in glomerular hemodynamics. This inhibition reduces plasma volume and lowers systolic blood pressure by approximately 3–6 mm Hg and diastolic blood pressure by approximately 1–2 mm Hg regardless of the individual’s hypertension status, leading to the activation of the sympathetic nervous system and RAAS and reduction in arterial stiffness. These effects may also be observed in patients with low eGFR [19][20][21]. The role of the SGLT1 and SGLT2 transporters within the nephron and SGLT2 inhibitors’ action are graphically presented in Figure 2.
Despite the fact that glucosuria reduces the mean plasma glucose concentrations and ameliorates glucotoxicity, resulting in improved cell function and insulin sensitivity [1], it is known that SGLT2 inhibitors have a mild impact on glycemic control in patients with T2DM who have normal kidney function. This outcome is achieved by stimulating the excretion of glucose with inhibition of the SGLT2 transporter in the S1 segment. Moreover, the accompanying reduction in caloric balance leads to an average weight loss of around 1–3 kg [22]. Although some of the weight loss can be attributed to increased sodium excretion, the primary cause is a decrease in body fat mass, and this effect is more pronounced in T2DM patients who have higher baseline hemoglobin A1c levels [23][24]. Additionally, glucosuria results in a metabolic shift towards a fasting state, which is characterized by an increase in the utilization of lipids and ketones as energy substrates. The metabolic shift caused by SGLT2 inhibitors may enhance energy utilization efficiency and stimulate low-energy cellular sensors, resulting in decreased hypoxia and improved mitochondrial function at both the cellular and organ levels [25][26]. It was found that SGLT2 inhibitors provide kidney protection by reducing inflammation and oxidative stress, as shown by changes in biomarkers related to cytokine/chemokine profiles and AGEs [27]. Recent studies of SGLT2 inhibitors have demonstrated improvements in endothelial function and reductions in local ROS generation [28][29]. Lee et al. found, in the cultured proximal tubular cells, that empagliflozin enhanced mitochondrial biogenesis and the balance of mitochondrial fission and fusion proteins, reducing ROS and also decreasing expression of TGF-β [30]. Additionally, they consistently reduce tubular injury markers and inflammatory mediators, including interleukin-6, nuclear factor-κB, kidney injury molecule 1, and profibrotic factors, such as TGF-β and fibronectin [13][31][32][33]. SGLT2 inhibitors may also attenuate kidney hypoxia by reducing the energy expenditure involved in tubular sodium and glucose reabsorption, leading to increases in erythropoietin production and hematopoiesis [31][34][35].
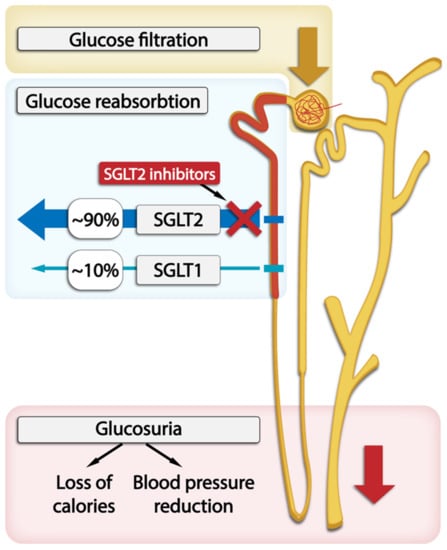

Figure 2. The location of SGLT1 and SGLT2 transporters within the nephron and SGLT2 inhibitors’s action in glucose excretion by the kidneys.
It needs to be pointed out that T2DM alone may also be an independent risk factor for acute kidney injury (AKI). AKI is more frequent in T2DM patients when they are undergoing surgery, taking some specific medications, or have sepsis or septic shock. On the other hand, AKI may also manifest as acute exacerbation of DKD. There has been a continuous concern about potential negative effects of SGLT2 inhibitors on the risk and prognosis of AKI due to the changes in kidney function associated with their usage, such as significant hypovolemia and different types of diabetic ketoacidosis (DKA). However, the results from clinical trials, observational studies, and meta-analyses have consistently shown no increase in the risk of AKI or negative effects of AKI associated with SGLT2 inhibitors. On the contrary, SGLT2 inhibitors were reported to reduce the risk of AKI by 30–40% [36]. In terms of CKD, SGLT2 inhibitors’ capacity to halt the loss of GFR with aging may be associated with a lower risk and better prognosis of AKI. However, for individuals with an already significantly low eGFR, the initial decrease in eGFR commonly observed when starting treatment with SGLT2 inhibitors may further increase the likelihood of experiencing an acute adverse event. Therefore, the recommendations clearly state that we can introduce SGLT2 inhibitors when eGFR is ≥45 mL/min, and they are not endorsed or recommended in ESRD. Accordingly, in patients who undergo AKI, it is advisable to stop the use of an SGLT2 inhibitor to prevent possible worsening of low plasma volume, low blood pressure, or low glomerular perfusion [37].
3. Treatment with SGLT2 Inhibitors and Kidney Outcomes
Several large clinical trials investigated clinical outcomes of treatment with SGLT2 inhibitors; however, kidney outcomes were mostly evaluated as secondary endpoints. On the other hand, some of these studies also included patients without T2DM.
The CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) study was the first double-blind, randomized, placebo-controlled clinical trial that included 4401 patients with T2DM and albuminuric CKD treated with ACE inhibitors or ARBs. All subjects had HbA1c levels from 6.5 to 12.0%, eGFR of 30 to <90 mL/min per 1.73 m2, albuminuria (30–500 mg/mmol), and were randomized into a canagliflozin or placebo group. The composite outcome included either ESKD (dialysis, transplantation, or an estimated GFR of 15 mL/min per 1.73 m2 sustained for at least 30 days based on central laboratory assessment) or a doubling of serum creatinine level from baseline (average of randomization and prerandomization) sustained for at least 30 days, or a death caused by kidney or cardiovascular disease. The study was stopped early because of clearly demonstrated superiority of canagliflozin, showing a 30% relative reduction in the primary kidney outcome by canagliflozin (HR: 0.70; 95% CI: 0.59–0.82). In addition, there was significant evidence of benefits in regard to the secondary outcome of kidney transplant, dialysis, or kidney-related mortality (HR: 0.72; 95% CI: 0.54–0.97) [38]. Two double-blind, randomized studies were conducted concurrently as a part of the CANagliflozin cardioVascular assessment study (CANVAS) program. The CANVAS and CANVAS-R (renal) studies included 10,142 patients with or without baseline CKD. A 27% lower risk in progression of albuminuria (HR:0.73; 95% CI: 0.67–0.79) and a 40% (HR: 0.60; 95% CI: 0.47–0.77) lower risk for a composite kidney outcome consisting of ≥40% reduction in eGFR, need for KRT, or death from kidney cause were observed in the canagliflozin group compared to placebo [39].
The DECLARE-TIMI 58 (Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58) study was predominantly designed to evaluate the cardiovascular safety of dapagliflozin. The composite outcome consisting of a ≥40% decrease in eGFR to <60 mL/min per 1.73 m2, kidney failure, and death from cardiovascular or kidney cause was 4.3% in patients on dapagliflozin and 5.6% in the placebo group (HR: 0.76; 95% CI: 0.67–0.87) [40]. The cardiovascular safety of dapagliflozin was also the primary outcome in the DAPA-HR (Dapagliflozin and Prevention of Adverse-outcomes in Heart Failure) study. After a period of 18.2 months, a sustained ≥50% reduction in eGFR, kidney failure, or death from kidney cause in dapagliflozin and in the placebo arm were 1.2% and 1.6%, respectively (p = 0.17) [41]. The DELIGHT (An Exploratory Phase II/III, Randomized, Double-blind, Placebo Controlled, Parallel Design Study to Evaluate the Efficacy, Safety and Pharmacodynamics of Dapagliflozin and Dapagliflozin in Combination With Saxagliptin in CKD Patients With Type 2 Diabetes Mellitus and Albuminuria Treated With ACEi or ARB) study enrolled T2DM patients with HbA1c of 7–11%, UACR 30–3500 mg/g, and eGFR of 25–75 mL/min per 1.73 m2 who were on a stable dose of ACE inhibitors or ARBs. The patients were randomized into three treatment arms: 145 were treated with dapagliflozin, 155 with dapagliflozin and saxagliptin, and 148 received a placebo and were followed up every 4 weeks. Within the entire study period, reduced UACR was observed in the dapagliflozin and also in the dapagliflozin–saxagliptin arm. At the end of the study, which lasted 24 weeks, the difference in the mean UACR change from baseline was −21% (p = 0.011) in the dapagliflozin and −38% (p < 0.0001) in the dapagliflozin–saxagliptin arm. Furthermore, eGFR reductions were also observed at the end of the study, with differences in the mean change from baseline of –2.4 mL/min per 1·73 m2 (p = 0.011) in the dapagliflozin arm and –2.4 mL/min per 1.73 m2 (p = 0.0075) in the dapagliflozin–saxagliptin arm when compared to placebo [42]. A randomized, double-blind, placebo-controlled, multi-center clinical study DAPA-CKD (The Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease) enrolled 4094 patients with/without T2DM on a stable dose of an ACE inhibitor or ARBs. The eligible subjects had eGFR 25–75 mL/min per 1.73 m and UACR of 200–5000 mg/g. The primary composite outcome, which included a sustained decline in eGFR of at least 50%, kidney failure, or death from kidney or cardiovascular causes, was reduced by 39% with dapagliflozin (HR: 0.61; 95% CI: 0.51–0.72). These results were comparable in patients with and without T2DM [43].
EMPA-KIDNEY (Study of Heart and Kidney Protection With Empagliflozin) was also a double-blind, randomized, placebo-controlled study with a median duration of 2 years, which included 6600 patients with CKD and eGFR of ≥20 to <45 mL/min per 1.73 m2, or an eGFR of ≥45 to <90 mL/min per 1.73 m2 with UACR of at least 200 mg/g. Patients with or without T2DM treated with ACE inhibitors or ARBs were randomized into two groups. The patients in the first group were receiving 10 mg of empagliflozin, while the patients in the other group were receiving a placebo. The primary outcome was a composite of death from kidney or cardiovascular causes, ESKD, a persistent decline in eGFR <10 mL/min per 1.73 m2, and a sustained decline in eGFR of less than 40% from the baseline. The progression of kidney disease or death from cardiovascular causes appeared in 13.1% of the patients on empagliflozin and in 16.9% of the patients in the placebo group (HR, 0.72; 95% CI, 0.64 to 0.82; p < 0.001). There were no significant differences between both groups regarding the composite outcome of hospitalization for heart failure, death from cardiovascular causes, or death from any cause. However, the rate of hospitalization from any cause was lower in the empagliflozin group compared to the placebo group (HR, 0.86; 95% CI, 0.78 to 0.95; p = 0.003). Both groups experienced equal rates of major adverse events [44]. The EMPA-REG (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose) study included patients with advanced kidney disease with eGFR of 30 mL/min or more. Empagliflozin slowed the progression of kidney failure, and there were fewer clinically relevant kidney events in this group [45].
The EMPEROR-Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction) study included 3730 patients with/without T2DM and heart failure with an ejection fraction of 40% or less who had appropriate treatment for heart failure. The median follow-up at 21 months showed that annual reduction in eGFR was slower in the empagliflozin group than in the placebo group (p < 0.001). Furthermore, patients treated with empagliflozin had a lower risk of serious kidney outcome [46]. The EMPEROR-Preserved (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) study was a double-blind and randomized clinical trial that included 5988 patients with class II–IV heart failure and an ejection fraction of more than 40% who were randomized in receiving empagliflozin 10 mg or a placebo in addition to their standard therapy. The median follow-up at 26.2 months indicated a slower rate of decline in the eGFR in the empagliflozin group (p < 0.001) [47]. In the EMPEROR-Reduced and the EMPEROR-Preserved study, the HR values for major kidney events were 0.51 (95% CI, 0.33 to 0.79) and 0.95 (95% CI, 0.73 to 1.24), respectively [48].
The VERTIS-CV (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes) study included T2DM patients treated with ertugliflozin or a placebo. Although UACR was reduced, the study did not observe significant changes in composite kidney outcomes. eGFR was reduced after ertugliflozin treatment, but the values returned to baseline and were higher after 104 weeks [49].
The SCORED (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) study included a total of 10.584 T2DM patients with CKD (eGFR, 25 to 60 mL per minute per 1.73 m2) that were randomized into two study arms: 5292 were treated with sotagliflozin and 5292 received a placebo. Although this study was primarily designed to assess cardiovascular safety, the secondary end points included a composite kidney outcome of the first occurrence of a sustained decrease of ≥50% in the eGFR from baseline for ≥30 days, long-term dialysis, kidney transplantation, or sustained eGFR of <15 mL/min/1.73 m2 for ≥30 days. The kidney outcomes did not differ significantly between the two groups [50].
To summarize the kidney outcomes reported by all these randomized clinical studies, it can be concluded that, in T2DM patients with or without prevalent cardiovascular diseases, SGLT2 inhibitors may reduce albuminuria, progression of DKD, doubling of serum creatinine levels, and initiation of KRT to more than 40%.
The randomized data support the use of SGLT2 inhibitors in patients with CKD or heart failure regardless of diabetes status, primary kidney disease, or kidney function in order to reduce the risk of kidney disease progression and acute kidney injury [51][52].
Furthermore, researchers expect very promising results from the treatment of DKD with the novel and more selective non-steroidal mineralocorticoid receptor antagonist finerenone, which has more potent anti-inflammatory and anti-fibrotic effects on the kidney than spironolactone [53].
References
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334.
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883.
- National Kidney Foundation. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150.
- Faselis, C.; Katsimardou, A.; Imprialos, K.; Deligkaris, P.; Kallistratos, M.S.; Dimitriadis, K. Microvascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 117–124.
- Crasto, W.; Patel, V.; Davies, M.J.; Khunti, K. Prevention of Microvascular Complications of Diabetes. Endocrinol. Metab. Clin. North Am. 2021, 50, 431–455.
- Nitta, K.; Goto, S.; Masakane, I.; Hanafusa, N.; Taniguchi, M.; Hasegawa, T.; Nakai, S.; Wada, A.; Hamano, T.; Hoshino, J.; et al. Annual dialysis data report for 2017, JSDT Renal Data Registry: Survey methods, facility data, incidence, prevalence, and mortality. Ren. Replace. Ther. 2020, 6, 41.
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S83–S96.
- Nathan, D.M.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16.
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412.
- American Diabetes Association Professional Practice Committee. 11. Chronic Kidney Disease and Risk Management: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45 (Suppl. S1), S175–S184.
- Sasso, F.C.; Simeon, V.; Galiero, R.; Caturano, A.; De Nicola, L.; Chiodini, P.; Rinaldi, L.; Salvatore, T.; Lettieri, M.; Nevola, R.; et al. The number of risk factors not at target is associated with cardiovascular risk in a type 2 diabetic population with albuminuria in primary cardiovascular prevention. Post-hoc analysis of the NID-2 trial. Cardiovasc. Diabetol. 2022, 21, 235.
- Herrington, W.G.; Preiss, D.; Haynes, R.; von Eynatten, M.; Staplin, N.; Hauske, S.J.; George, J.T.; Green, J.B.; Landray, M.J.; Baigent, C.; et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: A rationale for the EMPA-KIDNEY study. Clin. Kidney J. 2018, 11, 749–761.
- Mima, A. Inflammation and Oxidative Stress in Diabetic Nephropathy: New Insights on Its Inhibition as New Therapeutic Targets. J. Diabetes Res. 2013, 2013, 248563.
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651.
- Van Bommel, E.J.; Muskiet, M.H.; van Baar, M.J.; Tonneijck, L.; Smits, M.M.; Emanuel, A.L.; Bozovic, A.; Danser, A.J.; Geurts, F.; Hoorn, E.J.; et al. The renal hemodynamic effects of the SGLT2 inhibitor dapagliflozin are caused by post-glomerular vasodilatation rather than pre-glomerular vasoconstriction in metformin-treated patients with type 2 diabetes in the randomized, double-blind RED trial. Kidney Int. 2020, 97, 202–212.
- Ott, C.; Jung, S.; Korn, M.; Kannenkeril, D.; Bosch, A.; Kolwelter, J.; Striepe, K.; Bramlage, P.; Schiffer, M.; Schmieder, R.E. Renal hemodynamic effects differ between antidiabetic combination strategies: Randomized controlled clinical trial comparing empagliflozin/linagliptin with metformin/insulin glargine. Cardiovasc. Diabetol. 2021, 20, 178.
- Cherney, D.Z.I.; Zinman, B.; Inzucchi, S.E.; Koitka-Weber, A.; Mattheus, M.; von Eynatten, M.; Wanner, C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: An exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017, 5, 610–621.
- Deng, D.; Yan, N. GLUT, SGLT, and SWEET: Structural and mechanistic investigations of the glucose transporters. Protein Sci. 2016, 25, 546–558.
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794.
- Thomas, M.C.; Cherney, D.Z.I. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018, 61, 2098–2107.
- Cherney, D.Z.; Perkins, B.A.; Soleymanlou, N.; Har, R.; Fagan, N.; Johansen, O.; Woerle, H.-J.; von Eynatten, M.; Broedl, U.C. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc. Diabetol. 2014, 13, 28.
- Lee, P.C.; Ganguly, S.; Goh, S.-Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: A review of evidence and underlying mechanisms. Obes. Rev. 2018, 19, 1630–1641.
- Bolinder, J.; Ljunggren, Ö.; Kullberg, J.; Johansson, L.; Wilding, J.; Langkilde, A.M.; Sugg, J.; Parikh, S. Effects of Dapagliflozin on Body Weight, Total Fat Mass, and Regional Adipose Tissue Distribution in Patients with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on Metformin. J. Clin. Endocrinol. Metab. 2012, 97, 1020–1031.
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46.
- Liu, H.; Sridhar, V.S.; Boulet, J.; Dharia, A.; Khan, A.; Lawler, P.R.; Cherney, D.Z. Cardiorenal protection with SGLT2 inhibitors in patients with diabetes mellitus: From biomarkers to clinical outcomes in heart failure and diabetic kidney disease. Metabolism 2022, 126, 154918.
- Liu, H.; Sridhar, V.S.; Montemayor, D.; Lovblom, L.E.; Lytvyn, Y.; Ye, H.; Kim, J.; Ali, M.T.; Scarr, D.; Lawler, P.R.; et al. Changes in plasma and urine metabolites associated with empagliflozin in patients with type 1 diabetes. Diabetes Obes. Metab. 2021, 23, 2466–2475.
- Dharia, A.; Khan, A.; Sridhar, V.S.; Cherney, D.Z. SGLT2 Inhibitors: The Sweet Success for Kidneys. Annu. Rev. Med. 2023, 74, 369–384.
- Kimura, Y.; Kuno, A.; Tanno, M.; Sato, T.; Ohno, K.; Shibata, S.; Nakata, K.; Sugawara, H.; Abe, K.; Igaki, Y.; et al. Canagliflozin, a sodium–glucose cotransporter 2 inhibitor, normalizes renal susceptibility to type 1 cardiorenal syndrome through reduction of renal oxidative stress in diabetic rats. J. Diabetes Investig. 2019, 10, 933–946.
- Alshnbari, A.S.; Millar, S.A.; O’sullivan, S.E.; Idris, I. Effect of Sodium-Glucose Cotransporter-2 Inhibitors on Endothelial Function: A Systematic Review of Preclinical Studies. Diabetes Ther. 2020, 11, 1947–1963.
- Lee, Y.H.; Kim, S.H.; Kang, J.M.; Heo, J.H.; Kim, D.-J.; Park, S.H.; Sung, M.; Kim, J.; Oh, J.; Yang, D.H.; et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am. J. Physiol. Physiol. 2019, 317, F767–F780.
- Dekkers, C.C.J.; Petrykiv, S.; Laverman, G.D.; Cherney, D.Z.; Gansevoort, R.T.; Heerspink, H.J.L. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes. Metab. 2018, 20, 1988–1993.
- Liu, H.; Sridhar, V.S.; Lovblom, L.E.; Lytvyn, Y.; Burger, D.; Burns, K.; Brinc, D.; Lawler, P.R.; Cherney, D.Z. Markers of Kidney Injury, Inflammation, and Fibrosis Associated With Ertugliflozin in Patients With CKD and Diabetes. Kidney Int. Rep. 2021, 6, 2095–2104.
- Mima, A. Mitochondria-targeted drugs for diabetic kidney disease. Heliyon 2022, 8, e08878.
- Heerspink, H.J.; Kosiborod, M.; Inzucchi, S.E.; Cherney, D.Z. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018, 94, 26–39.
- Hesp, A.C.; Schaub, J.A.; Prasad, P.V.; Vallon, V.; Laverman, G.D.; Bjornstad, P.; van Raalte, D.H. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020, 98, 579–589.
- Yu, S.M.-W.; Bonventre, J.V. Acute Kidney Injury and Progression of Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 166–180.
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diabetes Rep. 2022, 22, 39–52.
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306.
- Perkovic, V.; de Zeeuw, D.; Mahaffey, K.W.; Fulcher, G.; Erondu, N.; Shaw, W.; Barrett, T.D.; Weidner-Wells, M.; Deng, H.; Matthews, D.R.; et al. Canagliflozin and renal outcomes in type 2 diabetes: Results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018, 6, 691–704.
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357.
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008.
- Pollock, C.; Stefánsson, B.; Reyner, D.; Rossing, P.; Sjöström, C.D.; Wheeler, D.C.; Langkilde, A.M.; Heerspink, H.J.L. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2019, 7, 429–441.
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. DAPA-CKD Trial Committees and Investigators. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446.
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127.
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128.
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424.
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461.
- Packer, M.; Butler, J.; Zannad, F.; Pocock, S.J.; Filippatos, G.; Ferreira, J.P.; Brueckmann, M.; Jamal, W.; Zeller, C.; Wanner, C.; et al. Empagliflozin and Major Renal Outcomes in Heart Failure. N. Engl. J. Med. 2021, 385, 1531–1533.
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435.
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2021, 384, 129–139.
- Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801.
- Georgianos, P.I.; Vaios, V.; Eleftheriadis, T.; Papachristou, E.; Liakopoulos, V. Therapeutic Advances in Diabetic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 2803.
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26.
More
Information
Subjects:
Medicine, Research & Experimental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
615
Revisions:
2 times
(View History)
Update Date:
04 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




