Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lisheng Wang | -- | 1547 | 2023-08-03 03:59:29 | | | |
| 2 | Dean Liu | -1 word(s) | 1546 | 2023-08-03 05:36:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, W.; Ling, Y.; Sun, Y.; Xiao, F.; Wang, L. Extracellular Vesicles Derived from Mesenchymal Stem Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/47582 (accessed on 07 February 2026).
Zhang W, Ling Y, Sun Y, Xiao F, Wang L. Extracellular Vesicles Derived from Mesenchymal Stem Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/47582. Accessed February 07, 2026.
Zhang, Weiyuan, Yang Ling, Yang Sun, Fengjun Xiao, Lisheng Wang. "Extracellular Vesicles Derived from Mesenchymal Stem Cells" Encyclopedia, https://encyclopedia.pub/entry/47582 (accessed February 07, 2026).
Zhang, W., Ling, Y., Sun, Y., Xiao, F., & Wang, L. (2023, August 03). Extracellular Vesicles Derived from Mesenchymal Stem Cells. In Encyclopedia. https://encyclopedia.pub/entry/47582
Zhang, Weiyuan, et al. "Extracellular Vesicles Derived from Mesenchymal Stem Cells." Encyclopedia. Web. 03 August, 2023.
Copy Citation
Mesenchymal stem cell-derived extracellular vesicles (MSC-EVs) are biologically active substances secreted by MSCs into the extracellular matrix that play an immunomodulatory role in skin damage repair.
mesenchymal stem cells
cellular changes
extracellular vesicles
1. Introduction
Poor skin wound healing is an urgent concern in the field of trauma medicine. The application of traditional therapeutic methods, such as systemic anti-inflammatory drugs and traditional dressings, has not achieved breakthroughs in skin wound healing. Mesenchymal stem cells (MSCs) were found to possess great therapeutic potential for wound healing and skin regeneration [1]. MSCs, also known as mesenchymal stromal cells, are recognized as cell populations with diverse differentiation potential and are derived from fat, umbilical cord, amniotic fluid, placenta, skin, dental pulp, and many other tissues [2]. MSCs were initially reported by Friedenstein et al. for their ability to self-renew and undergo multilineal differentiation [3][4]. Subsequently, researchers found that MSCs secrete various small molecules, such as extracellular vesicles (EVs), cytokines, chemokines, growth factors, and interleukins (ILs), which can undergo endocytosis or bind to receptor surface proteins, transmit signals to the corresponding receptor cells, and mediate intercellular communication among cell types to change their biological behavior and participate in immune regulation [5][6][7][8][9].
Researchers reported that the paracrine function of MSCs enables them to acquire strong immune regulation capabilities and have attempted to apply them to cell therapy regimens for various human diseases [10]. Some effects, such as suppression of the local immune system, inhibition of fibrosis (scarring) and apoptosis, enhancement of angiogenesis, stimulation of mitosis, induction of tissue intrinsic repair cells, and stem cell differentiation, are different from those of MSCs that differentiate directly into repair tissue [11]. MSC-EVs have been shown to promote skin wound healing and accelerate this process through multiple mechanisms. These mechanisms comprise reducing inflammation, promoting angiogenesis, and promoting proliferation and migration of epithelial cells and fibroblasts. Consequently, MSC-EVs can be used as new biomarkers and therapeutic targets because of the functional molecules they encapsulate, which can simultaneously promote wound healing through multiple mechanisms and may be a promising method to replace cells for skin wound treatment [8].
2. Description of MSC-EVs
MSCs are often described as a highly heterogeneous population of stem and progenitor cells that expand into unisolated fibroids and mucinous cells in vitro [12]. Initially, a group of fibroblast-like cells, capable of differentiating into adipocytes, chondrocytes, and osteocytes, was isolated from the bone marrow of guinea pigs and mice, which influenced the microenvironment for the in vitro culture of hematopoietic stem cells (HSCs) [3][4]. These cells were later identified as MSCs in human tissues. In 2006, the International Society for Cell & Gene Therapy provided a clear definition for MSCs. They express surface molecules, including CD105, CD73, and CD90 but do not express surface molecules, including CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR, and can differentiate into osteoblasts, adipocytes, and chondroblasts in vitro [13]. More importantly, MSCs were later found to produce some “factors” through paracrine actions, which can play notable roles in immune regulation [14][15].
2.1. Classification, Labeling, Formation, and Delivery of MSC-EVs
MSC-EVs are a diverse family of particles composed of membrane-bound particles released from stem cells. Particles in this family are predominantly circular, isolated, or rarely aggregated into small clusters, have confinement membranes, and exhibit uniform electron transmission [16]. There are many types of MSC-EVs, and research scholars have followed two rules to classify them. The first rule is from the International Society of Extracellular Vesicles, which revised a new MSC-EV subtype nomenclature based on the physical properties, biochemical components, and cells of origin of EVs according to the latest information on EV research in 2018 [17]. Another method is simpler than that aforementioned and is a general classification according to the subtype of MSC-EVs, namely, exosomes (30–120 nm), microvesicles (MVs) (100–1000 nm), and apoptotic bodies (800–5000 nm) [18]. Markers of EVs include multiple proteins involved in endosome biogenesis, such as Alix, tumor susceptibility gene 101 protein (TSG101), tetraspanins (CD63, CD81, CD9), and lysosome-associated membrane proteins (LAMP1 and LAMP2) [19]. The membrane of MSC-EVs contains large amounts of cholesterol, sphingomyelin, ceramide, and various lipid molecules [20]. Additionally, the membrane surface of MSC-EVs was confirmed to contain both the characteristic surface markers of MSCs (CD29, CD105, and CD73) and the traditional markers of EVs (CD63, CD81, and CD9) [21]. EVs contain proteins, miRNAs, and lipids [22]. Regarding the formation of EVs, the budding theory, which refers to the formation of multivesicular bodies (MVBs) that fuse with the plasma membrane after mature endosomes sprout inward, is widely recognized. The buds released later are called EVs [8]. MSCs produce active EVs and release them into the cytoplasm of recipient cells, where they are captured by recipient cells through endocytosis, receptor–ligand binding, or direct binding and can transmit signals to recipient cells, guiding their biological behavior [23]. Under some conditions, small EVs were not easily distinguishable from exosomes, and some subpopulations of small EVs were similar in size to exosomes and were observed during direct budding from the plasma membrane [24]. MSC-EVs consist of many different molecules, such as nucleic acids (DNA, RNA, mRNA, and miRNA), pro-inflammatory and anti-inflammatory cytokines, enzymes, and various other proteins [24]. Some research scholars believe that MSC-EVs can not only dump self-secreted cytokines into the intercellular space for recognition by any cell containing the corresponding receptor but also deliver a small amount of cytokines directly to target cells, which is a surprising and more efficient delivery mechanism than that of MSCs [25].
2.2. MSCs and MSC-EVs
MSC-EVs may contain MSC-specific components and exert specific effects on recipient cells, similar to the therapeutic effect of MSCs to a certain extent [26]. EVs derived from MSCs have more advantages than that of MSCs. First, the phospholipid bilayer vesicles of EVs prevent themselves from being recognized as foreign objects by tissues and become complex carriers that protect enzymes, cytokines, and genetic material from degradation. Moreover, owing to the presence of cell-binding affinity proteins embedded on the surface of vesicles, EVs can show the same excellent delivery efficiency as MSCs. Second, EVs move freely in the blood because of their nanometer size, which can easily achieve membrane fusion of target cells and can penetrate the skin mucosal barrier, blood–brain barrier, and placental barrier, making them an ideal carrier for the delivery of active molecules and drugs [27]. However, the ratio of MSCs enriched to the target site through blood circulation after administration and the ratio of MSCs integrated to the damaged site in a short time are relatively low. Third, EVs are less immunogenic than MSCs because they do not express MHC-I or MHC-II antigens on their membrane surfaces; thus, tissues do not recognize these EVs as foreign, protecting their contents from degradation. However, MSCs express high levels of MHC-II when stimulated by inflammation, and treatment with MSCs was reported to be carcinogenic [28]. Fourth, EVs are highly modifiable as noncellular structures. By loading functional drugs, specific proteins, and non-coding RNAs, including miRNAs and siRNAs, EVs can replace cell therapy and become a new biotherapeutic method.
3. Mechanism of MSC-EVs in Promoting Wound Healing and Skin Regeneration
Skin wound healing is a series of physiological processes that begins after the normal anatomical structure or integrity of the skin is destroyed. Several studies have investigated the effects of MSC-EVs on wound healing and skin diseases. Different types of cells are involved in different stages of chronic wound healing (e.g., hemostasis, inflammation, proliferation, and remodeling), including immune cells involved in the regulation of inflammation, for example, macrophages and neutrophils T cells [29], and cells involved in tissue proliferation and remodeling, for example, fibroblasts, keratinocytes, and endothelial cells (Figure 1). In this chapter, researchers attempt to elucidate the immunomodulatory effects of MSC-EVs on different cell types.
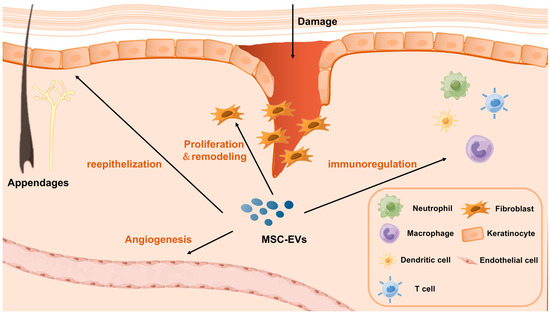
Figure 1. A schematic diagram illustrating the mechanism of MSC-EVs in promoting wound healing and skin regeneration.
4. Challenges in Applying MSC-EVs to Promote Wound Healing and Skin Regeneration
Cell therapy has made great strides in the clinical practice of skin damage repair, and an increasing number of clinical trials have reported the therapeutic effects of MSC-EVs. As a new therapeutic approach, MSC-EVs have many limitations that must be overcome before they can be used clinically. First, their effects are difficult to predict in vivo because of the tissue origin, concentration, number of doses, route and timing of MSC administration, and inflammatory state of the recipient. To predict the biological effects of MSC-EVs, a comprehensive characterization of MSC-EV content and standardization of experimental methods are essential. The MSCs donors used to generate EVs need to be planned and regulated, and a standard good manufacturing practice (GMP)-compliant MSC-EV isolation protocol needs to be developed and refined. Second, because MSC-EVs cover a relatively wide range involving microvesicles (MVs), apoptotic bodies, and exosomes, developers need to classify MSC-EVs and establish consistent, graded release criteria (e.g., particle size, loading, surface marker expression) before they can be injected into potential patients. Additionally, medical practitioners need to monitor the treatment process in the human body at any time, determine the markers that distinguish functional and non-functional EVs based on the efficacy of the treatment, and then report them back to researchers to pursue the production of functional-specific MSC-EVs. Third, the optimal dose of MSC-EVs in humans, the optimal route of administration of MSC-EVs, and the length of time that MSC-EVs remain in patients before being cleared by phagocytes remain unclear and need to be determined according to the treatment. Investigators must overcome these limitations to achieve MSC-EV-induced immunomodulation and regeneration.
References
- Salibian, A.A.; Widgerow, A.D.; Abrouk, M.; Evans, G.R. Stem Cells in Plastic Surgery: A Review of Current Clinical and Translational Applications. Arch. Plast. Surg. 2013, 40, 666–675.
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507.
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The Development of Fibroblast Colonies in Monolayer Cultures of Guinea-Pig Bone Marrow and Spleen Cells. Cell Prolif. 1970, 3, 393–403.
- Friedenstein, A.; Gorskaja, J.; Kulagina, N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274.
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236.
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933.
- Kourembanas, S. Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu. Rev. Physiol. 2015, 77, 13–27.
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177.
- Massa, M.; Croce, S.; Campanelli, R.; Abbà, C.; Lenta, E.; Valsecchi, C.; Avanzini, M.A. Clinical Applications of Mesenchymal Stem/Stromal Cell Derived Extracellular Vesicles: Therapeutic Potential of an Acellular Product. Diagnostics 2020, 10, 999.
- Wang, J.; Xia, J.; Huang, R.; Hu, Y.; Fan, J.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles alter disease out-comes via endorsement of macrophage polarization. Stem Cell Res. Ther. 2020, 11, 424.
- Caplan, A.; Dennis, J. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084.
- Koliaraki, V.; Prados, A.; Armaka, M.; Kollias, G. The mesenchymal context in inflammation, immunity and cancer. Nat. Immunol. 2020, 21, 974–982.
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Yang, C.; Chen, Y.; Li, F.; You, M.; Zhong, L.; Li, W.; Zhang, B.; Chen, Q. The biological changes of umbilical cord mesenchymal stem cells in inflammatory environment induced by different cytokines. Mol. Cell. Biochem. 2018, 446, 171–184.
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528.
- Del Fattore, A.; Luciano, R.; Pascucci, L.; Goffredo, B.M.; Giorda, E.; Scapaticci, M.; Fierabracci, A.; Muraca, M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015, 24, 2615–2627.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673.
- Zöller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55.
- Bazzoni, R.; Kamga, P.T.; Tanasi, I.; Krampera, M. Extracellular Vesicle-Dependent Communication Between Mesenchymal Stromal Cells and Immune Effector Cells. Front. Cell Dev. Biol. 2020, 8, 596079.
- Ramos, T.L.; Sánchez-Abarca, L.; Muntión, S.; Preciado, S.; Puig, N.; López-Ruano, G.; Hernández-Hernández, Á.; Redondo, A.; Ortega, R.; Rodríguez, C.; et al. MSC surface markers (CD44, CD73, and CD90) can identify human MSC-derived extracellular vesicles by conventional flow cytometry. Cell Commun. Signal. CCS 2016, 14, 2.
- Xu, S.; Liu, C.; Ji, H.-L. Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses. Stem Cells Transl. Med. 2019, 8, 344–354.
- Hur, Y.H.; Cerione, R.A.; Antonyak, M.A. Extracellular vesicles and their roles in stem cell biology. Stem Cells 2020, 38, 469–476.
- O’brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606.
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63.
- Schatz, D.; Vardi, A. Extracellular vesicles—New players in cell–cell communication in aquatic environments. Curr. Opin. Microbiol. 2018, 43, 148–154.
- Bang, C.; Thum, T. Exosomes: New players in cell-cell communication. Int. J. Biochem. Cell Biol. 2012, 44, 2060–2064.
- Nicolay, N.H.; Perez, R.L.; Debus, J.; Huber, P.E. Mesenchymal stem cells—A new hope for radiotherapy-induced tissue damage? Cancer Lett. 2015, 366, 133–140.
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955.
More
Information
Subjects:
Cell Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
539
Revisions:
2 times
(View History)
Update Date:
03 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




