Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amit Kumar | -- | 2722 | 2023-08-02 12:16:05 | | | |
| 2 | Sirius Huang | Meta information modification | 2722 | 2023-08-03 10:10:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Baranwal, J.; Barse, B.; Di Petrillo, A.; Gatto, G.; Pilia, L.; Kumar, A. Metal Nanoparticles and Their Application in Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/47547 (accessed on 08 February 2026).
Baranwal J, Barse B, Di Petrillo A, Gatto G, Pilia L, Kumar A. Metal Nanoparticles and Their Application in Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/47547. Accessed February 08, 2026.
Baranwal, Jaya, Brajesh Barse, Amalia Di Petrillo, Gianluca Gatto, Luca Pilia, Amit Kumar. "Metal Nanoparticles and Their Application in Cancer" Encyclopedia, https://encyclopedia.pub/entry/47547 (accessed February 08, 2026).
Baranwal, J., Barse, B., Di Petrillo, A., Gatto, G., Pilia, L., & Kumar, A. (2023, August 02). Metal Nanoparticles and Their Application in Cancer. In Encyclopedia. https://encyclopedia.pub/entry/47547
Baranwal, Jaya, et al. "Metal Nanoparticles and Their Application in Cancer." Encyclopedia. Web. 02 August, 2023.
Copy Citation
Nanotechnology has revolutionized medical research with new and improved materials for biomedical applications, with a particular focus on therapy and diagnostics. In cancer research, the application of metal nanoparticles as substitute chemotherapy drugs is growing. Metals exhibit inherent or surface-induced anticancer properties, making metallic nanoparticles extremely useful.
metal nanoparticle
nanotechnology
biomedical
cancer
1. Introduction
Cancer, a multifaceted illness, is becoming a global health crisis and the leading cause of death and disability [1][2]. Around 9.6 million individuals died from cancer in 2018, affecting 18.1 million people worldwide. More than two-thirds of the world’s malignancies can be expected to be diagnosed by the year 2040. About 30% of early deaths in individuals aged 30–69 are brought on by cancer [3]. Utilization of biological agents such as terpenoids, plant alkaloids, anti-metabolites, and DNA-damaging alkylating chemicals are used to treat cancer. Unfortunately, contemporary chemotherapy has several drawbacks, most of which are attributable to the lack of target specificity and defects resulting in deficiencies and inconsistent clinical outcomes. Since normal cells also proliferate rapidly, chemotherapeutics are toxic to normal cells in the bone marrow, macrophages, digestive tract, and hair follicles [4][5]. This results in chronic toxicity, including myelosuppression, thrombocytopenia, anaemia, mucositis, organ malfunction, and alopecia. Due to this, physicians may choose to delay, halt, or modify the dosage of the prescribed treatment [6][7]. In addition to toxicity, chemotherapeutic resistance decreases the effectiveness of anticancer agents [8].
Surgical, chemotherapeutic, and radiotherapeutic cancer treatments are now accessible and widely utilized, as seen in Figure 1. However, their target is not just cancer but also healthy cells, which is the most significant problem of modern cancer treatment. Nanoparticles are nontoxic, stable, and biocompatible compounds found in nature, allowing them to be used as an effective drug delivery technique [9].
Figure 1. Types of cancer treatment.
Metal nanoparticles (NPs) may overcome difficulties associated with traditional treatment. Reportedly, metal NPs perform a positive and potent function in cancer therapy by improving targeting, gene silencing, and medication delivery. Functionalized metal nanoparticles with targeted ligands enhance control over tumor energy deposition in tumors. In addition to their therapeutic use, metal NPs are employed to image cancer cells as a diagnostic tool. Not only do therapeutic systems based on metal NPs give simultaneous diagnosis and treatment, but they also permit regulated and targeted drug release, revolutionizing cancer treatment and control.
Recently, NPs and nanotechnology have drawn a lot of interest in cancer therapy. They receive a lot of attention in the field of cancer therapeutics because they may provide more effective and targeted drug delivery strategies to address the drawbacks of conventional chemotherapy [10][11]. Drug delivery at the target site is hampered by physical and metabolic obstacles [12]. Drug activity is hampered at the cancer level by cellular and non-cellular processes, which increases the risk of recurrence and mortality. Recent decades have seen a substantial increase in scientific interest in nanotechnology because of its distinct functional and physical characteristics [13]. Because of the unique physical and chemical characteristics of NPs, including their chemical composition, small size, vast surface area, and structure, all these applications are feasible and economical [14]. Applications of NPs may be advantageous in treating a number of diseases, including cancer [15]. Attractive potential for NP application includes diagnostics (nanoimaging), drug delivery systems (nanocarrier), and the medical use of NPs themselves [16][17][18].
2. Role of Nanoparticles for Cancer, Biomedical Properties, and Therapeutics
Nanotechnology has a growing potential for application in medical diagnosis and therapy. Nanotechnology advancements have developed novel and better nanomaterials for biomedical applications [19]. NPs are utilized in various applications due to their unique properties [20]. Multifunctional NPs can transport hydrophobic compounds, target disease cells both actively and passively, extend the time a drug is in the bloodstream, increase the entry and accumulation of pharmaceuticals at tumor sites, overcome drug resistance, increase the safety and tolerability of medications, and advance the development of other technologies [21][22]. NPs are used in medical applications because of their unique properties, such as quantum properties, a surface-to-mass ratio that is much higher than that of other particles, and the ability to absorb and transport other compounds, such as proteins and medicines. NPs can have variable compositions, as their beginning ingredients might be dextran, chitosan, biological lipids, phospholipids, lactic acid, or chemicals such as silica, metals, carbon, and other polymers [23][24][25][26][27]. Classes of NPs, along with their advantages and disadvantages, are shown in Figure 2.
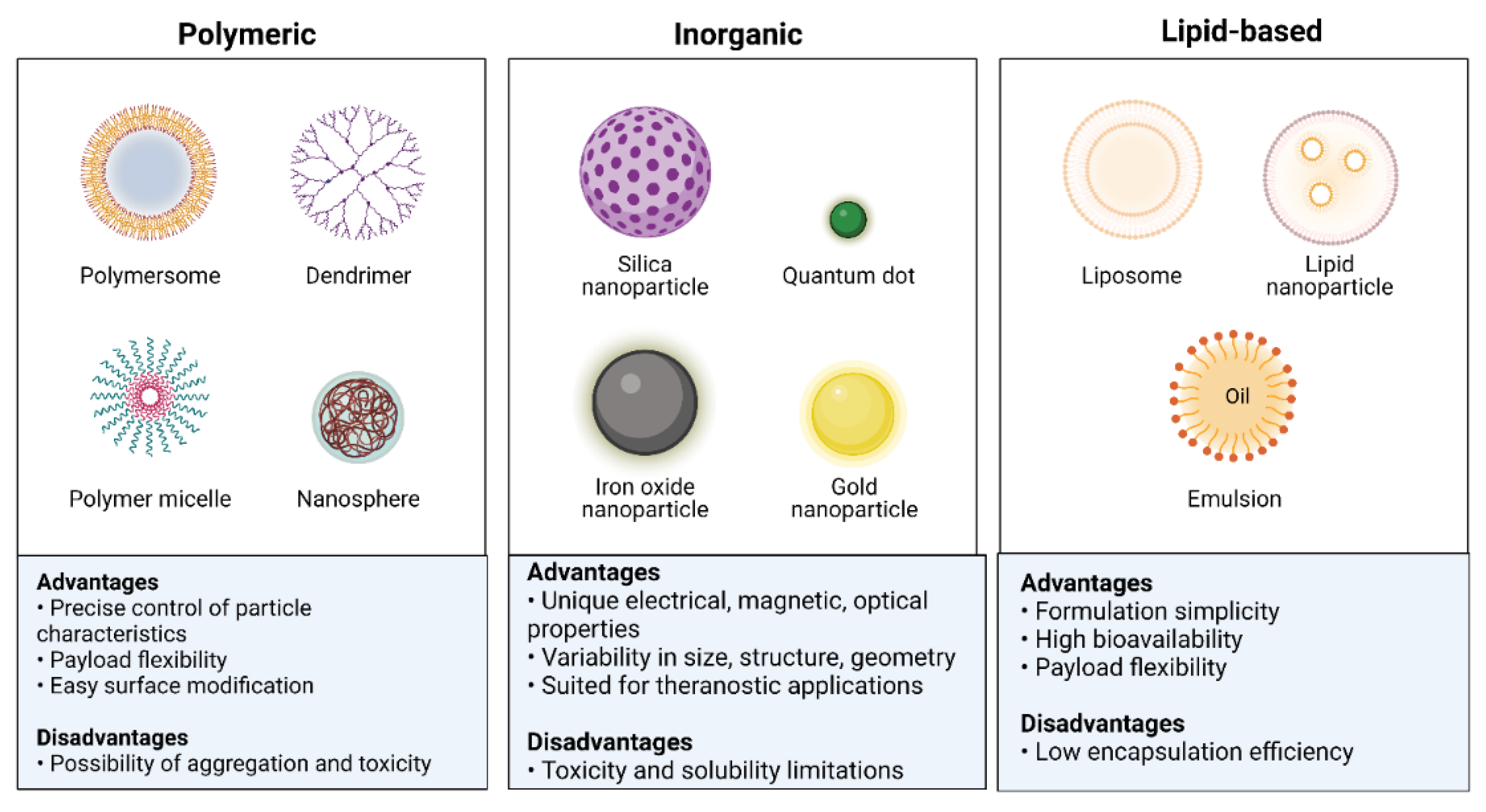
Figure 2. Classification of nanoparticles based on their physicochemical properties.
NPs are the perfect theranostic tools for tumor tracking and therapy because of their size (1–100 nm) and the high surface-to-volume ratio [28]. Effective medicine delivery is made possible by directly coupling NPs to various biomolecules, which also permits anatomical and functional imaging. Three distinct processes are involved in the delivery of NPs: (a) systemic localization without RES sequestration; (b) extravasation from intratumoral capillaries; and (c) diffusion and penetration into cancerous cells. NPs are effective anticancer weapons due to the high accumulation levels in tumor cells [29]. Manufacturing NPs bigger than 50 nm prevents RES sequestration [30]. Since solid tumors have a unique microenvironment, NP is desirable for imaging and drug delivery. The tumor microenvironment comprises blood vessels, inflammatory cells, signalling molecules, extracellular matrix, and lymphocytes. Blood vessels, inflammatory cells, signalling molecules, extracellular matrix, and lymphocytes make up the tumor microenvironment [31]. Solid tumors differ from healthy tissue in permeable microvascular environments with capillary pores between 120 and 1200 nm in size, which makes it easier for NP to penetrate tumors [32].
3. Metal Nanoparticles and Their Application in Cancer
The use of nanotechnology as a novel technique for cancer diagnosis, monitoring, and treatment has recently attracted interest in the biomedical community. Nanomaterials vary in diameter size from 1 to 1000 nm and display several distinctive features that differ from those seen in tiny particles or bulk materials. Nanomaterials have the potential to be used in several biological applications because of their substantial specific surfaces, high surface activity, robust antioxidant properties, outstanding biocompatibility, and solubility for molecular modifications. Liposomes, carbon nanotubes, polymeric micelles, graphs, quantum dots, metallic NPs, and magnetic NPs are often employed in biomedical applications. Previously, it has been demonstrated that using them extensively can improve treatment results [33].
3.1. Gold-Based Nanoparticles
The biological application of metallic nanoparticles, particularly gold nanoparticles (Au NPs), has sparked attention among numerous nanomaterials primarily due to its apparent benefits. Various Au NP forms, including spherical, rod-like, cage-like, and others, in diameters ranging from 1 nm to more than 100 nm, can be prepared rapidly. Au NPs’ form and size significantly impact optical and electrical characteristics [34]. Moreover, Au NPs have a negative charge, and many biomolecules, including genes and targeting ligands, can readily functionalize them [35]. Au NPs are harmless and biocompatible [36]. Surface plasmon resonance (SPR) bands are present in Au NPs, which also have an ultra-small size, a macroscopic quantum tunnelling effect, and a distinct surface effect [37]. Due to these unique characteristics, Au NPs have emerged as the most promising material for various biological applications, such as drug delivery, molecular imaging, and biosensing.
Photoacoustic Imaging
Photoacoustic imaging (PAI) is a biomedical imaging technology that employs endogenous and exogenous contrasts and provides insightful data on the cellular and molecular properties of the tissue. Because of their inherent and geometrically induced optical components, Au NPs as exogenous contrast agents hold significant potential for PA imaging. The most popular Au NPs forms for PA imaging include shells, prisms, spheres, rods, cages, stars, and blisters [38].
Gold nanorods are frequently employed as PAI contrast agents. Using PAI, it has previously been reported that Au/Ag activatable nanoparticles react to reactive oxygen and nitrogen species (RONS). The gold core can be preserved, while just some of the shell can be removed using RONS. The PAI signal is reactivated as a result of the etching. Iodide-doping of silver improves RONS sensitivity, allowing the detection of physiologically relevant levels in a mouse model [39].
3.2. Silver-Based Nanoparticles
Using silver (Ag) NPs as a safe and efficient method for treating cancer is challenging. Integrative studies involving physicists, chemists, engineers for creating NPs, and biologists who can evaluate the effects of Ag NPs in the cell systems they come in contact with, in aspects of cytotoxic effects and ability to damage malignant cells, are required [40]. Ag NPs are important and offer multiple advantages due to their size and shape-dependent features, such as optical, magnetic, chemical, and physical characteristics [41]. Ag NPs are included in numerous items, such as biosensors, composite fibers, antimicrobials, cosmetics, and electrical chemicals [42]. Additionally, Ag NPs can be utilized in cell electrodes, filters, nanocomposites, drug delivery, and medical imaging [42]. Ag is favoured over other NPs due to its superior light absorption, higher resolution, and stronger affinity for functionalization [43].
Like other noble metal NPs, Au NPs display exceptional SPR, making them ideal for usage in various fields, such as biosensing, catalysis, protein/gene transport, and photo-controlled delivery systems [44]. In addition to their antiproliferative effects on cancer cells, silver NPs can activate pathways that inhibit cell division and may be employed in the detection of many cancers like lung cancer, prostate cancer, hepatic cancer, cervical cancer, etc. Research on the antitumor effectiveness of Ag is still actively being conducted, despite technological advancements in its well-defined shapes and sizes and biocompatibility tests of both simple and coated Ag NPs.
3.3. Palladium-Based Nanoparticles
The development of palladium (Pd) based NPs is among the innovative nanomaterials that have significantly contributed to advancing the applications of noble metal nanomaterials in biomedicine. Pd-based nanomaterials have distinct advantages over other nanostructures made of noble metals. These advantages include biocompatibility and good photothermal stability. These characteristics make Pd-based nanomaterials stand out as exceptional and promising in biomedicine. After several years of research, Pd-based nanomaterials, such as Pd NSs, Pd@Au, Pd NPs, and Pt@Pt nanostructures, have been the subject of substantial investigation in the field of multimodal imaging-guided cancer treatment.
Pd-based nanomaterials have been noted to exhibit remarkable optical properties, high levels of biocompatibility, and high levels of stability in physiological conditions, all of which make them extremely promising for use in biomedical applications. Research on Pd-based nanomaterials started significantly later than that of other noble nanoparticles that have been extensively studied, such as gold (Au) and silver (Ag) nanomaterials. However, due to distinct qualities, including high photothermal conversion efficiency and photothermal stability, they have attracted a lot of attention in the nanomedicine sector. Pd-based nanomaterials display great near-infrared (NIR) absorption, a fast rate of photothermal conversion, outstanding biocompatibility, and exceptional photothermal stability. Pd nanosheets, porous/hollow Pd NPs, and Pd@M (M = Ag, Au, Pt, SiO2, ZIF-8) are examples of these nanomaterials. Pd-based nanoparticles have emerged as promising candidates for use as therapeutic agents and contrast agents in cancer imaging [45][46][47].
Pd nanosheets are typical examples of 2D nanomaterials with powerful NIR absorption, high photothermal conversion efficiency, outstanding photothermal stability, and a high level of biocompatibility. The diameters of Pd nanosheets (Pd NSs) can be easily altered to be anywhere between 5 and 120 nm while maintaining a consistent hexagonal shape [48][49]. The optical absorption peaks of Pd NSs shift depending on their size, but they are always found in the NIR range, thus offering exciting prospects for use in photothermal therapy (PTT). Table 1 summarises cancer therapies that utilize Pd-based nanoparticles, both as stand-alone PTT treatments and in combination with other therapeutic techniques [45].
Table 1. Summary of Pd-based nanoparticles for cancer treatment.
| Type of Therapy | Material | Reference |
|---|---|---|
| Photothermal therapy (PTT) | Pd NSs | [50][51] |
| Pd collora | [52] | |
| Pd@Ag nanoplates | [53] | |
| Pd@Au nanoplates | [54] | |
| Pd NS-CO Pd-TAT | [55] | |
| PTT and photodynamic therapy (PDT) | Pd@Pt-PEG-Ce6 | [56] |
| Pd@Ag@mSiO2-Ce6 | [57] | |
| Pd-PEI-Ce6 | [58] | |
| H-Pd NSs | [59] | |
| PLCs-HSA-ICG | [60] | |
| PTT and chemotherapy | Dox-loaded 8dc-Pd NPs | [61] |
| SPNS-DOX | [62] | |
| Pd@Au-PEG-Pt | [63] | |
| HMSS-NH2/DOX@Pd | [64] | |
| PTT and radiation therapy | [131I]PHPdNPs-DOX | [65] |
| 131I-Pd-PEG | [66][67] | |
| PTT and immunotherapy | Pd-CpG | [68] |
| PTT and hydrogen therapy | PdH0.2 nanocubes | [69] |
| PdH-MOF | [70] |
3.4. Iron Oxide-Based Nanoparticles
Because of their exceptional magnetic properties, surface-to-volume ratio perfect for successful functionalization, and biocompatibility, iron oxide NPs are often used in biological applications. Because these nanoparticles are now utilized in the medical system as contrast agents and heating mediators, most of the attention is focused on their development for MRI or magnetic particle hyperthermia. As a result, it is essential to keep improving and making new materials that are better and more reliable [71] for molecular imaging and biosensing.
Super Paramagnetic Iron Oxide Nanoparticles for Cancer Treatment
Superparamagnetic iron oxide nanoparticles (SPIONs) have drawn more attention due to their superior superparamagnetism, magnetic heating abilities, and improved magnetic resonance imaging (MRI). In vivo imaging, magnetic thermotherapy, and simultaneous delivery of anticancer treatments are only a few benefits of conjugating SPIONs with medications to create delivery nanosystems. Additional targeting moieties such as transferrin, hyaluronic acid, antibodies, aptamers, folate, and targeting peptides are coated onto the surface of SPIONs to improve the targeting efficacy of pharmaceuticals delivered by a delivery nanosystem based on SPIONs [72]. SPIONs exceptional MRI enhancement capabilities can be employed as tracers to depict the location and status of illness in the body, in addition to being deposited in cancer cells through the EPR effect and under an external magnetic field. Furthermore, SPIONs have a lower potential for toxicity than other inorganic NPs like their carbon- and gold-based counterparts because they can biodegrade into ferric ions in the human body, particularly in cells with acidic conditions (such as the lysosome and endosome) [73]. Theranostic agents based on SPION are essential for the delivery of therapeutic payloads such chemotherapeutic drugs and genes and for the diagnosis of cancer.
3.5. Copper-Based Nanoparticles
For biomedical applications, copper-based NPs have gained more interest. When exposed to a near-infrared laser, copper chalcogenide NPs display excellent near-infrared absorption, exhibit effective light-to-heat transformation, and selectively thermally destroy the tumor. Smaller copper NPs demonstrate the fluorescence signal and optical imaging capabilities. Additionally, copper-based NPs provide a flexible means of drug administration and image-guided treatment. Current developments in the biological use of copper-based NPs with an emphasis on cancer imaging and therapy have been discussed by Zhou et al. [74].
Copper NPs have more uses than Au and Ag NPs due to their reduced price, greater cytotoxic action against cancer cells at low doses, and prolonged stability period [75]. Novel copper-containing NIR-absorbing nano-formulations, such as copper selenide (Cu2-xSe) nanocrystals, nanocubes, monodispersed CuTe nanorods, nanoplates, and copper bismuth sulfide (Cu3BiS3) nanostructures, have all been fabricated and further confirmed for PTT. All the nanomolecules of copper discussed above have strong anticancer potential and improved photothermal heating efficiency [76].
3.6. Selenium-Based Nanoparticles
The trace element selenium (Se) is essential. It is included as selenocysteine, the most significant component of the active centre of selenoproteins’ enzymatic activity. Numerous selenoproteins have oxidoreductase activity and hence control the redox balance in the body. Se has a small therapeutic window and very fragile toxicity margins, but Se nanoparticles (SeNPs) have remarkably lower toxicity. SeNPs have been investigated for their potential therapeutic effects in a number of oxidative stress and inflammation-induced diseases, including cancer, diabetes, nephropathy, and arthritis. SeNPs serve as a desirable drug delivery system for a variety of medications. The impact of nanosizing on Se’s pharmacological action has been covered herein. Presently discussed is the function of SeNPs in the pharmacological defence against diverse inflammatory and oxidative stress-mediated situations. SeNPs’ potential impact on the pharmacokinetics and pharmacodynamics of selenoproteins, however, remains mainly unknown.
Human clinical trials validate the protective and curative functions of selenium in the initiation and progression of cancer. Diverse anticancer processes of selenium [77] can be divided into three major categories: thiol modification, chromatin binding and alteration, and reactive oxygen species (ROS) generation. Important factors affecting selenium’s biological activity, toxicity, and ability to prevent cancer are its amount and form [78]. Selenium’s anticancer activities have traditionally been linked to its organic form, particularly dietary selenium, and its antioxidant and pro-oxidant capabilities.
Studies on several malignancies, such as breast, lung, prostate, and colon cancers, demonstrate cancer-protective effects.
The most prevalent form of selenium in plants, seleniomethionine, was once believed to be the most chemopreventive and therapeutic form of selenium, but more recently, methylselenocysteine was discovered to have higher biological activity [79]. Since then, synthetic organoselenium species have been created that outperform their natural counterparts in terms of anticancer activity [80].
Despite their origins as an antioxidant, selenium-based medications like ebselen (Figure 3) have demonstrated potential anticancer action against breast, liver, and colon cancer cells. In Phase I clinical studies for the treatment of non-small cell lung cancer, ethaselen, a modified version of ebselen, has demonstrated better solubility [77][81]. According to studies, selenium can prevent cancer by preventing DNA damage brought on by the production of adducts caused by dimethylbenz(a) anthracene, which is a factor in the development of breast, colon, and liver cancers [82].
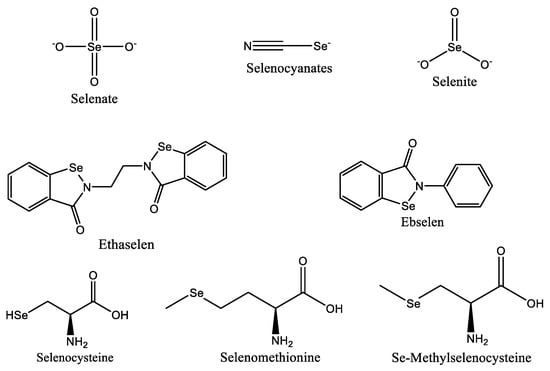
Figure 3. Selenium species with anticancer properties.
References
- Vinardell, M.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021.
- Conde, J.; Doria, G.; Baptista, P. Noble Metal Nanoparticles Applications in Cancer. J. Drug Deliv. 2012, 2012, 751075.
- World Health Organization. Global Centre for Traditional Medicine. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 15 May 2022).
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 939378.
- Zhao, G.; Rodriguez, L.B. Molecular targeting of liposomal nanoparticles to tumor microenvironment. Int. J. Nanomed. 2012, 8, 61–71.
- Nguyen, K.T. Targeted Nanoparticles for Cancer Therapy: Promises and Challenges. J. Nanomed. Nanotechnol. 2011, 2, 1000103e.
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbutts, T.; Frewin, C.; Fox, R.M.; Tattersall, M.H.N. On the receiving end—Patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208.
- Sharma, A.; Goyal, A.K.; Rath, G. Recent advances in metal nanoparticles in cancer therapy. J. Drug Target. 2017, 26, 617–632.
- Ho, B.N.; Pfeffer, C.M.; Singh, A.T. Update on Nanotechnology-based Drug Delivery Systems in Cancer Treatment. Anticancer Res. 2017, 37, 5975–5981.
- Prasad, R.; Pandey, R.; Varma, A.; Barman, I. Polymer-based nanoparticles for drug delivery systems and cancer therapeutics. In Natural Polymers for Drug Delivery; CABI: Wallingford, UK, 2017; pp. 53–70.
- Ficai, D.; Ficai, A. New Challenges in Cancer Treatment, from Novel Agents to Innovative Administration. Anti-Cancer Agents Med. Chem. 2019, 19, 4–5.
- Kumar, A.; Gatto, G.; Delogu, F.; Pilia, L. DFT study of complex (L = rubeanic acid) and its derived compounds with DNA purine bases. Chem. Phys. 2020, 530, 110646.
- Sack, M.; Alili, L.; Karaman, E.; Das, S.; Gupta, A.; Seal, S.; Brenneisen, P. Combination of Conventional Chemotherapeutics with Redox-Active Cerium Oxide Nanoparticles—A Novel Aspect in Cancer Therapy. Mol. Cancer Ther. 2014, 13, 1740–1749.
- Kumari, M.; Singh, S.P.; Chinde, S.; Rahman, M.F.; Mahboob, M.; Grover, P. Toxicity Study of Cerium Oxide Nanoparticles in Human Neuroblastoma Cells. Int. J. Toxicol. 2014, 33, 86–97.
- Sreena, R.; Nathanael, A.J. Biodegradable Biopolymeric Nanoparticles for Biomedical Applications-Challenges and Future Outlook. Materials 2023, 16, 2364.
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31.
- Balogh, L.P. Nano-Enabled Medical Applications; CRC Press: Boca Raton, FL, USA, 2020.
- Florence, A.T. “Targeting” nanoparticles: The constraints of physical laws and physical barriers. J. Control. Release 2012, 164, 115–124.
- Dessale, M.; Mengistu, G.; Mengist, H.M. Nanotechnology: A Promising Approach for Cancer Diagnosis, Therapeutics and Theragnosis. Int. J. Nanomed. 2022, 17, 3735–3749.
- Machado, S.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Utilization of food industry wastes for the production of zero-valent iron nanoparticles. Sci. Total Environ. 2014, 496, 233–240.
- Yang, Q.; Jones, S.W.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading Immune Cell Uptake and Clearance Requires PEG Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol. Pharm. 2014, 11, 1250–1258.
- Zeineldin, R.; Syoufjy, J. Cancer Nanotechnology: Opportunities for Prevention, Diagnosis, and Therapy. In Cancer Nanotechnology: Methods and Protocols; Humana Press: New York, NY, USA, 2017; pp. 3–12.
- Jani, P.; Subramanian, S.; Korde, A.; Rathod, L.; Sawant, K.K. Theranostic Nanocarriers in Cancer: Dual Capabilities on a Single Platform. In Functional Bionanomaterials: From Biomolecules to Nanoparticles; Springer: Cham, Switzerland, 2020; pp. 293–312.
- Nikolova, M.; Slavchov, R.; Nikolova, G. Nanotechnology in Medicine. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Springer: Cham, Switzerland, 2020; pp. 533–546.
- Saxena, S.K.; Nyodu, R.; Kumar, S.; Maurya, V.K. Current Advances in Nanotechnology and Medicine. In NanoBioMedicine; Springer: Singapore, 2020; pp. 3–16.
- Mukherjee, A.; Bhattacharyya, S. Nanotechnology in Medicine. In Biotechnology Business—Concept Delivery; Springer: Cham, Switzerland, 2020; pp. 57–64.
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001.
- Pedrosa, P.; Vinhas, R.; Fernandes, A.; Baptista, P. Gold Nanotheranostics: Proof-of-Concept or Clinical Tool? Nanomaterials 2015, 5, 1853–1879.
- De, J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149.
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release 2013, 172, 782–794.
- Barar, J.; Omidi, Y. Dysregulated pH in Tumor Microenvironment Checkmates Cancer Therapy. Bioimpacts 2013, 3, 149–162.
- Omidi, Y.; Barar, J. Targeting tumor microenvironment: Crossing tumor interstitial fluid by multifunctional nanomedicines. Bioimpacts 2014, 4, 55–67.
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445.
- Verissimo, T.V.; Santos, N.T.; Silva, J.R.; Azevedo, R.B.; Gomes, A.J.; Lunardi, C.N. In vitro cytotoxicity and phototoxicity of surface-modified gold nanoparticles associated with neutral red as a potential drug delivery system in phototherapy. Mater. Sci. Eng. C 2016, 65, 199–204.
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799.
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253.
- Kumar, A.; Zhang, X.; Liang, X.-J. Gold nanoparticles: Emerging paradigm for targeted drug delivery system. Biotechnol. Adv. 2013, 31, 593–606.
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320.
- Mantri, Y.; Davidi, B.; Lemaster, J.E.; Hariri, A.; Jokerst, J.V. Iodide-doped precious metal nanoparticles: Measuring oxidative stress in vivo via photoacoustic imaging. Nanoscale 2020, 12, 10511–10520.
- Vlăsceanu, G.M.; Marin, Ş.; Ţiplea, R.E.; Bucur, I.R.; Lemnaru, M.; Marin, M.M.; Grumezescu, A.M.; Andronescu, E. Silver nanoparticles in cancer therapy. In Nanobiomaterials in Cancer Therapy; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 29–56.
- Kent, R.D.; Vikesland, P.J. Controlled Evaluation of Silver Nanoparticle Dissolution Using Atomic Force Microscopy. Environ. Sci. Technol. 2012, 46, 6977–6984.
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; et al. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2014, 42, 173–180.
- Nedelcu, I.-A.; Ficai, A.; Sonmez, M.; Ficai, D.; Oprea, O.; Andronescu, E. Silver Based Materials for Biomedical Applications. Curr. Org. Chem. 2014, 18, 173–184.
- Qureshi, A.T.; Monroe, W.T.; Dasa, V.; Gimble, J.M.; Hayes, D.J. miR-148b–Nanoparticle conjugates for light mediated osteogenesis of human adipose stromal/stem cells. Biomaterials 2013, 34, 7799–7810.
- Chen, A.; Ostrom, C. Palladium-Based Nanomaterials: Synthesis and Electrochemical Applications. Chem. Rev. 2015, 115, 11999–12044.
- Chen, M.; Wu, B.; Yang, J.; Zheng, N. Small Adsorbate-Assisted Shape Control of Pd and Pt Nanocrystals. Adv. Mater. 2012, 24, 862–879.
- Chen, X.; Shi, S.; Wei, J.; Chen, M.; Zheng, N. Two-dimensional Pd-based nanomaterials for bioapplications. Sci. Bull. 2017, 62, 579–588.
- Huang, X.; Tang, S.; Mu, X.; Dai, Y.; Chen, G.; Zhou, Z.; Ruan, F.; Yang, Z.; Zheng, N. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat. Nanotechnol. 2010, 6, 28–32.
- Chen, M.; Chen, S.; He, C.; Mo, S.; Wang, X.; Liu, G.; Zheng, N. Safety profile of two-dimensional Pd nanosheets for photothermal therapy and photoacoustic imaging. Nano Res. 2016, 10, 1234–1248.
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble Metals on the Nanoscale: Optical and Photothermal Properties and Some Applications in Imaging, Sensing, Biology, and Medicine. Acc. Chem. Res. 2008, 41, 1578–1586.
- Fang, W.; Tang, S.; Liu, P.; Fang, X.; Gong, J.; Zheng, N. Pd Nanosheet-Covered Hollow Mesoporous Silica Nanoparticles as a Platform for the Chemo-Photothermal Treatment of Cancer Cells. Small 2012, 8, 3816–3822.
- Huang, X.; Tang, S.; Yang, J.; Tan, Y.; Zheng, N. Etching Growth under Surface Confinement: An Effective Strategy to Prepare Mesocrystalline Pd Nanocorolla. J. Am. Chem. Soc. 2011, 133, 15946–15949.
- Huang, X.; Tang, S.; Liu, B.; Ren, B.; Zheng, N. Enhancing the Photothermal Stability of Plasmonic Metal Nanoplates by a Core-Shell Architecture. Adv. Mater. 2011, 23, 3420–3425.
- Chen, M.; Tang, S.; Guo, Z.; Wang, X.; Mo, S.; Huang, X.; Liu, G.; Zheng, N. Core-Shell Nanoplates as Theranostic Agents for In-Vivo Photoacoustic Imaging, CT Imaging, and Photothermal Therapy. Adv. Mater. 2014, 26, 8210–8216.
- Tang, S.; Huang, X.; Zheng, N. Silica coating improves the efficacy of Pd nanosheets for photothermal therapy of cancer cells using near infrared laser. Chem. Commun. 2011, 47, 3948–3950.
- Wei, J.; Li, J.; Sun, D.; Li, Q.; Ma, J.; Chen, X.; Zhu, X.; Zheng, N. A Novel Theranostic Nanoplatform Based on for Enhanced Photodynamic Therapy by Modulating Tumor Hypoxia Microenvironment. Adv. Funct. Mater. 2018, 28, 1706310.
- Shi, S.; Zhu, X.; Zhao, Z.; Fang, W.; Chen, M.; Huang, Y.; Chen, X. Photothermally enhanced photodynamic therapy based on mesoporous @mSiO2 nanocarriers. J. Mater. Chem. B 2013, 1, 1133–1141.
- Zhao, Z.; Shi, S.; Huang, Y.; Tang, S.; Chen, X. Simultaneous Photodynamic and Photothermal Therapy Using Photosensitizer-Functionalized Pd Nanosheets by Single Continuous Wave Laser. ACS Appl. Mater. Interfaces 2014, 6, 8878–8885.
- Li, S.; Gu, K.; Wang, H.; Xu, B.; Li, H.; Shi, X.; Huang, Z.; Liu, H. Degradable Holey Palladium Nanosheets with Highly Active 1D Nanoholes for Synergetic Phototherapy of Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 5649–5656.
- Sun, D.; Huang, Y.; Zhang, X.; Peng, J.; Li, J.; Ming, J.; Wei, J.; Chen, X.; Zheng, N. A Pd corolla–human serum albumin–indocyanine green nanocomposite for photothermal/photodynamic combination therapy of cancer. J. Mater. Chem. B 2018, 6, 6969–6976.
- Gil, Y.-G.; Kang, S.; Chae, A.; Kim, Y.-K.; Min, D.-H.; Jang, H. Synthesis of porous Pd nanoparticles by therapeutic chaga extract for highly efficient tri-modal cancer treatment. Nanoscale 2018, 10, 19810–19817.
- Tang, S.; Chen, M.; Zheng, N. Multifunctional ultrasmall Pd nanosheets for enhanced near-infrared photothermal therapy and chemotherapy of cancer. Nano Res. 2014, 8, 165–174.
- Shi, S.; Chen, X.; Wei, J.; Huang, Y.; Weng, J.; Zheng, N. Platinum(iv) prodrug conjugated nanoplates for chemotherapy and photothermal therapy. Nanoscale 2016, 8, 5706–5713.
- Chen, X.; Zhu, X.; Xu, T.; Xu, M.; Wen, Y.; Liu, Y.; Liu, J.; Qin, X. Targeted hexagonal Pd nanosheet combination therapy for rheumatoid arthritis via the photothermal controlled release of MTX. J. Mater. Chem. B 2019, 7, 112–122.
- Song, M.; Liu, N.; He, L.; Liu, G.; Ling, D.; Su, X.; Sun, X. Porous hollow palladium nanoplatform for imaging-guided trimodal chemo-, photothermal-, and radiotherapy. Nano Res. 2018, 11, 2796–2808.
- Chen, M.; Guo, Z.; Chen, Q.; Wei, J.; Li, J.; Shi, C.; Xu, D.; Zhou, D.; Zhang, X.; Zheng, N. Pd nanosheets with their surface coordinated by radioactive iodide as a high-performance theranostic nanoagent for orthotopic hepatocellular carcinoma imaging and cancer therapy. Chem. Sci. 2018, 9, 4268–4274.
- Guo, Z.; Chen, M.; Peng, C.; Mo, S.; Shi, C.; Fu, G.; Wen, X.; Zhuang, R.; Su, X.; Liu, T.; et al. pH-sensitive radiolabeled and superfluorinated ultra-small palladium nanosheet as a high-performance multimodal platform for tumor theranostics. Biomaterials 2018, 179, 134–143.
- Hess, K.L.; Medintz, I.L.; Jewell, C.M. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 2019, 27, 73–98.
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241.
- Zhou, G.; Wang, Y.S.; Jin, Z.; Zhao, P.; Zhang, H.; Wen, Y.; He, Q. Porphyrin–palladium hydride MOF nanoparticles for tumor-targeting photoacoustic imaging-guided hydrogenothermal cancer therapy. Nanoscale Horiz. 2019, 4, 1185–1193.
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910.
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting strategies for superparamagnetic iron oxide nanoparticles in cancer therapy. Acta Biomater. 2020, 102, 13–34.
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44.
- Zhou, M.; Tian, M.; Li, C. Copper-Based Nanomaterials for Cancer Imaging and Therapy. Bioconjug. Chem. 2016, 27, 1188–1199.
- Nagajyothi, P.C.; Muthuraman, P.; Sreekanth, T.V.M.; Kim, D.H.; Shim, J. Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017, 10, 215–225.
- Mandal, A. Copper Nanomaterials as Drug Delivery System against Infectious Agents and Cancerous Cells. J. Appl. Life Sci. Int. 2017, 15, 1–8.
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1642–1660.
- El-Bayoumy, K.; Sinha, R. Molecular chemoprevention by selenium: A genomic approach. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 591, 224–236.
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium Compounds, Apoptosis and Other Types of Cell Death: An Overview for Cancer Therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672.
- Maiyo, F.; Singh, M. Selenium nanoparticles: Potential in cancer gene and drug delivery. Nanomedicine 2017, 12, 1075–1089.
- Jing, F.; Fu, X.; Li, S.; Li, B.; Zhao, J.; Wang, X.; Liu, Y.; Chen, B. Synthesis and in Vitro Antiproliferative Evaluation of Novel Hybrids from 1,3,4-Thiadiazole and Benzisoselenazolone. Chem. Pharm. Bull. 2015, 63, 431–437.
- El-Bayoumy, K. The protective role of selenium on genetic damage and on cancer. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2001, 475, 123–139.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
859
Revisions:
2 times
(View History)
Update Date:
03 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




