Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bhupendra G Prajapati | -- | 1809 | 2023-08-02 08:12:15 | | | |
| 2 | Camila Xu | Meta information modification | 1809 | 2023-08-02 08:15:25 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kumar, A.; Yadav, S.; Pramanik, J.; Sivamaruthi, B.S.; Jayeoye, T.J.; Prajapati, B.G.; Chaiyasut, C. Fabrication Methods for Composite Films/Coatings. Encyclopedia. Available online: https://encyclopedia.pub/entry/47530 (accessed on 08 March 2026).
Kumar A, Yadav S, Pramanik J, Sivamaruthi BS, Jayeoye TJ, Prajapati BG, et al. Fabrication Methods for Composite Films/Coatings. Encyclopedia. Available at: https://encyclopedia.pub/entry/47530. Accessed March 08, 2026.
Kumar, Akash, Sangeeta Yadav, Jhilam Pramanik, Bhagavathi Sundaram Sivamaruthi, Titilope John Jayeoye, Bhupendra G. Prajapati, Chaiyavat Chaiyasut. "Fabrication Methods for Composite Films/Coatings" Encyclopedia, https://encyclopedia.pub/entry/47530 (accessed March 08, 2026).
Kumar, A., Yadav, S., Pramanik, J., Sivamaruthi, B.S., Jayeoye, T.J., Prajapati, B.G., & Chaiyasut, C. (2023, August 02). Fabrication Methods for Composite Films/Coatings. In Encyclopedia. https://encyclopedia.pub/entry/47530
Kumar, Akash, et al. "Fabrication Methods for Composite Films/Coatings." Encyclopedia. Web. 02 August, 2023.
Copy Citation
Chitin is made by joining N-acetyl glucosamine residues through β (1–4) glycosidic linkages and is the second-most prevalent natural polysaccharide after cellulose. Chitosan and its derivatives exhibit anti-hypoxic, adaptogenic, immunostimulant, antiviral, antibacterial, radioprotective, and hemostatic properties. In addition, they are nontoxic, biocompatible, and biodegradable.
fabrication
chitosan
nanocomposites
antimicrobial
polysaccharide
1. Introduction
Chitin is made by joining N-acetyl glucosamine residues through β (1–4) glycosidic linkages and is the second-most prevalent natural polysaccharide after cellulose [1][2]. Chitin (Figure 1) is a naturally occurring structured crystalline microfibril in arthropods’ exoskeletons, fungi, and yeast cell walls. The main commercial sources of α-chitin are crabs and prawn shells [3]. Squid comprises the β-form chitin, which is highly sensitive to deacetylation and has stronger reactivity and solubility, as well as more affinity to solvents and better swelling capacity than α-chitin [4]. The γ-form of chitin is generally found in fungi and yeast [5].
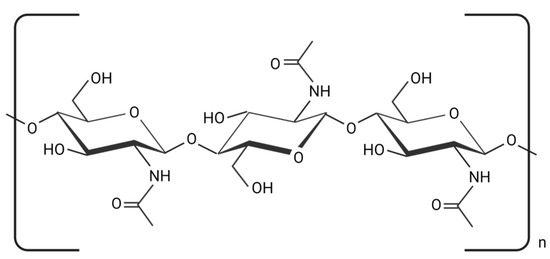
Figure 1. The chemical structure of chitin (created using BioRender.com on 4 July 2023).
N-acetylglucosamine and glucosamine residues are copolymerized to form chitosan. It can be produced by deacetylating chitin using strong alkalis, which hydrolyzes the acetamide groups. Several chemical processes are involved in converting chitin into chitosan (CH). Demineralization, deproteinization, and deacetylation are the main steps in extracting chitosan (Figure 2).
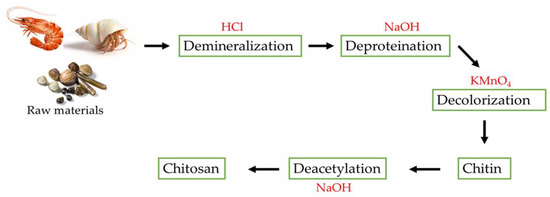
Figure 2. The steps in the synthesis of chitosan.
Chitosan and its derivatives exhibit anti-hypoxic, adaptogenic, immunostimulant, antiviral, antibacterial, radioprotective, and hemostatic properties. In addition, they are nontoxic, biocompatible, and biodegradable [6][7]. Since chitosan sulfate possesses anticoagulant properties, it could be used to produce drugs that have an anticoagulant effect [8][9]. Further, sulfated chitosan acts as an antioxidant [10]. Chitosan enhances the amount of insulin and is a potential way to manage diabetes [11][12]. Chitosan in immunotherapy is recommended as a potential anticancer drug that inhibits the development of infections and tumor cells while promoting humoral and cellular immunity [13][14]. Chitosan-based materials are used to treat wounds and promote the growth of granulation tissue and fibroblasts [15].
Chitosan products may create transparent films to enhance foods’ quality and shelf life. They are extremely dense and have antibacterial properties due to active amino groups [16][17]. Chitosan may be utilized to make edible films that minimize water loss and delay ripening due to its ability to build a durable, flexible, and partially permeable film. These qualities make chitosan unique from other edible coatings [18][19]. Apart from the above-stated advantages, chitosan-based films have disadvantages, including poor UV-light barrier features and mechanical attributes. Chitosan films’ hydrophilicity renders them incredibly vulnerable to moisture. Therefore, different kinds of natural substances, such as phenolic compounds, essential oils, plant extracts, or other biopolymers, can be added to improve the mechanical, physical, and biological properties of chitosan-based films [20]. CH-based materials are employed in various industries (Figure 3), including food, medicine, agriculture, and cosmetics [21][22].
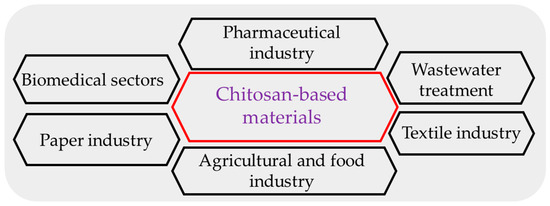
Figure 3. The application of chitosan in different sectors.
2. Fabrication Methods for Composite Films/Coatings
2.1. Solution Casting
This process is a commonly utilized method to produce chitosan coatings and films. First, it is dissolved in a solution, then the mixture is evaporated [23]. The process is affordable and easy, and the polymer structure is produced due to intermolecular electrostatic and hydrogen bonding [24]. However, the film becomes brittle due to this intermolecular entanglement. To enhance the mechanical characteristics of the films, several plasticizers (such as sugars, sorbitol, and glycerol) are added [25]. It is quite challenging to use this method on a commercial scale, and for this, there is still a need for further improvement. There are various steps in the fabrication process: Chitosan is first dissolved in an acid solution (pH less than 6.0), then it is blended, mixed, or crosslinked with other biopolymers, fillers, and functional materials at a different proportion, then the mixture is stirred to obtain a homogeneous viscous solution, then it is filtered, sonicated, or centrifuged to remove any air bubbles and insoluble particles. After this, the solution is cast or poured onto the surface for drying. After complete drying, the film is peeled off. Films are often created using the solution-casting process on a laboratory scale; however, further study is required to determine if it would be feasible to utilize solution-casting at a large scale [26][27]. The general method to fabricate the film from the solution casting method is shown in Figure 4.
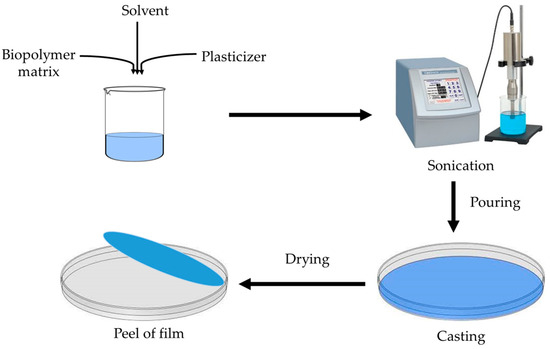
Figure 4. The solution-casting method of film formation.
The cast films of the chitosan blend and fucose-rich exopolysaccharide showed excellent characteristics such as biodegradability, antibacterial activity, and gas barrier [28]. As a result, the film may be used as a packaging material that extends the shelf life of foods.
2.2. Layer-by-Layer Assembly
Due to its versatility and ability to integrate the functional features of various polymers, layer-by-layer assembly has been widely studied in producing nanocomposite films for effectively managing material properties and functionality. It is a technique for making multi-layered films that do not require any complicated equipment. In layer-by-layer assembly, surface modification primarily depends on the mutual attraction and deposition of alternating polyelectrolytes [29][30]. Artificial polymers, polysaccharides, proteins, and other biopolymers that carry net charge can be considered polyelectrolytes. Deposition can be accomplished by spraying solutions onto the substrate or submerging them in different polyelectrolyte solutions. Both approaches have the potential for scalability. Layer-by-layer deposition may create active packaging films and coatings by incorporating the active agents [31][32]. The general method to fabricate the coating on food materials through the layer-by-layer method is shown in Figure 5. It has been claimed that the layer-by-layer method, combined with other techniques, successfully preserves food quality and extends shelf life [33].
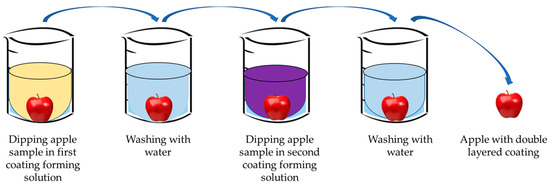
Figure 5. Layer-by-layer method of coating foods.
2.3. Extrusion
Most commercial plastic packaging films are made via extrusion techniques. Due to fast fabrication time and less energy-intensive nature, extrusion is frequently chosen over solution casting techniques [34][35]. The extrusion process includes several steps, such as preparing and mixing raw materials. Blending the mixture in an extruder for palettization, cutting the extrudates into pellets via a pelletizer, then drying the pellets and extruding them into the sheets via another extruder. Finally, film extruders blow the mixed resins into a film (Figure 6).
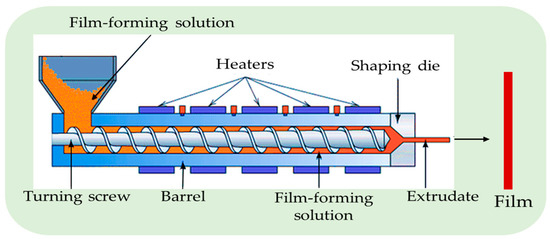
Figure 6. The extrusion method for the preparation of the film (adapted and recreated from Alsadi et al. [36]).
The films manufactured through extrusion have adequate mechanical and thermal characteristics. It is a potential method for film manufacture, but more research must be conducted to form chitosan-based films via this technique [37]. The extrusion method produces an antimicrobial film from a mixture of chitosan, starch, and poly (lactic acid) to preserve food with high water activity [38].
2.4. Coatings (Spraying, Dipping, or Spreading)
The coating can be applied to the skin to extend the shelf life of fresh items like fruits, vegetables, fish, meat, etc. [39]. The most common methods of food coating are spraying, dipping, or spreading (Figure 7). With a few instruments, such as a brush or spatula, spread coating is accomplished. The coating is a useful method to prevent microbial growth, enhance the shelf life, and maintain quality keeping [40].
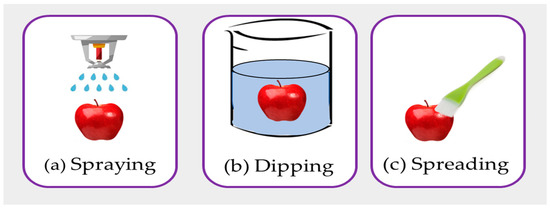
Figure 7. The different methods of coating such as (a) spraying, (b) dipping, and (c) spreading.
The coating procedure entails several steps:
-
Preparation of raw materials through mixing the right proportions of chitosan and fillers.
-
Preparation of coating samples through various methods such as irradiating, heating, mixing, and steam flash pasteurizing.
-
Sanitizing of the food samples through sodium hypochlorite.
-
Application of the chitosan-based composite solutions to food through the sterile spreader.
-
Drying under specific circumstances.
-
Packaging and storage in suitable conditions [41].
Antimicrobial components may migrate from the films to the food to increase the shelf life of food [42]. In an active packaging system, the coating is formed by dipping or spraying methods. In dipping methods, food is dipped into a previously made acidic chitosan solution that also contains preservatives and plasticizers to enhance the efficiency of the coating or film [43]. The coating process typically involves dipping the food for a short period in the composite solutions, draining the excess solution, and then drying the samples. Food products have been coated with one or more layers using various dipping techniques. However, double-dipping was preferred over three or more dipping in certain circumstances. This method is easy and affordable because there is no need for complicated equipment, and it gives high preservation efficiency. Similar procedures are followed for spray coating, except spraying is conducted with a sprayer supported by compressed air [44]. While dipping and spraying processes are easy, economical, and often used in most food manufacturing lines, these methods can hinder the food’s sensory qualities [45]. Therefore, it is strongly advised to employ this approach carefully to apply chitosan-based biopolymer coatings or films. These methods are used for coating fruits and vegetables to improve their shelf life [46].
2.5. Crosslinking
Two polymers are linked together via a crosslink developed by a chemical process, covalent or ionic bonds, or weaker bonding interactions [47] (Figure 8). The component polymers in the crosslinked composite may exhibit new characteristics when combined while retaining their unique characteristics. An interpenetrating polymer network (IPN) is one sort of crosslink that has been extensively studied. Among other chemical substances, cross-linkers include polymers [48], oxides [49], metals [50], and amino acids [51]. Various polymers may successfully create an IPN with the chitin-based material, including polymers made of epoxides, alcohols, and carboxylic acids. However, some of these polymers may affect membrane development, making their usage undesirable [52]. Crosslinking techniques can be used to manufacture food packaging films. The food packaging film was developed using a mixture of chitosan and gelatin with genipin as a crosslinked filler and a hybridization of quercetin and rosemary essential oil. The film has excellent UV protective, antioxidant, and antibacterial properties. Thus, the developed film can be employed as active food packaging materials [53].
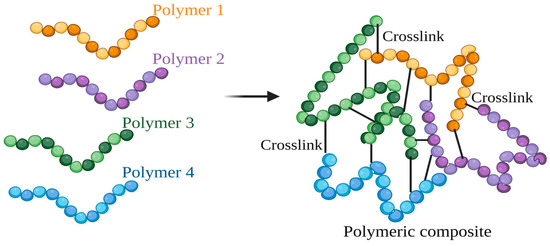
Figure 8. The schematic of the crosslinking for developing polymeric composite (created using BioRender.com on 4 July 2023).
2.6. Electrospinning
A spinneret, a collector (a grounded wire), and a high-voltage power source comprise a simple electrospinning system (Figure 9). Spinneret (which contains polymer solution) has a blunt-tip needle and a syringe pump that regulates the flow of the polymer solution [54]. To control the structure of electrospun nanofibers (coaxial setup for core-sheath and hollow nanofibers [55]) or to increase the throughput of electrospun nanofibers (multi-needle electrospinning setup [56]), numerous advanced electrospinning setups have been developed [57]. Typically, the electrospinning procedure may be broken down into four steps:
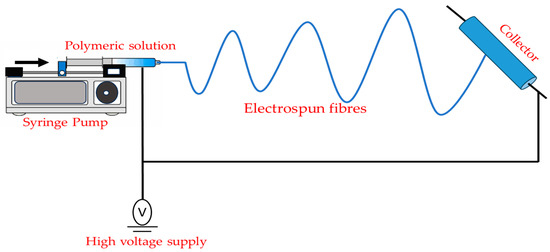
Figure 9. Schematic representation of the fundamental electrospinning arrangement.
- (1)
-
Formation of the cone-shaped jet from a pendant droplet by charging it in an electric field.
- (2)
-
Lengthening of the charged jet.
- (3)
-
Stretching and thinning of the charged jet under the electric field causes bending instability.
- (4)
-
Solidification and collection of the jet as solid fibers on a grounded collector [54].
The development of chitosan-based fibers for wound healing will largely depend on incorporating suitable copolymers. However, electrospinning still has technical difficulties producing chitosan-based fibers [58].
This section summarizes the various fabrication methods used (such as solution-casting, layer-by-layer, extrusion, coating, crosslinking, and electrospinning) to create chitosan-based materials that may be used in the biomedical and food industries. Fabrication methods can be used to develop a chitosan-based composite to enhance the mechanical, UV-shielding, antibacterial, and antioxidant properties. As a result, chitosan composites are suggested as potential materials to be utilized in the biomedical and food sectors.
References
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98.
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073.
- Younes, I.; Rinaudo, M.; Harding, D.; Sashiwa, H. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174.
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367.
- Sagheer, F.A.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Marine Sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419.
- Mohite, P.; Shah, S.R.; Singh, S.; Rajput, T.; Munde, S.; Ade, N.; Prajapati, B.G.; Paliwal, H.; Mori, D.D.; Dudhrejiya, A.V. Chitosan and chito-oligosaccharide: A versatile biopolymer with endless grafting possibilities for multifarious applications. Front. Bioeng. Biotechnol. 2023, 11, 1190879.
- Fatullayeva, S.; Tagiyev, D.; Zeynalov, N.; Mammadova, S.; Aliyeva, E. Recent Advances of Chitosan-Based Polymers in Biomedical Applications and Environmental Protection. J. Polym. Res. 2022, 29, 259.
- Dimassi, S.; Tabary, N.; Chai, F.; Blanchemain, N.; Martel, B. Sulfonated and Sulfated Chitosan Derivatives for Biomedical Applications: A Review. Carbohydr. Polym. 2018, 202, 382–396.
- Imran, M.; Sajwan, M.; Alsuwayt, B.; Asif, M. Synthesis, Characterization and Anticoagulant Activity of Chitosan Derivatives. Saudi Pharm. J. 2020, 28, 25–32.
- Charernsriwilaiwat, N.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. In Vitro Antioxidant Activity of Chitosan Aqueous Solution: Effect of Salt Form. Trop. J. Pharm. Res. 2012, 11, 235–242.
- Tzeng, H.P.; Liu, S.H.; Chiang, M.T. Antidiabetic Properties of Chitosan and Its Derivatives. Mar. Drugs 2022, 20, 784.
- Priyanka, D.N.; Prashanth, K.V.H.; Tharanathan, R.N. A Review on Potential Anti-Diabetic Mechanisms of Chitosan and Its Derivatives. Carbohydr. Polym. Technol. Appl. 2022, 3, 100188.
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 795–816.
- Ding, J.; Guo, Y. Recent Advances in Chitosan and Its Derivatives in Cancer Treatment. Front. Pharmacol. 2022, 13, 888740.
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598.
- Nguyen, T.T.; Thi Dao, U.T.; Thi Bui, Q.P.; Bach, G.L.; Ha Thuc, C.N.; Ha Thuc, H. Enhanced Antimicrobial Activities and Physiochemical Properties of Edible Film Based on Chitosan Incorporated with Sonneratia caseolaris (L.) Engl. Leaf Extract. Prog. Org. Coat. 2020, 140, 105487.
- Kumar, A.; Hasan, M.; Mangaraj, S.; Pravitha, M.; Verma, D.K.; Srivastav, P.P. Trends in Edible Packaging Films and Its Prospective Future in Food: A Review. Appl. Food Res. 2022, 2, 100118.
- Tokatlı, K.; Demirdöven, A. Effects of Chitosan Edible Film Coatings on the Physicochemical and Microbiological Qualities of Sweet Cherry (Prunus avium L.). Sci. Hortic. 2020, 259, 108656.
- Hu, W.; Sarengaowa; Feng, K. Effect of Edible Coating on the Quality and Antioxidant Enzymatic Activity of Postharvest Sweet Cherry (Prunus avium L.) during Storage. Coatings 2022, 12, 581.
- Bigi, F.; Haghighi, H.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of Chitosan-Hydroxypropyl Methylcellulose Blend Films Enriched with Nettle or Sage Leaf Extract for Active Food Packaging Applications. Food Hydrocoll. 2021, 120, 106979.
- Prajapati, B. Chitosan A Marine Medical Polymer and Its Lipid Lowering Capacity. Internet J. Health 2008, 9, 1–6.
- Sun, J.; Li, Y.; Cao, X.; Yao, F.; Shi, L.; Liu, Y. A Film of Chitosan Blended with Ginseng Residue Polysaccharides as an Antioxidant Packaging for Prolonging the Shelf Life of Fresh-Cut Melon. Coatings 2022, 12, 468.
- El-Hefian, E.A.; Nasef, M.M.; Yahaya, A.H. Preparation and Characterization of Chitosan/Polyvinyl Alcohol Blends—A Rheological Study. J. Chem. 2010, 7, S349–S357.
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209.
- Becerra, J.; Sudre, G.; Royaud, I.; Montserret, R.; Verrier, B.; Rochas, C.; Delair, T.; David, L. Tuning the Hydrophilic/Hydrophobic Balance to Control the Structure of Chitosan Films and Their Protein Release Behavior. AAPS PharmSciTech 2017, 18, 1070–1083.
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Kumar, S. Lagerstroemia Speciosa Fruit-Mediated Synthesis of Silver Nanoparticles and Its Application as Filler in Agar Based Nanocomposite Films for Antimicrobial Food Packaging. Food Packag. Shelf Life 2018, 17, 99–106.
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-Biofilm Activity and Food Packaging Application of Room Temperature Solution Process Based Polyethylene Glycol Capped Ag-ZnO-Graphene Nanocomposite. Mater. Sci. Eng. C 2018, 91, 743–753.
- Wu, Y.; Luo, X.; Li, W.; Song, R.; Li, J.; Li, Y.; Li, B.; Liu, S. Green and biodegradable composite films with novel antimicrobial performance based on cellulose. Food Chem. 2016, 197, 250–256.
- Tu, H.; Wu, G.; Yi, Y.; Huang, M.; Liu, R.; Shi, X.; Deng, H. Layer-by-Layer Immobilization of Amphoteric Carboxymethyl Chitosan onto Biocompatible Silk Fibroin Nanofibrous Mats. Carbohydr. Polym. 2019, 210, 9–16.
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of Chitosan-TiO2 Composite Film with Efficient Antimicrobial Activities under Visible Light for Food Packaging Applications. Carbohydr. Polym. 2017, 169, 101–107.
- Guzmán, E.; Mateos-Maroto, A.; Ruano, M.; Ortega, F.; Rubio, R.G. Layer-by-Layer Polyelectrolyte Assemblies for Encapsulation and Release of Active Compounds. Adv. Colloid Interface Sci. 2017, 249, 290–307.
- Rouster, P.; Dondelinger, M.; Galleni, M.; Nysten, B.; Jonas, A.M.; Glinel, K. Layer-by-Layer Assembly of Enzyme-Loaded Halloysite Nanotubes for the Fabrication of Highly Active Coatings. Colloids Surf. B 2019, 178, 508–514.
- Tzeng, P.; Stevens, B.; Devlaming, I.; Grunlan, J.C. Polymer–Graphene Oxide Quadlayer Thin-Film Assemblies with Improved Gas Barrier. Langmuir 2015, 31, 5919–5927.
- Estevez-Areco, S.; Guz, L.; Famá, L.; Candal, R.; Goyanes, S. Bioactive Starch Nanocomposite Films with Antioxidant Activity and Enhanced Mechanical Properties Obtained by Extrusion Followed by Thermo-Compression. Food Hydrocoll. 2019, 96, 518–528.
- Wang, W.; Zhang, H.; Jia, R.; Dai, Y.; Dong, H.; Hou, H.; Guo, Q. High Performance Extrusion Blown Starch/Polyvinyl Alcohol/Clay Nanocomposite Films. Food Hydrocoll. 2018, 79, 534–543.
- Alsadi, J.; Obaidat, M.T.; Ismail, R.; Trrad, I.; Aljamal, M.; Dahim, M.; Abuaddous, M.; Khodier, M.; Hatamleh, R.; Al-Mattarneh, H. Box–Behnken Design for Polycarbonate-Pigment Blending: Applications and Characterization Techniques. Polymers 2022, 14, 4860.
- Matet, M.; Heuzey, M.C.; Ajji, A.; Sarazin, P. Plasticized Chitosan/Polyolefin Films Produced by Extrusion. Carbohydr. Polym. 2015, 117, 177–184.
- Bie, P.; Liu, P.; Yu, L.; Li, X.; Chen, L.; Xie, F. The properties of antimicrobial films derived from poly(lactic acid)/starch/chitosan blended matrix. Carbohydr. Polym. 2013, 98, 959–966.
- Vargas, M.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Edible Chitosan Coatings for Fresh and Minimally Processed Foods. In Emerging Food Packaging Technologies, 1st ed.; Yam, K.L., Lee, D.S., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 66–95.
- Yu, D.; Jiang, Q.; Xu, Y.; Xia, W. The Shelf Life Extension of Refrigerated Grass Carp (Ctenopharyngodon idellus) Fillets by Chitosan Coating Combined with Glycerol Monolaurate. Int. J. Biol. Macromol. 2017, 101, 448–454.
- Pushkala, R.; Raghuram, P.K.; Srividya, N. Chitosan Based Powder Coating Technique to Enhance Phytochemicals and Shelf Life Quality of Radish Shreds. Postharvest Biol. Technol. 2013, 86, 402–408.
- Kashiri, M.; Cerisuelo, J.P.; Domínguez, I.; López-Carballo, G.; Muriel-Gallet, V.; Gavara, R.; Hernández-Muñoz, P. Zein Films and Coatings as Carriers and Release Systems of Zataria Multiflora Boiss. Essential Oil for Antimicrobial Food Packaging. Food Hydrocoll. 2017, 70, 260–268.
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of Fungal Chitosan Incorporated with Pomegranate Peel Extract as Edible Coating for Microbiological, Chemical and Sensorial Quality Enhancement of Nile Tilapia Fillets. Int. J. Biol. Macromol. 2017, 99, 499–505.
- Meng, X.H.; Qin, G.Z.; Tian, S.P. Influences of Preharvest Spraying Cryptococcus laurentii Combined with Postharvest Chitosan Coating on Postharvest Diseases and Quality of Table Grapes in Storage. LWT-Food Sci. Technol. 2010, 43, 596–601.
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368.
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan-based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Li, B.; Shan, C.L.; Zhou, Q.; Fang, Y.; Wang, Y.L.; Xu, F.; Han, L.R.; Ibrahim, M.; Guo, L.B.; Xie, G.L.; et al. Synthesis, characterization, and antibacterial activity of crosslinked chitosan-glutaraldehyde. Mar. Drugs 2013, 11, 1534–1552.
- Salehi, R.; Arami, M.; Mahmoodi, N.M.; Bahrami, H.; Khorramfar, S. Novel biocompatible composite (chitosan–zinc oxide nanoparticle): Preparation, characterization and dye adsorption properties. Colloids Surf. B Biointerfaces 2010, 80, 86–93.
- Vasconcelos, H.L.; Camargo, T.P.; Gonçalves, N.S.; Neves, A.; Laranjeira, M.C.; Fávere, V.T. Chitosan crosslinked with a metal complexing agent: Synthesis, characterization and copper (II) ions adsorption. React. Funct. Polym. 2008, 68, 572–579.
- Tsai, W.B.; Chen, Y.R.; Liu, H.L.; Lai, J.Y. Fabrication of UV-crosslinked chitosan scaffolds with conjugation of RGD peptides for bone tissue engineering. Carbohydr. Polym. 2011, 85, 129–137.
- Yeh, J.T.; Chen, C.L.; Huang, K.S. Synthesis and properties of chitosan/SiO2 hybrid materials. Mater. Lett. 2007, 61, 1292–1295.
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769.
- Xue, C.; Wilson, L.D. An Overview of the Design of Chitosan-Based Fiber Composite Materials. J. Compos. Sci. 2021, 5, 160.
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Ganan-Calvo, A.M. Micro/nano encapsulation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698.
- Ding, B.; Kimura, E.; Sato, T.; Fujita, S.; Shiratori, S. Fabrication of blend biodegradable nanofibrous nonwoven mats via multi-jet electrospinning. Polymer 2004, 45, 1895–1902.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415.
- Sapkota, S.; Chou, S.F. Electrospun Chitosan-based Fibers for Wound Healing Applications. J. Biomater. 2020, 4, 51–57.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
02 Aug 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





