1. Pain
Between 25% and 40% of older patients with cancer experience pain daily
[1]. Pain is associated with increased dependence on activities of daily living, risk of falls, malnutrition, limitation in social activity, and increased risk of depression
[2][3][4]. Since the etiology of pain in older adults with cancer is multifactorial, they benefit from a multidisciplinary team approach, including a geriatrician, a palliative care physician, a physical medicine and rehabilitation physician, physical therapists, and a psychiatrist or psychologist when appropriate
[2][3][4][5]. The evaluation of pain in older adults may be a challenge since some (with and without cognitive impairment) may have difficulty responding to numerical pain scales. Other verbal descriptor scales, pain thermometers, and facial pain scales may have greater validity in older populations
[2][4][6].
Treatment plans for older patients with cancer should incorporate non-pharmacological interventions such as massage, relaxation techniques, exercise, and rehabilitation
[3][7][8]. Acetaminophen should be considered as a first-line treatment for pain management due to its proven efficacy and good safety profile
[7]. Non-steroidal anti-inflammatory drugs should be administered at the lowest dose, for the shortest possible time, while monitoring for possible adverse effects (AEs)
[7]. Although older people are more sensitive to the analgesic properties of opioids, they are also at higher risk of toxicity, and require close monitoring for AEs and adequate laxative therapy
[7]. A good strategy is to use low-dose drug combinations (in which each analgesic acts by a different mechanism) to improve analgesic efficacy
[4][7]. Adjuvants are recommended in neuropathic pain and in patients with associated depression. Serotonin and norepinephrine reuptake inhibitors are a good option since they would have analgesic effects superior to those of traditional selective serotonin reuptake inhibitors
[9].
2. Anorexia/Cachexia
Cancer cachexia is a multifactorial syndrome defined by an ongoing loss of skeletal muscle mass that cannot be reversed by conventional nutritional support and leads to progressive functional impairment
[10]. It is associated with an increase in chemotherapy toxicity, increased mortality, and QoL impairment
[10]. The presence of anorexia/loss of appetite is associated with an increased risk of malnutrition, sarcopenia, care requirements, hospitalization, falls, and impaired cognition
[11]. Anorexia is difficult to control, with the first step being the identification and treatment of issues which may interfere with appetite, such as a dry mouth, pain, or nausea
[8].
No widely approved drug for the treatment of cancer cachexia is available. Neither glucocorticoids nor megestrol acetate improve cancer anorexia-cachexia syndrome beyond a few weeks, and may cause AEs, particularly among older adults
[12][13]. Promising drugs include ghrelin secretagogues, such as anamorelin, which significantly stimulate appetite in patients with cancer; however, in patients aged ≥65 years, the effects of these drugs are less significant, and their use does not lead to improved handgrip strength or decreased mortality and/or disability
[14][15].
In older patients with cancer, the best strategy to ameliorate cachexia can be a multimodal approach including exercise, nutritional support and, in some cases, medications. While these recommendations are part of guidelines issued by the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO), there is limited evidence regarding their use in older individuals
[16][17].
The single-arm NEXTAC-ONE trial examined a multimodal intervention (exercise and nutrition for eight weeks), in 30 patients aged ≥70 years with advanced lung or pancreatic cancer, demonstrating feasibility and low rate of adverse effects
[18]. The NEXTAC-TWO study is underway, which is a randomized clinical trial aimed at determining the efficacy of this intervention
[19]. In the ENeRgy clinical trial, 45 patients aged ≥65 years with advanced cancer were enrolled and randomized to a personalized program based on exercise and nutrition or standard care for eight weeks
[20]. The trial demonstrated feasibility, but was not powered to assess effects on nutritional, functional or QoL outcomes. Qualitative findings demonstrated the positive impact of the intervention, so the ENeRgise study is under development and aims to improve statistical power by recruiting a larger number of patients
[20].
3. Dyspnea
Dyspnea is a subjective experience of respiratory discomfort consisting of qualitatively distinct sensations that vary in intensity
[21]. Patients with reversible causes of dyspnea, such as pleural effusion, infection, exacerbation of pulmonary disease, pulmonary embolism, or treatment-induced pneumonitis, should receive targeted treatments
[22][23].
Systemic opioids should be offered when non-pharmacologic interventions fail to relieve dyspnea
[22][23][24]. Although there is a lack of information on the use of opioids as a treatment for dyspnea in older adults, data suggest that doses required are usually lower than those for treating pain. Since dyspnea is associated with increased anxiety, relaxation techniques and breathing retraining should be performed
[8]. If this is not enough, short-acting benzodiazepines can be offered in combination with opioids
[25], bearing in mind that these drugs increase the risk of falls, contributing to mortality and morbidity
[26]. Non-pharmacologic alternatives should always be considered, limiting opioid and benzodiazepine use to the lowest dose and shortest possible duration.
4. Delirium
Delirium is a fluctuating disturbance in attention and awareness that represents a decline from baseline status, accompanied by cognitive dysfunction
[8]. Delirium incidence ranges from 3% to 45% among inpatients with cancer, increasing to 59% to 88% near the end-of-life
[27]. The risk of delirium increases with age
[28][29], and its presence is associated with increased morbidity, mortality, length of stay, and need for long-term care
[30]. According to psychomotor characteristics and level of arousal, delirium is divided into three subtypes: hyperactive, hypoactive, and mixed type
[31]. The most common type of delirium in patients with advanced cancer requiring hospitalization due to poor symptom control is hypoactive delirium, which is the most difficult to diagnose and the hardest to treat, with a mortality of up to 81% during hospitalization (compared with 14% for hyperactive delirium)
[32]. The etiology of delirium is multifactorial, ranging from infections to medication-related AEs. In patients with cancer, the possibility of central nervous system metastases or AEs of cancer treatment, including immunotherapy, should always be considered
[32][33].
Delirium may interfere with the diagnosis and management of other symptoms, such as pain, which in turn may worsen delirium
[8]. It also interferes with the patient’s decision-making capacity, making shared decision-making significantly more complex
[34]. The main treatment consists of non-pharmacological interventions focused on prevention
[35][36]. However, when non-pharmacological interventions are insufficient, treatment with psychotropic drugs such as antipsychotics medications may be necessary
[33].
5. Nausea
The etiology of nausea in patients with cancer is not limited to AEs of treatment, and other causes such as opioid toxicity, bowel obstruction, or constipation must be excluded
[8]. Other causes of nausea may be particularly relevant in older adults, since data show they have a lower risk of chemotherapy-induced nausea and vomiting (CINV) than their younger counterparts
[37]. In addition, older adults are more likely to receive reduced chemotherapy dosing, which may also lead to reduced risk of CINV.
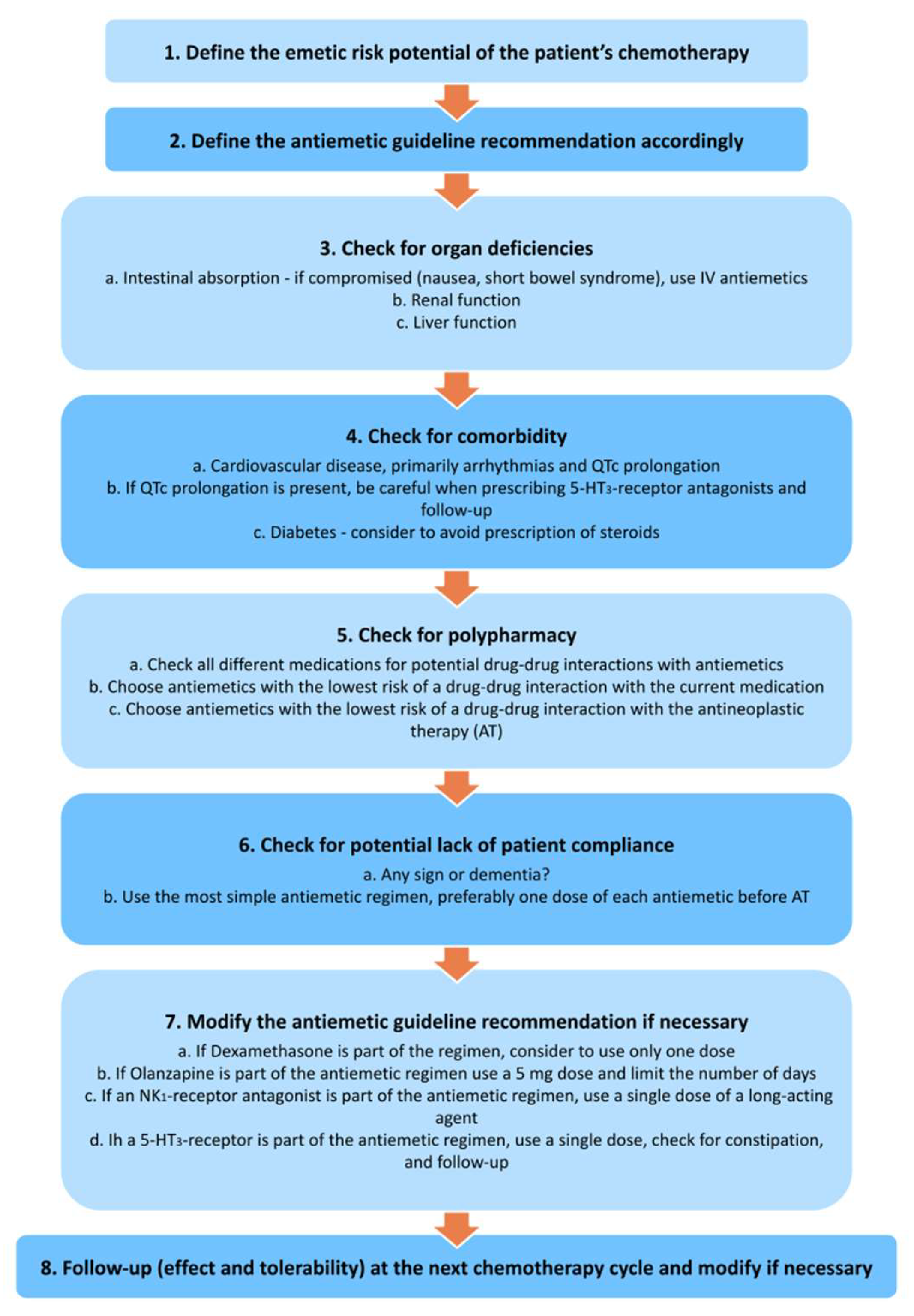
None of the current antiemetic guidelines include specific recommendations for older patients receiving chemotherapy, since there is little evidence for it’s use in older individuals
[38][39][40]. It seems reasonable to deprescribe or adjust the dosing of antiemetics in older adults with cancer, although this needs to be investigated in controlled trials. Single-dose dexamethasone, for example, has the same efficacy as multiple doses in older adults (excluding cisplatin-containing schedules)
[41][42].
Figure 1 illustrates recommendations for prescribing antiemetics in older patients with cancer
[37].
Figure 1. Recommendations for prescribing antiemetics in older patients with cancer
[36].