Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alan J. Stewart | -- | 2311 | 2023-07-25 11:45:54 | | | |
| 2 | Lindsay Dong | Meta information modification | 2311 | 2023-07-26 05:32:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium Deficiency and Cardiometabolic Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/47237 (accessed on 07 February 2026).
Fritzen R, Davies A, Veenhuizen M, Campbell M, Pitt SJ, Ajjan RA, et al. Magnesium Deficiency and Cardiometabolic Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/47237. Accessed February 07, 2026.
Fritzen, Remi, Amy Davies, Miriam Veenhuizen, Matthew Campbell, Samantha J. Pitt, Ramzi A. Ajjan, Alan J. Stewart. "Magnesium Deficiency and Cardiometabolic Disease" Encyclopedia, https://encyclopedia.pub/entry/47237 (accessed February 07, 2026).
Fritzen, R., Davies, A., Veenhuizen, M., Campbell, M., Pitt, S.J., Ajjan, R.A., & Stewart, A.J. (2023, July 25). Magnesium Deficiency and Cardiometabolic Disease. In Encyclopedia. https://encyclopedia.pub/entry/47237
Fritzen, Remi, et al. "Magnesium Deficiency and Cardiometabolic Disease." Encyclopedia. Web. 25 July, 2023.
Copy Citation
Magnesium (Mg2+) has many physiological functions within the body. These include important roles in maintaining cardiovascular functioning, where it contributes to the regulation of cardiac excitation–contraction coupling, endothelial functioning and haemostasis. The haemostatic roles of Mg2+ impact upon both the protein and cellular arms of coagulation.
cardiovascular disease
magnesium
metal ion dyshomeostasis
nutrient deficiency
1. Introduction
Magnesium is an essential nutrient required by all forms of life [1]. In mammalian cells, Mg2+ is an abundant cation present at concentrations ranging from 5 to 20 mmol/L [2]. In the plasma, the magnesium concentration is a little lower at around 1 mmol/L. Many different reference values for serum magnesium have been proposed (as reviewed in [3]), which collectively suggest that the concentration ranges somewhere between ~0.6 and ~1.2 mmol/L in healthy humans. The Canadian Health Measure Survey Cycle 3, conducted in 2012–2013, measured serum magnesium in subjects aged 3–79 years. They reported that 9.5% to 16.6% of adults and 15.8% to 21.8% of adolescents (12–19 years) had serum magnesium concentrations < 0.75 mmol/L [4], which is a level currently accepted as an indication of magnesium deficiency. However, it has recently been suggested that this indicative value is likely to be too low and should be raised to <0.85, as values in this range are associated with increased health risks [3][5].
Mg2+ has many physiological functions, such as maintaining DNA and RNA stability, as well as regulating cellular proliferation, bone metabolism, and neuromuscular functioning [6][7], regulating inflammation [8], and maintaining haemostasis (Figure 1). Mg2+ is a co-factor for many enzymes [9]. These include protein kinases which are commonly utilised to regulate gene transcription in response to extracellular stimuli [10]. Mg2+ is also required for the structure and functioning of DNA and RNA polymerases [11][12]. These polymerases are not only involved in nucleic acid synthesis, but some are also involved in DNA repair and genome maintenance. Virtually all enzymes taking part in mismatch repair, nucleotide repair, and base excision repair use Mg2+ as a co-factor. Given that defects in genome maintenance pathways are considered a hallmark of many cancers, magnesium deficiency might contribute to oncogenesis [1]. Moreover, magnesium deficiency has been shown to be associated with diverse pathologies including (pre)diabetes mellitus, platelet hyper-reactivity, pre-eclampsia, acute myocardial infarction and even some therapies [13][14].
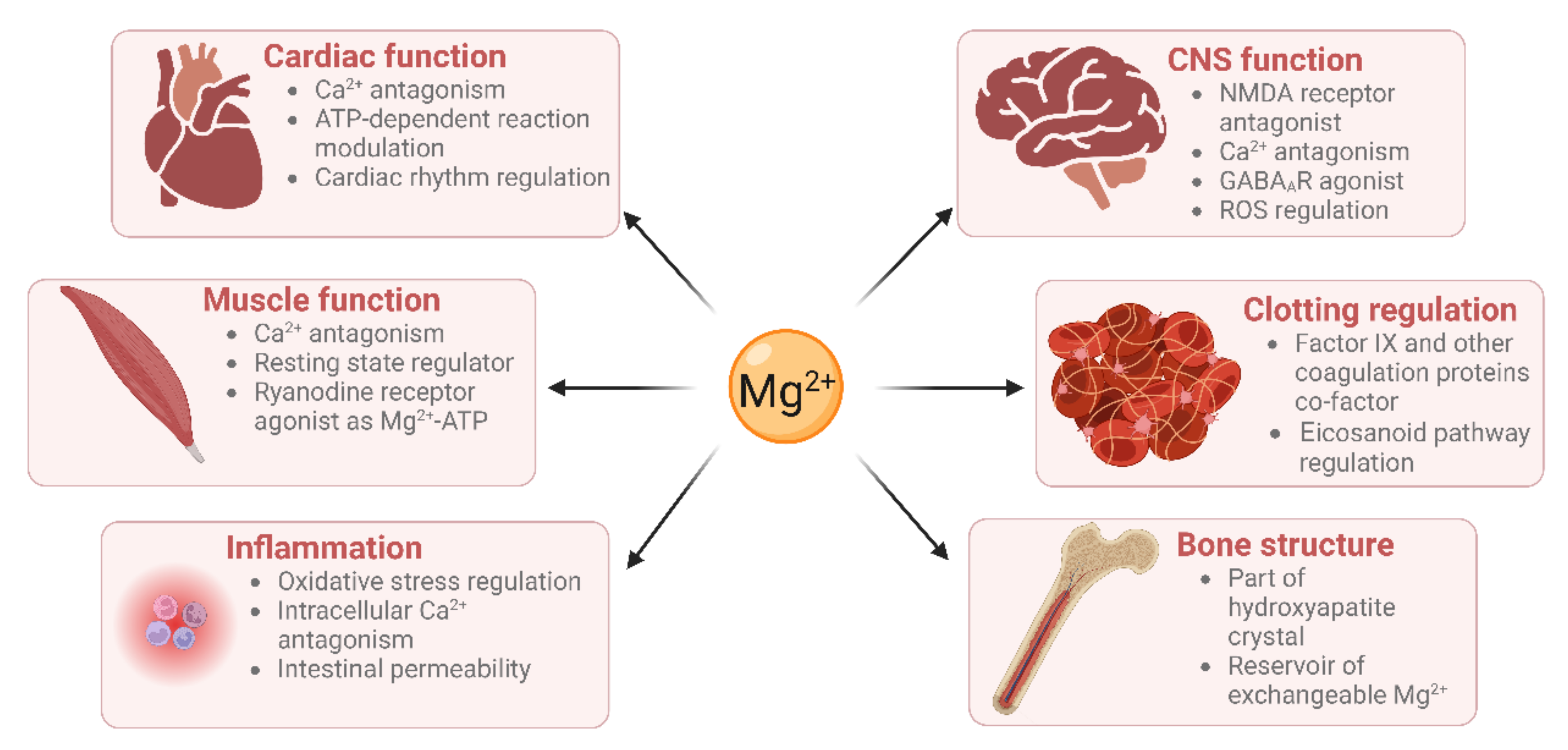
Figure 1. Magnesium has roles in many physiological processes (created using BioRender).
2. Magnesium Homeostasis
Magnesium homeostasis in the body largely depends on the collective actions of the intestine, skeleton and kidneys. The intestine is responsible for dietary uptake, the skeleton storage of ~50–60% of total Mg2+ in the form of hydroxyapatite, while the kidneys regulate its urinary excretion [1]. Magnesium can be found in all cells in the body [15][16], and it is particularly prevalent within mitochondria, the nucleus, and the endo/(sarco)-plasmic reticulum. The binding of Mg2+ by phospholipids, proteins, nucleic acids, chromatin, and nucleotides is thought to explain the presence of such high Mg2+ concentrations in these organelles [17].
Mg2+ absorption in the gut occurs via two separate pathways. Firstly, bulk absorption through the small intestine is thought to be regulated in a paracellular manner, since absorption correlates linearly with luminal Mg2+ concentrations [18][19]. Secondly, fine-tuning in the cecum and colon occurs transcellularly and involves the transient receptor potential (TRPM)-6 and 7 channels on the luminal enterocyte membrane for cell uptake [20] and the cyclin M4 transporter/exchanger on the basolateral membrane for Na+-dependent Mg2+ extrusion [21]. In contrast to other minerals, intestinal Mg2+ absorption is poorly regulated and depends mainly on intake [22][23]. Thus, overall Mg2+ maintenance and homeostasis are most likely regulated through excretion.
Sixty percent of the body’s total magnesium is stored in bones where it plays a structural role [24]. Two-thirds of this are stored within hydroxyapatite crystals. This portion is not readily available but is likely released following bone resorption [25]. Mg2+ binds at the surface of crystalline hydroxyapatite and aids in modulating crystal size and formation [26]. The quantity of magnesium present in the surface of the crystals is correlated with the plasma magnesium concentration, as demonstrated in studies with kidney disease patients [27]. This surface magnesium is a reservoir of readily exchangeable Mg2+ ions. Mg2+ deficiency affects the structure of bone, causing large hydroxyapatite crystals. It affects the cells involved in bone turnover, osteoblasts, and osteoclasts. Dietary magnesium intake has been linked to bone mineral density [28], and serum magnesium levels are strongly associated with an increased risk of fractures [28] and osteoporosis [29].
The excretion of Mg2+ is essentially regulated by filtration and reabsorption in the kidney [1]. Urinary Mg2+ excretion increases when magnesium intake is in excess, whereas the kidney conserves Mg2+ in the case of magnesium deprivation. Approximately one-tenth of total body magnesium is filtered by the kidney in a 24 h period [30]. A total of 10–15% of the filtered Mg2+ is reabsorbed in the proximal tubule by a passive process [31]. The majority of filtered Mg2+ (65%) is reabsorbed in the thick ascending loop of Henle [32], which is mediated by a paracellular mechanism dependent on the transepithelial potential generated by NaCl absorption. Thus, factors that impair NaCl reabsorption, such as diuretics and extracellular fluid volume expansion, increase Mg2+ excretion [33].
3. Magnesium Concentration Measurement and Supplementation
Magnesium deficiency/insufficiency can present a diagnostic challenge, as patients may have a “normal” serum magnesium concentration but have relatively low levels of skeletal or cellular magnesium [28]. An indicator of intracellular magnesium status is the measurement of magnesium retention after acute magnesium loading. This is also known as the magnesium retention test. A magnesium deficiency is indicated if a patient has <80% excretion (over 24 h) of an infused magnesium load (2.4 mg/kg of lean body weight given over the initial 4 h) [34][35]. Additional tests for magnesium deficiency involve measuring the magnesium/creatinine ratio in spot urine or in 24 h urine collections [28]. It is also possible to directly measure magnesium in the urine; this can be used to gain insight into kidney functioning and magnesium wasting. A 24 h urinary magnesium level of > 24 mg is indicative of magnesium wasting [36].
Several studies have linked magnesium intake with the presence of certain cardiometabolic conditions [37][38][39][40][41][42][43][44]. However, it is important to consider the bioavailability of magnesium when analysing food intake, as it can vary greatly depending on the overall composition of the food as well as the quantity of magnesium present [45]. Low magnesium intake is particularly concerning in Western countries. For instance, about 75% of the Spanish population declared a dietary magnesium intake of less than 80% the recommended level [46][47].
Magnesium deficiency is commonly associated with other conditions including diabetes, obesity, infection, and malnutrition, while some commonly used therapies, such as proton pump inhibitors, can also cause significant magnesium deficiency [48]. Different magnesium salts have been used via multiple administration routes to treat some of the conditions linked to magnesium deficiency. However, single studies comparing the effect of different salts are rare. A recent randomised controlled trial by Schutten and colleagues compared the effect of magnesium citrate, oxide, and sulfate on arterial stiffness, measured as carotid-to-femoral pulse wave velocity, in 164 slightly obese or overweight but otherwise healthy patients over a period of 24 weeks [49]. Compared to placebo, they did not observe any significant effect with all three magnesium salts on carotid-to-femoral pulse wave velocity or blood pressure at 24 weeks compared with placebo. However, a subgroup analysis showed an amelioration in people with a higher baseline value, although the low number of participants in this subgroup did not allow a firm conclusion related to the different salts administered to be drawn.
4. Magnesium Deficiency in Obesity and Diabetes
4.1. Type 1 Diabetes
T1DM is an autoimmune condition which leads to the destruction of β cells in the pancreas, resulting in a reduction in insulin production [50]. Exogenous insulin is required to treat people with T1DM to maintain normal serum glucose levels and magnesium deficiency (serum magnesium < 0.66 mmol/L has been reported in 4–38% of T1DM patients) [42]. When compared with age-matched controls, the mean plasma magnesium concentration was significantly lower in patients with T1DM [51]. The correlation between low magnesium and T1DM was particularly evident in female patients [51]. There is no evidence to suggest a direct mechanistic link between insulin and hypomagnesemia, although insulin might have an indirect role in the renal clearance of Mg2+.
Poorly controlled T1DM can lead to severe damage to the kidneys, eyes, and blood vessels [50]. Glycaemic control over the previous three months can be indicated by an HbA1c (glycated haemoglobin) test. HbA1c levels have been shown to negatively correlate with serum magnesium concentration in people with T1DM, suggesting that poor glycaemic control leads to hypomagnesaemia [50][51].
4.2. Type 2 Diabetes and Obesity
T2DM is a condition characterised by a combination of defective insulin secretion and increased resistance to insulin by peripheral tissues [52][53]. Once T2DM has developed, individuals require treatment to reduce their serum blood glucose levels, including lifestyle advice and medications [54]. T2DM can develop more slowly than T1DM and can progress through a pre-diabetic phase. Metabolic syndrome refers to a collective of conditions including hypertension, insulin resistance, central obesity and atherogenic dyslipidaemia is a risk factor for T2DM [55][56]. Lifestyle factors such as diet and exercise are associated with metabolic syndrome as well as genetic and other environmental factors [57][58][59].
It has been suggested that hypomagnesaemia is caused by diabetes rather than contributing to T2DM onset, which is based on the findings of a cohort study reporting hypomagnesaemia (<0.7 mmol/L) being more common in patients with T2DM but not pre-diabetes [60]. However, other cohort studies challenge this. Indeed, the 2015 dose–response meta-analysis of prospective cohort studies published by Fang and colleagues found an inverse correlation between magnesium intake and T2DM [61]. The number of pooled participants totalled about 26,300 cases of T2DM with follow-ups ranging from 4 to 30 years, and the dietary magnesium intake was self-reported using a validated food frequency questionnaire.
5. Cardiovascular Roles of Magnesium
5.1. Cardiac Muscle Contraction
Mg2+ is an integral regulator of muscle contraction. Muscle contraction is a Ca2+-dependent process. Mg2+ can compete with Ca2+ for the binding sites on proteins involved in contraction, including the type-2 ryanodine receptor (RyR2), troponin C and myosin [62]. Before contraction, Mg2+ occupies all binding sites available in the myocyte, as its cytoplasmic concentration is 10,000-times higher than that of Ca2+. During excitation–contraction coupling, Ca2+ enters the cell, and Mg2+ is displaced from RyR2, allowing the channel to open and to release Ca2+ from the sarcoplasmic reticulum. The release of Ca2+ from intracellular stores can displace Mg2+ from the myosin head and troponin C, enabling contraction of the muscle [1].
Secondly, the effects of Mg2+ on the myocardium are protective against ischaemia and arrhythmia. The anti-ischaemic effects are the results of several factors. As with its vasodilatory properties, it prevents Ca2+ overload by competing for the same binding sites. It lowers the heart rate and contractility as well as catecholamine-induced oxygen demand. Moreover, it modulates ATP-dependent reactions and acts as an antioxidant to prevent long-term damage to the myocardium [63][64].
5.2. Vascular Functioning
The vascular system is the collective of vessels in the body which carry blood to and from tissues. Large blood vessels consist of three layers of tissue supported by the extracellular matrix. These are the adventitia where innervation is found, the media where smooth muscle cells are located, and the intima, which is lined by the endothelium and is in contact with the blood [65]. Studies support the concept that Mg2+ is important for several aspects of vascular functioning.
Vasodilation and vasoconstriction refer to the widening and narrowing of blood vessels, respectively. These processes allow blood flow to be matched to tissue demands. Mg2+ has been seen to improve blood flow in various vascular beds by dilating blood vessels [66][67][68]. Evidence suggests that this occurs in part because Mg2+ antagonises the transport of Ca2+ into contractile smooth muscle cells [69][70]. Multiple mechanisms may be involved [71]. These include the direct binding of Mg2+ to ion channels to block their activity [72] as well as the binding of Mg2+ to the plasma membrane, changing the surface charge and subsequently the opening of voltage-gated calcium channels [71].
5.3. Haemostasis
Magnesium is involved in haemostasis as a co-factor for factor IX and membrane-bound coagulation proteins and as a regulator of the eicosanoid synthesis pathway, which produces inflammatory mediators including prostaglandins and thromboxane. Factor IX is part of the intrinsic pathway of the coagulation cascade, it activates factor X and is activated by activated factor VIII. The activation of factor IX is Ca2+-dependent [73]. Mutation of the factor IX gene is a hallmark of haemophilia B, a blood clotting disorder which is life threatening and shortens life expectancy [74]. Mg2+ has been shown to stabilise the native conformation of factor IX, and consequently to increase its activity [75].
Furthermore, during the initial stages of the coagulation process, when endothelial cell membranes are exposed to the blood stream, blood coagulation proteins reversibly interact with these membranes to trigger the coagulation cascade. Seven coagulation enzymes are bound to the cell surface through their γ-carboxyglutamate-rich (GLA) domains. GLA domain folding is dependent on both Ca2+ and Mg2+. The binding of these metal ions leads to the exposure of hydrophobic residues that ultimately help integration into the membrane bilayer.
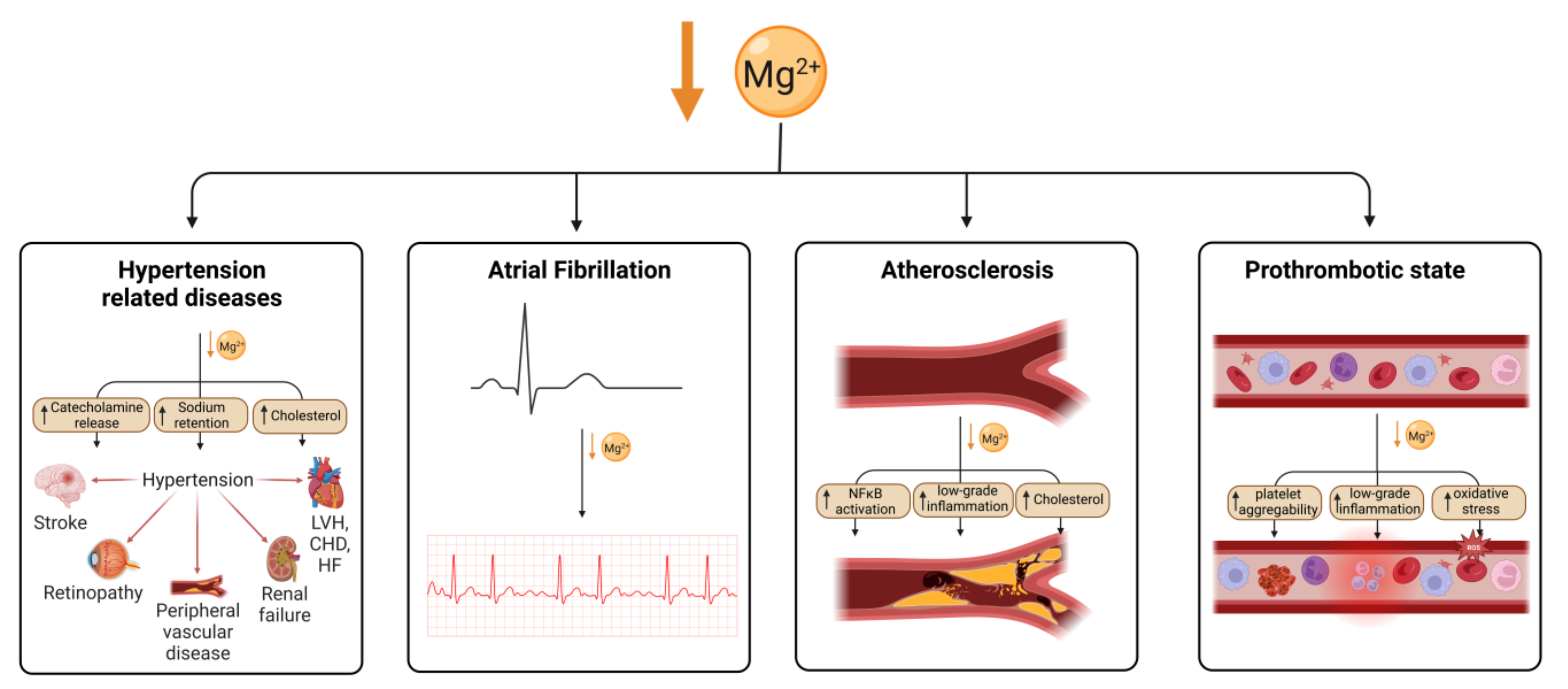
6. Effects of Magnesium Deficiency on the Cardiovascular System
Magnesium is an essential nutrient for cardiovascular health, acting to regulate vascular smooth muscle, cardiac conduction, vascular endothelial cell functioning, and thrombosis. Hypomagnesaemia and low dietary magnesium intake increase the likelihood of developing coronary artery disease (CAD) [76]. Hypomagnesaemia has been associated with hypertension, which can lead to congestive heart failure (CHF) or CAD (Figure 2). However, this could be confounded by diuretic medications to treat heart failure, which reduce serum magnesium levels in people with heart failure.

Figure 2. Magnesium deficiency leads to cardiovascular disease through multiple mechanisms. Mg deficiency leads to hypertension through an increase in catecholamine release, sodium retention and cholesterol, which is in turn a risk for several cardiovascular conditions. Arterial fibrillation has also been shown to be associated with Mg deficiency as well as atherosclerosis, which is thought to be caused by an increase in NFκB signalling, low-grade inflammation and cholesterol. Mg deficiency has also been linked to an increase in thrombotic risk. Created using BioRender.
References
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46.
- Rude, R. Magnesium disorders. In Fluids and Electrolytes; Kokko, J., Tannen, R., Eds.; W.B. Saunders Company: Philadelphia, PA, USA, 1996; pp. 421–445.
- Rosanoff, A.; West, C.; Elin, R.; Micke, O.; Baniasadi, S.; Barbagallo, M.; Campbell, E.; Cheng, F.C.; Costello, R.B.; Gamboa-Gomez, C.; et al. Recommendation on an updated standardization of serum magnesium reference ranges. Eur. J. Nutr. 2022, 61, 3697–3706.
- Bertinato, J.; Wang, K.C.; Hayward, S. Serum magnesium concentrations in the Canadian population and associations with diabetes, glycemic regulation, and insulin resistance. Nutrients 2017, 9, 296.
- Micke, O.; Vormann, J.; Kraus, A.; Kisters, K. Serum magnesium: Time for a standardized and evidence-based reference range. Magnes. Res. 2021, 34, 84–89.
- Maier, J.A.M.; Locatelli, L.; Fedele, G.; Cazzaniga, A.; Mazur, A. Magnesium and the brain: A focus on neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022, 24, 223.
- Kirkland, A.E.; Sarlo, G.L.; Holton, K.F. The role of magnesium in neurological disorders. Nutrients 2018, 10, 730.
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44.
- Andreini, C.; Bertini, I.; Cavallaro, G.; Holliday, G.L.; Thornton, J.M. Metal ions in biological catalysis: From enzyme databases to general principles. J. Biol. Inorg. Chem. 2008, 13, 1205–1218.
- Buelens, F.P.; Leonov, H.; de Groot, B.L.; Grubmüller, H. ATP-magnesium coordination: Protein structure-based force field evaluation and corrections. J. Chem. Theory Comput. 2021, 17, 1922–1930.
- Brautigam, C.A.; Steitz, T.A. Structural and functional insights provided by crystal structures of DNA polymerases and their substrate complexes. Curr. Opin. Struct. Biol. 1998, 8, 54–63.
- Suh, W.C.; Leirmo, S.; Record, M.T., Jr. Roles of Mg2+ in the mechanism of formation and dissociation of open complexes between Escherichia coli RNA polymerase and the lambda PR promoter: Kinetic evidence for a second open complex requiring Mg2+. Biochemistry 1992, 31, 7815–7825.
- Salehidoost, R.; Taghipour Boroujeni, G.; Feizi, A.; Aminorroaya, A.; Amini, M. Effect of oral magnesium supplement on cardiometabolic markers in people with prediabetes: A double blind randomized controlled clinical trial. Sci. Rep. 2022, 12, 18209.
- Al Alawi, A.M.; Majoni, S.W.; Falhammar, H. Magnesium and human health: Perspectives and research directions. Int. J. Endocrinol. 2018, 2018, 9041694.
- Romani, A.; Marfella, C.; Scarpa, A. Cell magnesium transport and homeostasis: Role of intracellular compartments. Miner. Electrolyte Metab. 1993, 19, 282–289.
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferré, S.; Maier, J.A. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64.
- Romani, A.M.P. Intracellular magnesium homeostasis. In Magnesium in the Central Nervous System; Vink, R., Nechifor, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011.
- Karbach, U.; Rummel, W. Cellular and paracellular magnesium transport across the terminal ileum of the rat and its interaction with the calcium transport. Gastroenterology 1990, 98, 985–992.
- Quamme, G.A. Recent developments in intestinal magnesium absorption. Curr. Opin. Gastroenterol. 2008, 24, 230–235.
- Schlingmann, K.P.; Waldegger, S.; Konrad, M.; Chubanov, V.; Gudermann, T. TRPM6 and TRPM7—Gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 2007, 1772, 813–821.
- Yamazaki, D.; Funato, Y.; Miura, J.; Sato, S.; Toyosawa, S.; Furutani, K.; Kurachi, Y.; Omori, Y.; Furukawa, T.; Tsuda, T.; et al. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: A mouse model. PLoS Genet. 2013, 9, e1003983.
- Hardwick, L.L.; Jones, M.R.; Buddington, R.K.; Clemens, R.A.; Lee, D.B. Comparison of calcium and magnesium absorption: In vivo and in vitro studies. Am. J. Physiol. Gastrointest. Liver Physiol. 1990, 259, G720–G726.
- Schweigel, M.; Martens, H. Magnesium transport in the gastrointestinal tract. Front. Biosci. 2000, 5, D666–D677.
- Walser, M. Magnesium metabolism. Ergeb. Physiol. 1967, 59, 185–296.
- Alfrey, A.C.; Miller, N.L. Bone magnesium pools in uremia. J. Clin. Investig. 1973, 52, 3019–3027.
- Salimi, M.H.; Heughebaert, J.C.; Nancollas, G.H. Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1985, 1, 119–122.
- Cunningham, J.; Rodríguez, M.; Messa, P. Magnesium in chronic kidney disease Stages 3 and 4 and in dialysis patients. Clin. Kidney J. 2012, 5, i39–i51.
- Ismail, A.A.A.; Ismail, Y.; Ismail, A.A. Chronic magnesium deficiency and human disease; time for reappraisal? QJM 2018, 111, 759–763.
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A. Magnesium and osteoporosis: Current state of knowledge and future research directions. Nutrients 2013, 5, 3022–3033.
- Taal, M.W.; Brenner, B.M.; Rector, F.C. Brenner & Rector’s the Kidney, 9th ed.; Elsevier: Amsterdam, The Netherlands; Saunders: Philadelphia, PA, USA, 2012.
- Le Grimellec, C.; Roinel, N.; Morel, F. Simultaneous Mg, Ca, P, K, Na and Cl analysis in rat tubular fluid. II. During acute Mg plasma loading. Pflug. Arch. 1973, 340, 197–210.
- Brunette, M.G.; Vigneault, N.; Carriere, S. Micropuncture study of magnesium transport along the nephron in the young rat. Am. J. Physiol. 1974, 227, 891–896.
- Ryan, M.P.; Devane, J.; Ryan, M.F.; Counihan, T.B. Effects of diuretics on the renal handling of magnesium. Drugs 1984, 28, 167–181.
- Gullestad, L.; Midtvedt, K.; Dolva, L.O.; Norseth, J.; Kjekshus, J. The magnesium loading test: Reference values in healthy subjects. Scand. J. Clin. Lab. Investig. 1994, 54, 23–31.
- Holm, C.N.; Jepsen, J.M.; Sjøgaard, G.; Hessov, I. A magnesium load test in the diagnosis of magnesium deficiency. Hum. Nutr. Clin. Nutr. 1987, 41, 301–306.
- Rosner, M.H.; Ha, N.; Palmer, B.F.; Perazella, M.A. Acquired Disorders of Hypomagnesemia. Mayo Clin. Proc. 2023, 98, 581–596.
- Cheteu Wabo, T.M.; Wu, X.; Sun, C.; Boah, M.; Ngo Nkondjock, V.R.; Kosgey Cheruiyot, J.; Amporfro Adjei, D.; Shah, I. Association of dietary calcium, magnesium, sodium, and potassium intake and hypertension: A study on an 8-year dietary intake data from the National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2022, 16, 74–93.
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925.
- Ford, E.S.; Li, C.; McGuire, L.C.; Mokdad, A.H.; Liu, S. Intake of dietary magnesium and the prevalence of the metabolic syndrome among U.S. adults. Obesity 2007, 15, 1139–1146.
- Kesteloot, H.; Joossens, J.V. Relationship of dietary sodium, potassium, calcium, and magnesium with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension 1988, 12, 594–599.
- van Leer, E.M.; Seidell, J.C.; Kromhout, D. Dietary calcium, potassium, magnesium, and blood pressure in the Netherlands. Int. J. Epidemiol. 1995, 24, 1117–1123.
- He, K.; Liu, K.; Daviglus, M.L.; Morris, S.J.; Loria, C.M.; van Horn, L.; Jacobs, D.R., Jr.; Savage, P.J. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation 2006, 113, 1675–1682.
- Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayón-Orea, C.; Fresán, U.; Barbagallo, M.; Ruiz-Canela, M.; Martínez-González, M.A. Low dietary magnesium and overweight/obesity in a Mediterranean population: A detrimental synergy for the development of hypertension. The SUN project. Nutrients 2020, 13, 125.
- Jiao, Y.; Li, W.; Wang, L.; Jiang, H.; Wang, S.; Jia, X.; Wang, Z.; Wang, H.; Zhang, B.; Ding, G. Relationship between dietary magnesium intake and metabolic syndrome. Nutrients 2022, 14, 2013.
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136.
- Rude, R.K. Magnesium. In Modern Nutrition in Health and Disease, 11th ed.; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MA, USA, 2012; pp. 159–175.
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168.
- Epstein, M.; McGrath, S.; Law, F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N. Engl. J. Med. 2006, 355, 1834–1836.
- Schutten, J.C.; Joris, P.J.; Groendijk, I.; Eelderink, C.; Groothof, D.; van der Veen, Y.; Westerhuis, R.; Goorman, F.; Danel, R.M.; de Borst, M.H.; et al. Effects of magnesium citrate, magnesium oxide, and magnesium sulfate supplementation on arterial stiffness: A randomized, double-blind, placebo-controlled intervention trial. J. Am. Heart Assoc. 2022, 11, e021783.
- Rodrigues, A.K.; Melo, A.E.; Domingueti, C.P. Association between reduced serum levels of magnesium and the presence of poor glycemic control and complications in type 1 diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2020, 14, 127–134.
- Sobczak, A.I.S.; Stefanowicz, F.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Total plasma magnesium, zinc, copper and selenium concentrations in type-I and type-II diabetes. Biometals 2019, 32, 123–138.
- Markovits, N.; Loebstein, R.; Halkin, H.; Bialik, M.; Landes-Westerman, J.; Lomnicky, J.; Kurnik, D. The association of proton pump inhibitors and hypomagnesemia in the community setting. J. Clin. Pharmacol. 2014, 54, 889–895.
- Kietsiriroje, N.; Pearson, S.; Campbell, M.; Ariëns, R.A.S.; Ajjan, R.A. Double diabetes: A distinct high-risk group? Diabetes Obes. Metab. 2019, 21, 2609–2618.
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275.
- Shin, J.A.; Lee, J.H.; Lim, S.Y.; Ha, H.S.; Kwon, H.S.; Park, Y.M.; Lee, W.C.; Kang, M.I.; Yim, H.W.; Yoon, K.H.; et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J. Diabetes Investig. 2013, 4, 334–343.
- Iafusco, D.; Franceschi, R.; Maguolo, A.; Guercio Nuzio, S.; Crinò, A.; Delvecchio, M.; Iughetti, L.; Maffeis, C.; Calcaterra, V.; Manco, M. From metabolic syndrome to type 2 diabetes in youth. Children 2023, 10, 516.
- Balkhiyarova, Z.; Luciano, R.; Kaakinen, M.; Ulrich, A.; Shmeliov, A.; Bianchi, M.; Chioma, L.; Dallapiccola, B.; Prokopenko, I.; Manco, M. Relationship between glucose homeostasis and obesity in early life-a study of Italian children and adolescents. Hum. Mol. Genet. 2022, 31, 816–826.
- Carroll, S.; Dudfield, M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sport. Med. 2004, 34, 371–418.
- Gorodeski Baskin, R.; Alfakara, D. Root Cause for Metabolic Syndrome and Type 2 Diabetes: Can Lifestyle and Nutrition Be the Answer for Remission. Endocrinol. Metab. Clin. N. Am. 2023, 52, 13–25.
- Simmons, D.; Joshi, S.; Shaw, J. Hypomagnesaemia is associated with diabetes: Not pre-diabetes, obesity or the metabolic syndrome. Diabetes Res. Clin. Pract. 2010, 87, 261–266.
- Fang, X.; Wang, K.; Han, D.; He, X.; Wei, J.; Zhao, L.; Imam, M.U.; Ping, Z.; Li, Y.; Xu, Y.; et al. Dietary magnesium intake and the risk of cardiovascular disease, type 2 diabetes, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. BMC Med. 2016, 14, 210.
- Greising, S.M.; Gransee, H.M.; Mantilla, C.B.; Sieck, G.C. Systems biology of skeletal muscle: Fiber type as an organizing principle. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 457–473.
- Kolte, D.; Vijayaraghavan, K.; Khera, S.; Sica, D.A.; Frishman, W.H. Role of magnesium in cardiovascular diseases. Cardiol. Rev. 2014, 22, 182–192.
- Zdanowicz, M.M.; Barletta, M.A. Protective role of magnesium in catecholamine-induced arrhythmia and toxicity in vitro. Magnes. Res. 1991, 4, 153–162.
- Pugsley, M.K.; Tabrizchi, R. The vascular system. An overview of structure and function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340.
- Haenni, A.; Johansson, K.; Lind, L.; Lithell, H. Magnesium infusion improves endothelium-dependent vasodilation in the human forearm. Am. J. Hypertens. 2002, 15, 10–15.
- Landau, R.; Scott, J.A.; Smiley, R.M. Magnesium-induced vasodilation in the dorsal hand vein. BJOG 2004, 111, 446–451.
- Murata, T.; Dietrich, H.H.; Horiuchi, T.; Hongo, K.; Dacey, R.G., Jr. Mechanisms of magnesium-induced vasodilation in cerebral penetrating arterioles. Neurosci. Res. 2016, 107, 57–62.
- Yoshimura, M.; Oshima, T.; Matsuura, H.; Ishida, T.; Kambe, M.; Kajiyama, G. Extracellular Mg2+ inhibits capacitative Ca2+ entry in vascular smooth muscle cells. Circulation 1997, 95, 2567–2572.
- Gilbert, T.M.; Pashley, D.H.; Anderson, R.W. Response of pulpal blood flow to intra-arterial infusion of endothelin. J. Endod. 1992, 18, 228–231.
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cation channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Biochem. Biophys. 2007, 458, 73–89.
- Lansman, J.B.; Hess, P.; Tsien, R.W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J. Gen. Physiol. 1986, 88, 321–347.
- Lawson, J.H.; Mann, K.G. Cooperative activation of human factor IX by the human extrinsic pathway of blood coagulation. J. Biol. Chem. 1991, 266, 11317–11327.
- Sidonio, R.F., Jr.; Malec, L. Hemophilia B (factor IX deficiency). Hematol. Oncol. Clin. N. Am. 2021, 35, 1143–1155.
- Sekiya, F.; Yoshida, M.; Yamashita, T.; Morita, T. Magnesium(II) is a crucial constituent of the blood coagulation cascade. Potentiation of coagulant activities of factor IX by Mg2+ ions. J. Biol. Chem. 1996, 271, 8541–8544.
- Tangvoraphonkchai, K.; Davenport, A. Magnesium and cardiovascular disease. Adv. Chronic Kidney Dis. 2018, 25, 251–260.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
26 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




