Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manoj Kumar Tripathi | -- | 2438 | 2023-07-25 08:52:12 | | | |
| 2 | Conner Chen | Meta information modification | 2438 | 2023-07-27 05:06:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Paliwal, S.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Payasi, D.K.; Tiwari, P.N.; Singh, K.; Yadav, R.K.; Asati, R.; Chauhan, S. Abiotic Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/47207 (accessed on 07 February 2026).
Paliwal S, Tripathi MK, Tiwari S, Tripathi N, Payasi DK, Tiwari PN, et al. Abiotic Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/47207. Accessed February 07, 2026.
Paliwal, Shruti, Manoj Kumar Tripathi, Sushma Tiwari, Niraj Tripathi, Devendra K. Payasi, Prakash N. Tiwari, Kirti Singh, Rakesh Kumar Yadav, Ruchi Asati, Shailja Chauhan. "Abiotic Stress" Encyclopedia, https://encyclopedia.pub/entry/47207 (accessed February 07, 2026).
Paliwal, S., Tripathi, M.K., Tiwari, S., Tripathi, N., Payasi, D.K., Tiwari, P.N., Singh, K., Yadav, R.K., Asati, R., & Chauhan, S. (2023, July 25). Abiotic Stress. In Encyclopedia. https://encyclopedia.pub/entry/47207
Paliwal, Shruti, et al. "Abiotic Stress." Encyclopedia. Web. 25 July, 2023.
Copy Citation
To achieve agricultural sustainability, it is critical to develop and nurture products that are tolerant to rising abiotic pressures caused by climate variability. Numerous abiotic and biotic stressors that constantly threaten plants have an impact on their outputs. Through intricate endogenous signalling networks and numerous modifications, the plant reacts to these stressors. Under these environmental circumstances, the plant’s output and reproductive success are determined by the interactions between these networks. Linseed, like other crops, if subjected to a variety of abiotic stresses, might have reduce yields.
Linum usitatissimum L.
biotic and abiotic stresses
integrated breeding
1. Abiotic Stress
To achieve agricultural sustainability, it is critical to develop and nurture products that are tolerant to rising abiotic pressures caused by climate variability [1]. Numerous abiotic and biotic stressors that constantly threaten plants have an impact on their outputs. Through intricate endogenous signalling networks and numerous modifications, the plant reacts to these stressors. Under these environmental circumstances, the plant’s output and reproductive success are determined by the interactions between these networks. Linseed, like other crops, if subjected to a variety of abiotic stresses, might have reduce yields [2]. The different abiotic stresses influencing linseed are summarised in the following subsections.
2. Drought Tolerance
Drought is considered one ofthe most pervasive and harmful abiotic factors that impact agricultural output. Drought, on average, affects crop growth, production, and quality by more than 50% [3][4][5][6]. By 2050, nearly fifty percent of land that is usable for agriculture is anticipated to have disastrous effects on plant development [7]. Soil moisture deficiency can have a substantial impact on the flax output capacity, oil quantity and fatty acid arrangement, and fibre quality indicators [8][9][10].
According to Hu and Xiong [11], drought causes normal metabolism to be disrupted by decreased leaf growth, oxidative damage, increased membrane lipid peroxidation, leaf senescence, and abscission. Flax can tolerate drought conditions more effectively than numerous other oil and food crops due to its hardiness [12]; however, flax plants lose a lot of water due to their high transpiration coefficients, which range from 787 to 1093 [13]. To generate optimal yields, fibre flax requires at least 600–650 mm of yearly precipitation, with at least 110–150 mm falling during the vegetative season [14][15]. Since drought is an unpredictable and irregular environmental event, genotypes with high yield potential and drought tolerance may be selected [16].
To overcome these limitations and enhance flax yields, research activities based on conventional breeding and later transgenic approaches were initiated [17]. Understanding the adaptation processes and identifying the underlying genes, markers, and QTLs might help to improve the genetics and production of linseed in semi-arid and arid areas because drought tolerance is a complicated polygenic trait [1]. A limited number of studies have been published identifying the accessions of flax that are resistant to drought [18][19][20] and genome-wide analyses of drought-induced gene expression [15]. Linseed has a shallow root system as compared to other oilseed crops, such as safflower, rapeseed, and sunflower. Therefore, understanding the root system architecture is crucial for improving flax’s ability to absorb water. Many crops, including rice, wheat, and maize, have recently demonstrated the significance of specific root characteristics for efficient water and nutrient absorption under water-stress conditions [21][22][23][24], but knowledge is still somewhat limited in flax. Among different morpho-physiological characteristics, plant height, biomass, seed colour, lignin content, seed yield, leaf absolute water content, and leaf relative water content have been found to be associated with drought tolerance in linseed [25][26].
Plants have many adaptations for surviving in conditions of drought and/or water shortage [27][28]. It is crucial to comprehend these processes in order to create agricultural plants that are resistant to such circumstances [29]. Quéro et al. [30] tried to investigate the processes underlying b-aminobutyric acid (BABA)-induced drought adaptation in plants. BABA causes a reorganisation of the solute content in flax leaves that results in an increased accumulation of proline and nonstructural carbohydrates, as well as a drop in inorganic solutes, according to metabolomic and ionomic profiling of the leaves [2]. In one of the previous studies, it was discovered that BABA therapy caused alterations that made flax plants more resistant to drought stress [30]. Under various irrigation regimes, Ansari et al. [31] investigated the relationship between the crop and mycorrhizal fungi. In a recent study conducted by Liu et al. [32], it was discovered that, in contrast to various plant species, flax mycorrhizal fungus may promote development under both stress and non-stress conditions.
Shivaraj et al. [2] explained the effects of various amounts of titanium dioxide (TiO2) at the nanoscale (10–25 nm) on flax development under stress and non-stress circumstances. It was discovered that TiO2 at modest concentrations facilitated the development of flax in water-scarce environments. Additionally, flax plants treated with nano-TiO2 at a concentration of 100 mg L−1 contained higher seed oil and protein contents. As a result, it has been concluded that the application of nano-TiO2 particles at modest concentrations can reduce the harm caused by drought stress to flax plants as well as increase drought tolerance. Crops that are resistant to duress are developed using both forward and backward genetic methods. In the same setting, the drought-responsive element binding protein 2A (DREB2A) gene was inserted into the plant genome to create a flax cv. Blanka cell line that is drought-tolerant [17].
3. Salinity Tolerance
Numerous variables, such as obscene irrigation, rock weathering, little precipitation, ion exchange, high surface evaporation, and poor cultivation practices, have contributed to the exponential increase in soil salinity in recent years [33][34][35]. According to estimates from Shrivastava and Kumar [36], greater than fifty percent of arable land will be salinised by around 2050. Currently, salty conditions impact around 33% of irrigated land and 20% of all cultivated land. According to Dubey et al. [33], soil salinity–alkalinity in flax causes delayed germination and emergence, reduced seedling survival, erratic crop development, and decreased yield. In a few investigations, flax germplasm was tested for resistance to salinity–alkalinity stressors, and salinity-tolerant lines were found based on biomass, germination, seedling traits, and the K+/Na+ ratio [37][38][39][40][41]. Wu et al. [42] discovered genes in flax that increase the root length, improve membrane damage, and alter the ion distribution to confer salt tolerance. Flax may be cultivated on agricultural terrain where other crops cannot since it can withstand pH levels up to 9.
All agricultural plants face genuine threats from rising soil salinity and alkalinity, among which alkaline-salt stress is more harmful than neutral-salt stress. Considering this, understanding the processes that control plant resistance to saline–alkaline stress has emerged as a hotly debated area of plant science. The impact of these circumstances on the sprouting of the flax plant was examined by Guo et al. [43]. In three experimental circumstances, they discovered that germination decreased with a rise in the ionic concentrations for each of the 10 common flax genotypes they examined. As anticipated, it was discovered that low-concentration treatments with neutral salt and alkaline salt had little impact on germination in all varieties [32].
All varieties of plants responded the most negatively to alkaline-salt stress, and higher concentrations of this treatment completely prevented germination. When five linseed genotypes were developed after being treated with neutral salt and alkaline salt, it was discovered that they had greater salt tolerance [43]. Digital gene expression in flax plants under alkaline-salt stress, neutral-salt stress, and alkaline stress was examined by Yu et al. [44]. In their experiment, it was discovered that under neutral-salt stress, carbohydrate metabolism was impacted, while under alkaline-salt stress, photosynthesis and thereaction to biotic stimuli were badly compromised. In some of the studies, the differential expression of important factors was implicated in responses to abiotic stressors, like WRKY, abscisic acid (ABA), ion channels, and mitogen-activated protein kinase (MAPKK) [2][16]. More of these differentially expressed genes were triggered by alkaline-salt stress than by alkaline stress or neutral-salt stress, indicating that a greater number of genes are involved in regulating the alkaline-salt stress pathway.
Salinity stress has a molecular foundation that is still poorly known. Therefore, Yu et al. [45] used deep sequencing to analyse small RNAs and the degradome in samples that had been exposed to three stress conditions in order to understand the genetic basis of salinity tolerance in flax. Small RNA target genes that control reactions to cues were discovered to be induced. Using transcriptome analysis and degradation genome sequencing, 29 showed opposite expression patterns. The development of climate-smart flax is progressingforall stressors, and 243 miRNA–target combinations have been identified. Two miRNAs, miR398a and miR530, have been reported to be linked to salt stress tolerance in flax. Furthermore, in a separate experiment, Wei et al. [46] discovered that flax seedlings of HIZ019, YOI254, and Tianxin3 had higher salt tolerance because of some important biochemical alterations in flax seedlings under salt stress.
4. Heavy Metal Stress Tolerance
Heavy metal buildup in vegetation is caused by heavy metal contamination of the earth. Heavy metals in the dietary chain are consequently biomagnified. One such heavy metal that can be detrimental to both plants and animals, even in minute quantities, is cadmium. Plants have evolved specialised regulatory mechanisms that allow them to bind metals to compounds like phytochelatins and metallothionein to create complexes (metallothionein group III). Numerous studies have demonstrated that linen clothing tolerates cadmium pollution well. Even though the toxic effect of cadmium restricts its use for both food and medicine, the use of metal-accumulating plants for the phytoremediation of contaminated soils opens a novel and hopeful path towards improving the resilience of their cultivars and variants to Cd stress [2]. Over the past ten years, extensive research has been conducted on the mechanisms that drivecadmium tolerance in plants. High Cd concentrations reduced root development and, to different degrees, increased the generation of membrane permeability, hydrogen peroxide (H2O2), protein oxidation, and lipid peroxides. Additionally, it was discovered that there had been a substantial change in the effectiveness of antioxidant and scavenging enzymes.
The impact of salicylic acid on the antioxidant defence system in flax plants was examined by Belkadhi et al. [47]. Salicylic acid, on the other hand, was discovered to lessen the toxic effects of Cd on the lipid composition of membranes, the antioxidant system, and root development [32]. Belkadhi et al. [48] discovered that a preliminary treatment with salicylic acid preferentially safeguarded plastidial lipids due to greater amounts of polyunsaturated fatty acids in flax. These findings imply that under Cd stress, salicylic acid-pretreated flax plantlets had increased membrane integrity. Salicylic acid boosts root antioxidant mechanisms in flax. Kaplan et al. [49] investigated the effects of cadmium stress on the fatty acid composition of flax in the presence of mycorrhizal fungi. They discovered that seeds from plants cultivated in mycorrhizal fungi contained more unsaturated (18:1, 18:2, and 18:3) fatty acids overall. At 15 ppm of Cd, these impacts became more obvious (the amounts of 18:1, 18:2, and 18:3 were increased by 169, 370, and 150%, respectively). These findings indicate that once the level of Cd in seeds hits a certain level, this heavy metal increases the effectiveness of the enzymes that control the transformation of saturated fatty acids into unsaturated fatty acids.
Although zinc (Zn) is a crucial ingredient needed for the healthy growth and development of plants, too much of it can also be harmful. Significant attempts have been made to comprehend the tolerance of plants to zinc in flax. Grant et al. [50] discovered that flax cultivars are better at transferring Zn through shoots to seeds than Cd. The use of plant species for phytoremediation is determined by the transfer of metal ions to seeds. Plants are also using a variety of biotechnological techniques to limit the absorption or transfer of the metal ions in plant cells. Smykalova et al. [51] investigated how flax varieties could collect and move Ca and Zn in an in vitro culture made from hypocotyl tissues. The flax varieties discovered by Smykalova et al. [51] are those that are tolerant to zinc, and increasing the amount of zinc and decreasing the amount of cadmium in the kernel will be crucial for increasing the nutritional value of linseed.
The toxic effects of heavy metals like copper (Cu), zinc (Zn), cadmium (cd), nickel (Ni), lead (Pb), chromium (Cr), arsenic (As), and cobalt (Co) on seed germination in various varieties of flax have been assessed in another important research conducted by Soudek et al. [52]. Various heavy metals manifested their harmful effects in the following order: As > Cu > Cd > Co > Cr > Ni > Pb > Zn. In fact, a lot of these studies point to the effective use of flax’s potential for phytoremediation, especially when cultivated for fibre production. The growing of flax on heavy-metal-contaminated soils may be made easier with a thorough grasp of the mechanisms underlying heavy metal tolerance [2].
5. Cold Stress Tolerance
Many cool-season perennial crops’ outputs are influenced by two significant physiological processes: vernalisation and photoperiodism. Darapuneni et al. [53] examined the flowering response of various flax genotypes under two photoperiod and vernalisation conditions in growth chamber research. The findings imply that the photoperiod, vernalisation, and genetics significantly influence early blooming in flax. For frigid climate regions like the Upper Midwest of the US and Canada, the early-blooming trait is more important. However, owing to the hot spring and summer temperatures in some places, such as Texas, flax is produced in the autumn. Flax varieties demonstrated genotypic interactions with the photoperiod and vernalisation.
In particular, Texas flax genotypes of the winter variety were susceptible to both vernalisation and blooming photoperiods. The flowering times of most other spring-grown flax genotypes were unchanged by the vernalisation treatments, whereas Texas genotypes postponed anthesis for 7 days or longer in unvernalised seedlings [16]. The majority of other genotypes were unaffected by circadian rhythms (photoperiodism)in vernalised seedlings, while Texas cultivars delayed anthesis for a period of twelve days or longer under vernalised and short-day conditions. Screening for vernalisation and photoperiodic sensitivity in Texas genotypes, as well as the introgression of these characteristics into newly adopted spring-grown genotypes, is required for the development of high-yielding flax genotypes for production sites in the southern Great Plains [2].
6. Heat Tolerance
Particularly in tropical and subtropical climates, heat stress negatively impacts physiological processes, development (Figure 1), growth, and yield [54]. According to several studies [55][56][57][58], a prolonged duration of heat stress (40 °C for 5–7 days) in relation to flowering duration may have a substantial effect on flax’s boll development, pollen viability, pollen production, flowering, seed oil quality, quantity, and seed set. Flax fibre does not need high temperatures. Flax grows best and produces the highest-quality fibre under circumstances that are damp, overcast, and moderately chilly (18–20 °C). High temperatures, especially terminal heat, which is limiting for flax growth, cause poor adaptation of superior fibre linseed genotypes to hotter climates. Although there have not been many studies on how higher temperatures affect flax development, physiological processes, and yields [58][59], the molecular dissection is also unclear.
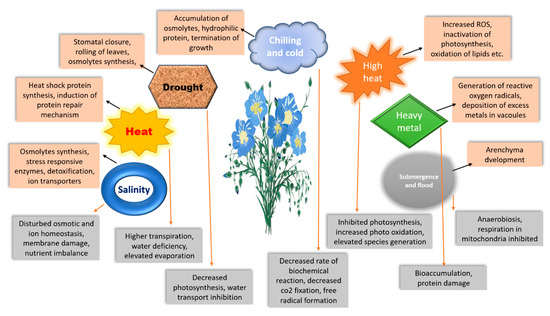
Figure 1. Schematic representation of abiotic stress regulation mechanisms by plants. The orange boxes represent coping mechanisms, while grey boxes represent disturbances in plant physiological response.
References
- Asati, R.; Tripathi, M.K.; Tiwari, S.; Yadav, R.K.; Tripathi, N. Molecular approaches in the development of drought tolerance in chickpea. Life 2022, 12, 1846.
- Shivaraj, S.M.; Dhakate, P.; Sonah, H.; Vuong, T.; Nguyen, H.T.; Deshmukh, R. Progress toward development of climate-smart flax: A perspective on omics-assisted breeding. In Genomic Designing Climate-Smart Oilseed Crops; Springer: Cham, Switzerland, 2019; pp. 239–274.
- Mishra, N.; Tripathi, M.K.; Tripathi, N.; Tiwari, S.; Gupta, N.; Sharma, A. Screening of soybean genotypes against drought on the basis of gene-linked microsatellite markers. In Book Innovations in Science and Technology; BP International: West Bengal, India, 2022; Volume 3, pp. 49–61.
- Tripathi, M.K.; Tripathi, N.; Tiwari, S.; Mishra, N.; Sharma, A.; Tiwari, S.; Singh, S. Identification of Indian soybean (Glycine max Merr.) genotypes for drought tolerance and genetic diversity analysis using SSR markers. Scientists 2023, 3, 31–46.
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147.
- Nasir, M.W.; Toth, Z. Effect of Drought Stress on Potato Production: A Review. Agronomy 2022, 12, 635.
- Molotoks, A.; Stehfest, E.; Doelman, J.; Albanito, F.; Fitton, N.; Dawson, T.P.; Smith, P. Global projections of future cropland expansion to 2050 and direct impacts on biodiversity and carbon storage. Glob. Chang. Biol. 2018, 24, 5895–5908.
- Cui, Z.; Yan, B.; Gao, Y.; Wu, B.; Wang, Y.; Wang, H.; Xu, P.; Zhao, B.; Cao, Z.; Zhang, Y.; et al. Agronomic cultivation measures on productivity of oilseed flax: A review. Oil Crop Sci. 2022, 7, 53–62.
- Zare, S.; Mirlohi, A.; Saeidi, G.; Sabzalian, M.R.; Ataii, E. Water stress intensified the relation of seed color with lignan content and seed yield components in flax (Linum usitatissimum L.). Sci. Rep. 2021, 11, 23958.
- Fila, G.; Bagatta, M.; Maestrini, C.; Potenza, E.; Matteo, R. Linseed as a dual-purpose crop: Evaluation of cultivar suitability and analysis of yield determinants. J. Agric. Sci. 2018, 156, 162–176.
- Hu, H.; Xiong, L. Genetic engineering and breeding of drought-resistant crops. Annu. Rev. Plant Biol. 2014, 65, 715–741.
- Stavropoulos, P.; Mavroeidis, A.; Papadopoulos, G.; Roussis, I.; Bilalis, D.; Kakabouki, I. On the Path towards a “Greener” EU: A Mini Review on Flax (Linum usitatissimum L.) as a Case Study. Plants 2023, 12, 1102.
- Heller, K.; Byczyńska, M. The impact of environmental factors and applied agronomy on quantitative and qualitative traits of flax fiber. J. Nat. Fibers 2015, 12, 26–38.
- Kaur, V.; Yadav, R.; Wankhede, D.P. Linseed (Linum usitatissimum L.) genetic resources for climate change intervention and its future breeding. J. Appl. Nat. Sci. 2017, 9, 1112–1118.
- Dash, P.K.; Cao, Y.; Jailani, A.K.; Gupta, P.; Venglat, P.; Xiang, D.; Rai, R.; Sharma, R.; Thirunavukkarasu, N.; Abdin, M.Z.; et al. Genome-wide analysis of drought induced gene expression changes in flax (Linum usitatissimum). GM Crops Food 2014, 5, 106–119.
- Mohammed, A.S.; Atnaw, S.M.; Ramaya, A.V.; Alemayehu, G. A comprehensive review on the effect of ethers, antioxidants, and cetane improver additives on biodiesel-diesel blend in CI engine performance and emission characteristics. J. Energy Inst. 2023, 108, 101227.
- Tawfik, R.S.; Badr, A.; Sammour, R.; Ibrahim, U.; Matter, M.; Sakr, M. Improvement of flax drought tolerance using gene transfer. Plant Tissue Cult. Biotechnol. 2016, 26, 197–207.
- Asgarinia, P.; Mirlohi, A.; Saeidi, G.; MohamadiMirik, A.A.; Gheysari, M.; Razavi, V.S. Selection criteria for assessing drought tolerance in a segregating population of flax (Linum usitatissimum L.). Can. J. Plant Sci. 2016, 97, 424–437.
- Qi, X.S.; Wang, X.R.; Xu, J.; Zhang, J.P.; Mi, J. Drought-resistance evaluation of flax germplasm at adult plant stage. Sci. Agric. Sin. 2010, 43, 3076–3087.
- Sharma, J.C.; Tomar, S.S.; Shivran, R.K.; Prakash, C. Water requirement water use efficiency consumptive use yield and quality parameters of linseed (Linum usitatissimum L.) varieties as influenced by fertility levels irrigation scheduling. Adv. Life Sci. 2012, 1, 180–182.
- Kaur, V.; Yadav, S.K.; Wankhede, D.P.; Pulivendula, P.; Kumar, A.; Chinnusamy, V. Cloning and characterization of a gene encoding MIZ1, a domain of unknown function protein and its role in salt and drought stress in rice. Protoplasma 2020, 257, 475–487.
- Gowda, V.R.P.; Henry, A.; Yamauchi, A.; Shashidhar, H.E.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crop Res. 2011, 122, 1–13.
- Tuberosa, R.; Sanguineti, M.C.; Landi, P.; Giuliani, M.M.; Salvi, S.; Conti, S. Identification of QTLs for root characteristics in maize grown in hydroponics and analysis of their overlap with QTLs for grain yield in the field at two water regimes. Plant Mol. Biol. 2002, 48, 697–712.
- Manschadi, A.M.; Christopher, J.; deVoil, P.; Hammer, G.L. The role of root architectural traits in adaptation of wheat to water-limited environments. Funct. Plant Biol. 2006, 33, 823–837.
- Soto-Cerda, B.J.; Cloutier, S.; Gajardo, H.A.; Aravena, G.; Quian, R. Identifying drought-resilient flax genotypes and related candidate genes based on stress indices, root traits and selective sweep. Euphytica 2019, 215, 41.
- Soto-Cerda, B.J.; Cloutier, S.; Gajardo, H.A.; Aravena, G.; Quian, R.; You, F.M. Drought response of flax accessions and identification of quantitative trait nucleotides (QTNs) governing agronomic and root traits by genome-wide association analysis. Mol. Breed. 2020, 40, 15.
- Mishra, N.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Gupta, N.; Sharma, A. Morphological and physiological performance of Indian soybean genotypes in respect to drought. Legume Res. 2021, 1–9.
- Choudhary, M.L.; Tripathi, M.K.; Tiwari, S.; Pandya, R.K.; Gupta, N.; Tripathi, N.; Parihar, P. Screening of Pearl Millet Germplam Lines for Drought Tolerance Based on Morpho-physiological Traits and SSR Markers. Curr. J. Appl. Sci. Technol. 2021, 40, 46–63.
- Kachare, S.; Tiwari, S.; Tripathi, N. Expression of DREB1, RBCL, PIP, SGR genes and morpho-physiological changes under water stress in soybean. J. Plant Biochem. Biotechnol. 2022, 32, 338–355.
- Kariuki, L.W.; Masinde, P.; Githiri, S.; Onyango, A.N. Effect of water stress on growth of three linseed (Linum usitatissimum L.) varieties. SpringerPlus 2016, 5, 759.
- Ansari, A.; Razmjoo, J.; Karimmojeni, H. Mycorrhizal colonization and seed treatment with salicylic acid to improve physiological traits and tolerance of flaxseed (Linum usitatissimum L.) plants grown under drought stress. Acta Physiol. Plant 2016, 38, 34.
- Liu, N.; Wan, B.; Zhang, Z.; Fang, X.; Lin, X.; Wang, Y.; Tang, J.; Bai, X.; Li, Y.; Yao, Y.; et al. Self-healing waterborne polyurethane coatings with high transparence and haze via cellulose nanocrystal stabilized linseed oil Pickering emulsion. Int. J. Biol. Macromol. 2023, 235, 123830.
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. 2020, 48, 954–966.
- Singh, M.; Nara, U.; Kumar, A.; Choudhary, A.; Singh, H.; Thapa, S. Salinity tolerance mechanisms and their breeding implications. J. Genet. Eng. Biotechnol. 2021, 19, 173.
- Tao, R.; Ding, J.; Li, C.; Zhu, X.; Guo, W.; Zhu, M. Evaluating and Screening of Agro-Physiological Indices for Salinity Stress Tolerance in Wheat at the Seedling Stage. Front. Plant Sci. 2021, 12, 646175.
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131.
- Kocak, M.Z.; Göre, M.; Kurt, O. The effect of different salinity levels on germination development of some flax (Linum usitatissimum L.) varieties. J. Food Sci. Technol. 2022, 10, 657–662.
- El-Afry, M.M.; El-Okkiah, S.A.; El-Kady, E.S.A.; El-Yamanee, G.S.A. Exogenous application of ascorbic acid for alleviation the adverse effects of salinity stress in flax (Linum usitatissimum L.). Middle East J. 2018, 7, 716–739.
- Nasri, N.; Maatallah, S.; Saidi, I.; Lachaal, M. Influence of salinity on germination, seedling growth, ion content and acid phosphatase activities of Linum usitatissimum L. J. Anim. Plant Sci. 2017, 27, 517–521.
- Patil, N.M.; Datir, S.S.; Shah, P.V. Salt-induced physiological and biochemical changes in two varieties of Linum usitatissimum L. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 296–304.
- Kaya, M.D.; Day, S.; Cikili, Y.; Arslan, N. Classification of some linseed (Linum usitatissimum L.) genotypes for salinity tolerance using germination, seedling growth, and ion content. Chil. J. Agric. Res. 2012, 72, 27–32.
- Khan, N. Designing Genomic Solutions for Abiotic Traits in Flax (Linum usitatissimum L.). Ph.D. Thesis, University of Ottawa, Ottawa, ON, Canada, 2022. Submitted.
- Guo, R.; Zhou, J.; Ren, G.; Hao, W. Physiological responses of linseed seedlings to iso osmotic polyethylene glycol, salt, and alkali stresses. Agron. J. 2013, 105, 764–772.
- Miart, F.; Fontaine, J.X.; Mongelard, G.; Wattier, C.; Lequart, M.; Bouton, S.; Molinie, R.; Dubrulle, N.; Fournet, F.; Demailly, H. Integument-Specific Transcriptional Regulation in the Mid-Stage of Flax Seed Development Influences the Release of Mucilage and the Seed Oil Content. Cells 2021, 10, 2677.
- Yu, Y.; Chen, H.; Yang, Y.; Lou, D.; Liang, C.; Yuan, H.; Wu, G.; Xu, C. Identification and Characterization of Differentially Expressed microRNAs and Target Gene Related to Flax Stem Development. J. Nat. Fibers 2022, 19, 5974–5990.
- Wei, W.; Qian, D.; Qiaoling, J. Effects of NaCl stress on the biochemical characteristics in six species fiber flax seeding. Chin. Agric. Sci. Bull. 2013, 18, 017.
- Belkadhi, A.; De Haro, A.; Soengas, P.; Obregon, S.; Cartea, M.E.; Djebali, W.; Chaïbi, W. Salicylic acid improves root antioxidant defense system and total antioxidant capacities of flax subjected to cadmium. OMICS 2013, 17, 398–406.
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Positive effects of salicylic acid pretreatment on the composition of flax plastidial membrane lipids under cadmium stress. Environ. Sci. Pollut. Res. 2015, 22, 1457–1467.
- Kaplan, M.E.; Simmons, E.R.; Hawkins, J.C.; Ruane, L.G.; Carney, J.M. Influence of cadmium and mycorrhizal fungi on the fatty acid profile of flax (Linum usitatissimum) seeds. J. Sci. Food Agric. 2015, 95, 2528–2532.
- Grant, C.A.; Dribnenki, J.C.P.; Bailey, L.D. Cadmium and zinc concentrations and ratios in seed and tissue of solin (cv LinolaTM 947) and flax (cvs McGregor and Vimy) as affected by nitrogen and phosphorus fertiliser and Provide (Penicillium bilaji). J. Sci. Food Agric. 2000, 80, 1735–1743.
- Smykalova, I.; Vrbova, M.; Tejklova, E.; Vetrovcova, M.; Griga, M. Large scale screening of heavy metal tolerance in flax/linseed (Linum usitatissimum L.) tested in vitro. Ind. Crops Prod. 2010, 32, 527–533.
- Soudek, P.; Katrušáková, A.; Sedláček, L.; Petrová, Š.; Kočí, V.; Maršík, P.; Griga, M.; Vaněk, T. Effect of heavy metals on inhibition of root elongation in 23 cultivars of flax (Linum usitatissimum L.). Arch. Environ. Contamin. Toxicol. 2010, 59, 194–203.
- Darapuneni, M.K.; Morgan, G.D.; Ibrahim, A.M.; Duncan, R.W. Effect of vernalization and photoperiod on flax flowering time. Euphytica 2014, 195, 279–285.
- Ramirez-Villegas, J.; Molero Milan, A.; Alexandrov, N.; Asseng, S.; Challinor, A.J.; Crossa, J.; Eeuwijk, F.V.; Ghanem, M.E.; Grenier, C.; Heinemann, A.B.; et al. CGIAR modeling approaches for resource-constrained scenarios: I. Accelerating crop breeding for a changing climate. Crop Sci. 2020, 60, 547–567.
- Cross, R.H. Heat Stress Effects on Flowering and Reproduction in Linum usitatissimum (Flax). Master’s Thesis, University of Saskatchewan, Saskatoon, SK, Canada, 2002.
- Saha, D.; Mukherjee, P.; Dutta, S.; Meena, K.; Sarkar, S.K.; Mandal, A.B.; Dasgupta, T.; Mitra, J. Genomic insights into HSFs as candidate genes for high-temperature stress adaptation and gene editing with minimal off-target effects in flax. Sci. Rep. 2019, 9, 5581.
- Saha, D.; Shaw, A.K.; Datta, S.; Mitra, J. Evolution and functional diversity of abiotic stress-responsive NAC transcription factor genes in Linum usitatissimum L. Environ. Exp. Bot. 2021, 188, 104512.
- Cross, R.H.; McKay, S.A.B.; McHughen, A.G.; Bonham-Smith, P.C. Heat stress effects on reproduction and seed set in Linum usitatissimum L. (flax). Plant Cell Environ. 2003, 26, 1013–1020.
- Pokhrel, S.; Meyers, B.C. Heat-responsive microRNAs and phased small interfering RNAs in reproductive development of flax. Plant Direct. 2022, 6, 385.
More
Information
Subjects:
Agriculture, Dairy & Animal Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
801
Revisions:
2 times
(View History)
Update Date:
27 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




