Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Melvin R Hayden | -- | 3394 | 2023-07-25 03:34:11 | | | |
| 2 | Melvin R Hayden | Meta information modification | 3394 | 2023-07-25 03:39:50 | | | | |
| 3 | Catherine Yang | Meta information modification | 3394 | 2023-07-25 03:42:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hayden, M.R. Brain Endothelial Cell Activation and Dysfunction (BECact/dys). Encyclopedia. Available online: https://encyclopedia.pub/entry/47196 (accessed on 07 February 2026).
Hayden MR. Brain Endothelial Cell Activation and Dysfunction (BECact/dys). Encyclopedia. Available at: https://encyclopedia.pub/entry/47196. Accessed February 07, 2026.
Hayden, Melvin R.. "Brain Endothelial Cell Activation and Dysfunction (BECact/dys)" Encyclopedia, https://encyclopedia.pub/entry/47196 (accessed February 07, 2026).
Hayden, M.R. (2023, July 25). Brain Endothelial Cell Activation and Dysfunction (BECact/dys). In Encyclopedia. https://encyclopedia.pub/entry/47196
Hayden, Melvin R.. "Brain Endothelial Cell Activation and Dysfunction (BECact/dys)." Encyclopedia. Web. 25 July, 2023.
Copy Citation
Embryonic genetic mechanisms are present in the brain and ready to be placed into action upon cellular injury, termed the response to injury wound-healing (RTIWH) mechanism. When injured, regional brain endothelial cells initially undergo activation and dysfunction with initiation of hemostasis, inflammation (peripheral leukocytes, innate microglia, and perivascular macrophage cells), proliferation (astrogliosis), remodeling, repair, and resolution phases if the injurious stimuli are removed.
astrogliosis
brain endothelial cells
enlarged perivascular spaces
microgliosis
1. Introduction
The repair and remodeling of injured brain tissue during postnatal life are associated with the upregulation of some mechanisms that are characteristic of embryonic development [1]. These embryonic genetic memory mechanisms are present in brain cells and ready to be placed into action upon cellular injury. This genetically encoded mechanism may be termed the response to injury wound-healing (RTIWH) mechanism [2][3][4]. This recapitulated brain RTIWH mechanism consists of four basic phases: hemostasis, inflammation, proliferation, and remodeling, similar to peripheral wound-healing mechanisms [5][6]. However, the inflammatory and proliferative phases are unique in the brain, in that neuroglia play an active role [6]. The innate central nervous system (CNS) microglia and perivascular macrophages become reactive and undergo polarization in addition to the peripheral monocyte and monocyte-to-macrophage transformation. Because there are no fibrocytes/fibroblasts within the brain parenchyma, the astroglia become reactive and form an astroglia-derived gliosis scar instead of a fibrosis scar to protect the surrounding neuropil in injury states [6]. Additionally, brain injuries may be characterized as traumatic, radiation, metabolic, infectious, and hemodynamic stressors, and in this research the focus is primarily on metabolic injurious stimuli as occur in obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM) (Figure 1 and Figure 2) [1][2][3][4][5][6].

Figure 1. Multiple injurious stimuli to brain endothelial cells (BECs) in obesity, metabolic syndrome (MetS), and type 2 diabetes mellitus (T2DM). This representative illustration does not depict the thin luminal endothelial glycocalyx layer covering the BECs. Ang II = angiotensin 2; AGE/RAGE = advanced glycation end-products and its receptor RAGE; BEC = brain endothelial cell; BECact/dys = brain endothelial cell activation and dysfunction; LDL = low-density lipoprotein cholesterol; LPa = lipoprotein little a; LPS = lipopolysaccharide; pCC = peripheral cytokine/chemokine; ROS = reactive oxygen species and the reactive species interactome; T = transcytosis;  = radiation symbol.
= radiation symbol.
 = radiation symbol.
= radiation symbol.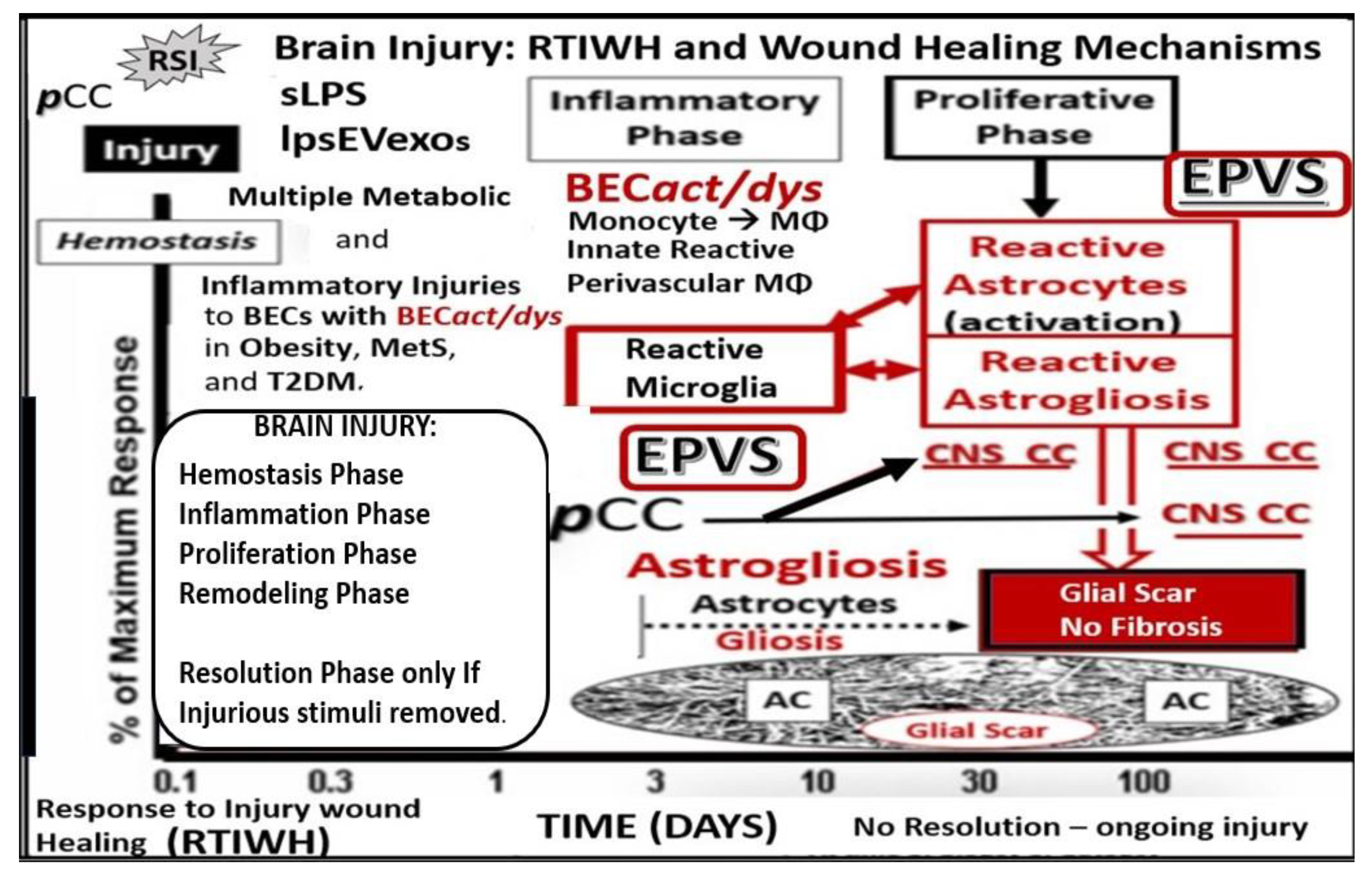
Figure 2. Comparison of peripheral response to injury wound healing (RTIWH) to the unique brain injury RTIWH mechanisms. This image depicts the RTIWH mechanisms as a result of the dual signaling by peripheral cytokines/chemokines (pCC), soluble lipopolysaccharide (sLPS), and LPS-enriched extracellular exosomes (lpsEVexos). This dual signaling of the brain endothelial cell(s) (BECs) results in BEC activation and dysfunction (BECact/dys), which in turn results in central nervous system (CNS) neuroinflammation with increased cnsCC, reactive polarized microglia, and reactive astroglia—reactive astrogliosis, which results in astrogliosis scarring instead of fibrosis scarring, since the brain does not have fibrocytes/fibroblasts in its parenchyma. Importantly, this RTIWH mechanism contributes to maladaptive remodeling and also contributes to the development of enlarged perivascular spaces (EPVSs). Figure adapted with permission by CC 4.0 [4].
Perivascular spaces (PVSs) or Virchow–Robin spaces are fluid-filled spaces that ensheath the pial arteries, within the subarachnoid space (SAS) that dive deep into the white matter of the brain as ensheathed precapillary arterioles, where they end in true capillaries (Figure 3) [7].

Figure 3. Illustration of the origins of the perivascular spaces (PVSs) and representative transmission electron micrograph (TEM) cross-sections of the precapillary arteriole, true capillary, and postcapillary venule. Panel (A) illustrates the whole brain with its surface pia arteries that dive deeply into the grey matter and deeply into the white matter, terminating in capillaries within the interstitial spaces (ISS). Panel (B) reveals the interaction of the PVSs to precapillary arterioles, which allow the vascular lumen influx of oxygen, nutrients, and solutes (red arrow), in addition to the influx of CSF via the PVSs (red arrow true capillaries with delivery of oxygen, nutrients, solutes, and the postcapillary venules that allow the removal of metabolic waste dioxide plus others), and PVSs that are responsible for the removal of the admixture of ISF and CSF, and metabolic waste (MW) that includes misfolded proteins (amyloid beta and tau neurofibrillary fibrils) (blue arrow) in the subarachnoid space (SAS). Panel (C) depicts how the CSF and its admixed metabolic waste from the PVS glymphatic system is transported to the dural venous sinus (DVS) and the systemic circulation. Panels (D–F) demonstrate representative and corresponding cross-sectional TEMs of corresponding labeled capillary images in Panel (B). Panel (D) demonstrates a precapillary arteriole with a PVS (pseudo-colored green). Panel (E) represents a true capillary without a PVS. Importantly, note that the pia membrane of the glia limitans abruptly ends at the true capillary and that the astrocyte endfeet end directly at the brain endothelial cell basement membrane (blue arrows) (numbers 1–7 indicate astrocyte numbers). Panel (F) depicts a postcapillary venule that appears to have a dilated PVS and is thus considered to be an enlarged PVS (EPVS). Note the presence of a resident perivascular macrophage cell within the EPVS. It is important to note that the PVS provides the conduit structure for the glymphatic draining system to deliver metabolic waste products to the CSF and the systemic circulation for disposal. This adapted and modified image is provided with permission by CC 4.0 [7]. AC = astrocyte; ACef = astrocyte endfeet; ACfp = astrocyte foot process end-feet; AQP4 = aquaporin four; Cl = capillary lumen; CSF = cerebrospinal fluid; EC = brain endothelial cell; ISF = interstitial fluid; N = nucleus; Pc = pericyte; PVS = perivascular space; Mt = mitochondria; RBC = red blood cell; rMΦ = resident reactive macrophage.
The arterial and precapillary PVSs are bounded abluminally by the pia membrane that remains adherent to the pial arteries and arterioles, and is adherent to the adjacent basal lamina of the astrocyte endfeet basement membranes to form the glia limitans, as these structures penetrate the grey and deeper white matter regions of the parenchyma. Further, PVSs serve as the delivery conduit for the CSF to admix with the interstitial fluid (ISF) and aid to promote movement within the interstitial space (ISS). The true capillaries are without a pial lining and do not have a PVS. The true capillaries transition to the postcapillary venules, which are also ensheathed by a PVS (without a pia membrane), that form the efflux conduit for the ISF and metabolic waste, known as the glymphatic system [8] (abluminally lined by the basal lamina of the astrocyte endfeet), to exit the brain parenchymal ISS and travel to the SAS and, hence, to the CSF, to be delivered to the systemic circulation via the arachnoid granulations and the dural venous sinuses (Figure 3B,C) [7]. These PVSs are capable of enlarging or dilating and are termed as either enlarged perivascular spaces (EPVSs) or dilated PVSs, once they are 1–3 μm by T-2 weighted magnetic resonance imaging (MRI) [7][9]. EPVSs can be visualized and identified by non-invasive MRI, in addition to microscopic techniques including transmission electron microscopy (TEM), and are becoming more clinically important (Figure 4) [7][9].
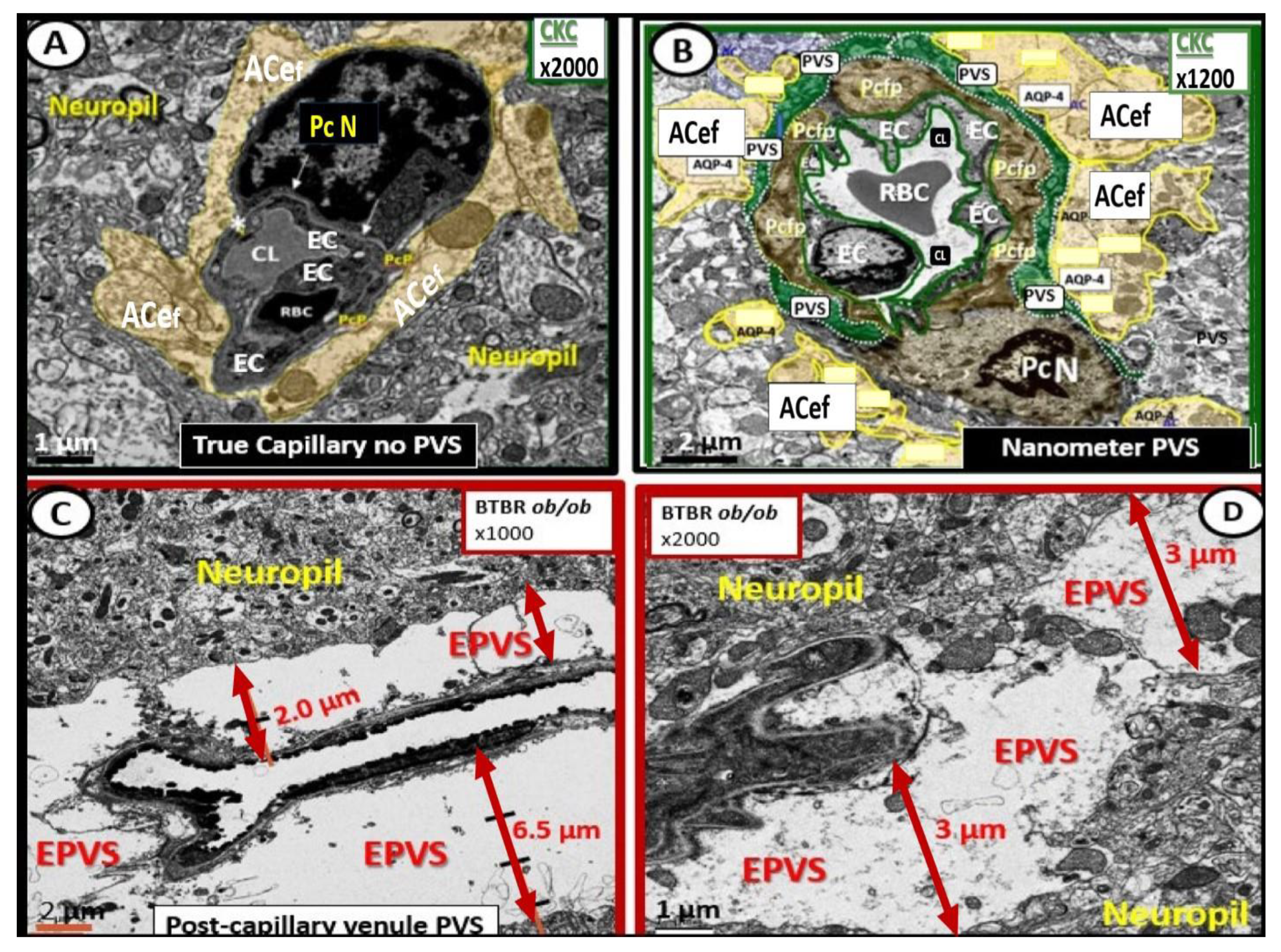
Figure 4. Comparison of normal true capillary and precapillary arterioles to postcapillary venules with enlarged perivascular spaces (EPVSs). Panel (A) demonstrates a true capillary with the astrocyte endfeet (ACef) abutting the shared basement membrane of the brain endothelial cell and pericyte (asterisk and closed arrows) without a pia mater membrane. Panel (B) demonstrates a precapillary arteriole with only a thinned perivascular space (PVS) (pseudo-colored green). Note that the abluminal perivascular space is still ensheathed by the pia matter and basal lamina of the adjacent ACef. Panels (C,D) each depict two different postcapillary venules with enlarged perivascular spaces (EPVS) between 2 and 6.5 μm. Modified image provided with permission by CC 4.0 [7]. Magnification ×1000; ×2000; scale bars = 2 and 1 μm in Panels (C,D) respectively. AC astrocyte; ACef = astrocyte endfeet; AQP 4 = aquaporin 4; Cl = capillary lumen; EC = brain endothelial cell; Pc = pericyte; Pc N = pericyte nucleus; Pcfp = pericyte foot process—endfeet; RBC = red blood cell.
EPVSs have been recognized as important remodeling changes in various neuropathologies [10]. EPVSs are known to associate with advancing age, hypertension, lacunes, microbleeds, intracerebral hemorrhages, cerebrocardiovascular disease with transient ischemic episodes and stroke, SVD, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADISIL), cerebral amyloid angiopathy, obesity, MetS, T2DM, white matter hyperintensities, late-onset Alzheimer’s disease (LOAD), sporadic Parkinson’s disease, and non-age-related multiple sclerosis [7][9][10][11][12][13][14][15][16][17][18][19].
Cerebral small vessel disease (SVD) presents clinically as lacunar strokes, which are responsible for at least 20% of ischemic strokes, while representing a major cause of vascular cognitive impairment and vascular dementia. Importantly, EPVSs are known to be a biomarker and prominent feature of both SVD and VaD, which are known to be associated with lacunar stroke, in addition to white matter hyperintensities WMH [11][15][19][20][21]. EPVSs are also known to be a biomarker for SVD once identified by MRI and associate with white matter hyperintensities and lacunes [22]. Prior studies have shown that EPVSs associate with worse executive function and information processing in healthy older adults [23] and are significantly more prevalent in those with mild cognitive impairment as compared to age-matched control subjects without mild cognitive impairment [24]. These findings suggest that EPVSs may be an early remodeling change in the development of SVD and impaired cognition [23][24].
As our global aging population continues to grow, EPVSs are becoming increasingly important abnormal structural findings, since they also relate to clinical extracranial atherosclerosis [7][25][26], neurovascular cerebromacrovascular and cerebromicrovascular disease, and age-related neurodegenerative diseases such as LOAD and sporadic Parkinson’s disease. In older community-dwelling individuals free of clinical dementia and stroke, SVD biomarkers including EPVSs, white matter hyperintensities, and lacunes are related to worse cognitive performance [27]. Importantly, EPVS have recently been determined to be a marker for an increased risk of cognitive decline and dementia, independent of other small vessel disease markers over a period of four years [28].
The MetS is a cluster of multiple interconnected risks and variables, which associate with an increased risk for developing atherosclerotic cerebrocardiovascular disease and T2DM [25][29][30]. Importantly, visceral adipose tissue (VAT), insulin resistance, and MetS are each associated with an increased risk for developing EPVSs [31][32][33]. Insulin resistance is a central core feature of the MetS; however, it is a risk factor that may be independent of the MetS for developing EPVSs (Figure 5) [25][34].

Figure 5. Metabolic syndrome (MetS), enlarged perivascular spaces (EPVSs), and cerebral small vessel disease (cSVD). The visceral adipose tissue (VAT), obesity, and hyperlipidemia (atherogenic dyslipidemia) located in the lower left-hand side of the letter X appears to drive the MetS and the other three arms of the letter X, which includes insulin resistance (IR) and the associated hyperinsulinemia, hypertension, vascular stiffening, and hyperglycemia, with or without manifest type 2 diabetes mellitus (T2DM). The global increase in obesity and the accumulation of visceral obesity is thought to be a major driver of the MetS that is related to the excessive metainflammation arising from VAT depots. Follow the prominent closed red arrows emanating from VAT to cerebrocardiovascular disease (CCVD), SVD, TIA, stroke, cerebral microbleeds, and hemorrhages. BEC activation and dysfunction (BECact/dys), with its proinflammatory and prooxidative properties, result in endothelial nitric oxide synthesis (eNOS) uncoupling with increased superoxide (O2•−) and decreased nitric oxide (NO) bioavailability. Importantly, note that obesity, MetS, T2DM, and decreased bioavailable NO interact to result in capillary rarefaction that may allow EPVS development, which are biomarkers for cerebral SVD. Figure adapted with permission by CC 4.0 [7][25]. AGE = advanced glycation end-products; RAGE = receptor for AGE; AGE/RAGE = advanced glycation end-products and its receptor interaction; βcell = pancreatic islet insulin-producing beta cell; FFA = free fatty acids—unsaturated long chain fatty acids; IGT = impaired glucose tolerance; LOAD = late-onset Alzheimer’s disease; ROS = reactive oxygen species; RSI = reactive species interactome; Sk = skeletal: TG Index = triglyceride/glucose index; TIA = transient ischemia attack.
There are four key features (hyperlipidemia, hyperinsulinemia, hypertension, and hyperglycemia) of the MetS, and each are interconnected to the central placement of insulin resistance (Figure 5) [25][29][30][34]. Globally, there exits an increase in obesity and the MetS due to an aging population, urbanization, sedentary lifestyles, and increased caloric high fat, sucrose, fructose, and glucose diets [25][29][30]. Further, our global population is now considered to be one of the oldest attained in our history [35]. Tucsek et al., have recently demonstrated that aging exacerbates obesity-induced neurovascular uncoupling, cerebromicrovascular capillary rarefaction, and cognitive decline in mice [36]. Thus, it is not surprising that aging is a reason for our observations of global increases in the MetS, EPVSs, and SVD [9][11][15][16][17][21][22][25][26][34][36].
Capillary rarefaction in the brain (loss of capillaries) has recently been found to be associated with an increase in obesity and the MetS [36][37][38]. Recently, Schulyatnikova and Hayden have hypothesized that capillary rarefaction may leave an empty space within the PVS that is subsequently filled with interstitial fluid [7]. This loss of capillaries within the PVS may allow for an increase in total fluid volume within the PVS when the capillary undergoes rarefaction and result in EPVSs (Figure 6) [7].

Figure 6. Cross- and longitudinal sections representative of pre- and postcapillary arterioles and venules with an encompassing surrounding perivascular space (PVS). Panel (A) depicts a cross-section of a capillary surrounded by a PVS (solid double red arrows) and its increase in total volume to become an enlarged perivascular space (EPVS) (dashed double red arrows), which represents capillary rarefaction. Panel (B) demonstrates a control longitudinal precapillary arteriole, postcapillary venule, and neurovascular unit (NVU) that runs through an encompassing PVS (light blue). Panel (C) depicts capillary rarefaction (CR) in a longitudinal view, and note how the volume of the PVS increases its total percentage volume once the capillary has undergone rarefaction as in obesity, metabolic syndrome, and type 2 diabetes mellitus. Panel (D) depicts the progression of a normal precapillary arteriole and postcapillary venule PVS to an EPVS once the capillary has undergone rarefaction, allowing for an increase in its total percentage volume of the PVS (1.–3.). Panels (B,C) provided with permission by CC 4.0 [7]. ACef = astrocyte endfeet; AQP4 = aquaporin 4; BEC = brain endothelial cells; BECact/dys = brain endothelial cell activation and dysfunction; CL =capillary lumen; EC = endothelial cell; lpsEVexos = lipopolysaccharide extracellular vesicle exosomes; NVU = neurovascular unit; Pcef = pericyte endfeet.
Capillary rarefaction (CR) is known to occur in multiple clinical situations, including: aging, hypertension, obesity, MetS, T2DM, and LOAD. Also, there are multiple proposed mechanisms that may co-occur to result in CR, including: oxidative–redox stress, inflammation, pericyte loss, BECact/dys, impaired angiogenesis (increased ratio of increased antiangiogenic factors/proangiogenic factors), microvessel emboli and decreased microvessel shear stress, increased microvessel tortuosity, and, in some cases, increased TGF-beta [39].
While this mechanistic hypothesis for an expansion of the PVS is possible, more research will be required for it to gain support as a mechanism for increased EPVS.
Obesity, MetS, and T2DM not only allow for the expansion of VAT depots to develop metainflammation and increased peripheral cytokines/chemokines (pCC) [7][25][34], but also provide a milieu for the development of gut microbiota dysbiosis with the secretion of pCC, soluble lipopolysacchride (LPS), and LPS extracellular exosomes to result in the bidirectional communication between the gut and the brain ‘microbiota-gut-brain axis’ in neuroinflammation due to the excessive dual signaling of NVU BECs [7][25][34][40]. Importantly, there may be a host of metabolic functions, gut microbiota, and the innate immune system (Figure 7) [7][25][40][41][42][43].

Figure 7. The triangulation of Metabolic function, gut dysbiosis, and the metabolic syndrome (MetS) allows the innate immune system, peripheral, proinflammatory cytokines and chemokines (pCC) to signal the neurovascular unit (NVU) brain endothelial cell(s) (BECs) resulting in BEC activation and dysfunction (BECact/dys) with subsequent neuroinflammation. This schematic demonstrates that gut microbiota dysbiosis, metabolic dysfunction, and an activated proinflammatory innate immune system are associated with obesity and metabolic syndrome (lower-left red box). Importantly, this triangulation also associates with the metainflammation that is produced in the obese visceral adipose tissue depots (upper-right red box). These two distinct sites of metainflammation (red boxes) and dual signaling of BECs will each signal the central nervous system brain endothelial cells to result in neuroinflammation to result in BECact/dys and contribute to enlarged perivascular spaces (EPVSs). Importantly, this dual signaling by the gut and visceral adipose tissue (VAT) of BECs may be synergistic in obesity and the MetS. Image provided with permission by CC 4.0 [34]. Db/db = obese, insulin resistance diabetic genetic mouse model; DIO = diet-induced obesity; BTBRob/ob = black and tan brachyuric ob/ob mouse model of obesity and diabetes; IR = insulin resistance; LR = leptin resistance; sLPS = soluble lipopolysaccharide; lpsEVexos = lipopolysaccharide extracellular vesicle exosomes; MΦ = macrophage; MetS = metabolic syndrome; pCC = peripheral cytokines/chemokines; T2DM = type 2 diabetes mellitus.
This dual signaling by these two disparate regions allows for heightened signaling of the BEC to result in BECact/dys as discussed in the following section [7][25][34].
2. Brain Endothelial Cell Activation and Dysfunction (BECact/dys)
BECs and their luminal endothelial glycocalyx serve as the sentinel gatekeeper cells of the BBB and CNS as the first effector cell that comes into direct contact with peripheral systemic solutes (ions, molecules, neurotoxins) and cells, including leukocytes between the blood and the brain parenchyma (Figure 1) [44]. Importantly, BECs are key to vascular hemostasis, tone, leukocyte recruitment, hormone trafficking, and fluid movement from the blood to the parenchymal interstitial space, in addition to their playing a central role in the development of EPVSs [34][45]. Further, they are activated by the dual peripheral signaling from the pCC and the peripheral soluble LPS and LPS-enriched small extracellular vesicles exosomes from the regional pCC VAT depot metainflammation and the regional dysbiosis microbiota, respectfully (Figure 8) [7][34].

Figure 8. Dual Signaling of BECs by peripheral cytokines/chemokines (pCC) and soluble lipopolysaccharide (sLPS) and LPS-enriched small extracellular vesicle exosome (lpsEVexos), to result in brain endothelial activation and dysfunction (BECact/dys). Note the insert lower-left, which depicts a small EVexo liberating multiple LPS-enriched vesicles and the metabolic signaling of BECs to result in central nervous system cytokines chemokines (cnsCC) and attracted microglia cells (atMGCs) via CCL5 (RANTES). BECact/dys results in neuroinflammation, blood–brain barrier (BBB) disruption, and enlarged perivascular spaces (EPVSs) in obesity, insulin resistance (IR), and the metabolic syndrome (MetS). This modified image is provided with permission by CC4.0 [34]. atMGC = attracted microglia cell; CCL5 = chemokine (C-C motif) ligand 5 (chemoattractant for microglia cells); CD14 = cluster of differentiation 14; CCR5 = C-C chemokine receptor type 5, also known as CD195; CL = capillary lumen; EC = endothelial cell, brain endothelial cells; ecGCx = endothelial glycocalyx; EV = extracellular vesicles; EVMp =EVmicroparticles or microvesicles; EVexo = EVexosomes; IL1-β = interleukin-1 beta; IL-6 = interleukin-6; LPS = lipopolysaccharide; LBP = lipopolysaccharide-binding protein; MAPK = mitogen-activated protein kinase; Mp = microparticles; N = nucleus; PI3K/AKT = phosphatidylinositol 3-kinase/protein kinase B; PM = plasma membrane; RANTES = regulated on activation, normal T cell expressed and secreted; TNFα = tumor necrosis alpha.
This dual signaling of BECs is associated with the increased expression of cell-surface adhesion molecules such a VCAM-1, ICAM-1, and E selectin, as defined in BECact/dys. Likewise, BEC dysfunction is defined as the decreased synthesis, release, and/or activity of endothelium-derived nitric oxide (NO), which results in decreased bioavailable NO [7][34][44][46][47].
BECact/dys is induced by: (1) proinflammatory cytokines, such as tumor necrosis alpha and interleukin-6; (2) turbulent blood flow, such as occurs at bifurcations and branch points of arteries; (3) advanced glycation end-products, which are elevated in hyperglycemia and aging; and (4) inflammatory stressors such as metainflammation pCC, from VAT depots and plasma membrane peptides of gram-negative bacteria such as soluble LPS and LPS extracellular exosomes from gut microbiota dysbiotic regions. These four functions are each important mediators of ECact/dys via the activation of the nuclear transcription factor of BEC (Figure 8). The following TEM images are examples of BEC activation [25]. BECact/dys is known to be induced by: (i) proinflammatory cytokines, such as tumor necrosis factor alpha and interleukin-6; (ii) turbulent blood flow, such as that which occurs at bifurcations and branch points of arteries; (iii) advanced glycation end-products, which are elevated in hyperglycemia and aging; and (iv) inflammatory stressors such as metainflammation and plasma membrane peptides of gram-negative bacteria such as soluble LPS and LPS extracellular vesicle exosomes. These four functions are each important mediators of BECact/dys via the signaling of the BEC nuclear transcription factor. The following TEM images are examples of BECact/dys (Figure 9) [7][25][34][46].
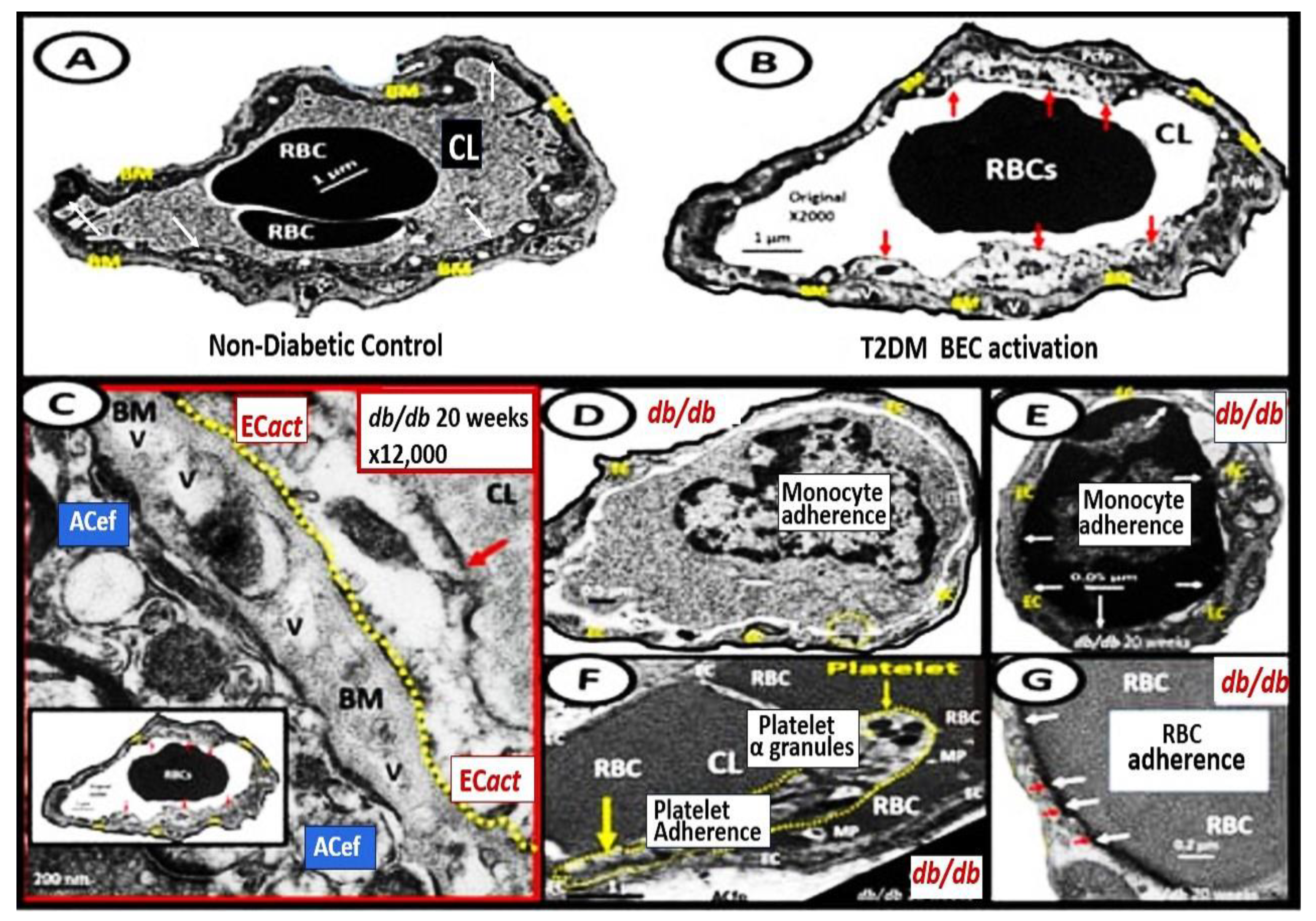
Figure 9. Representative transmission electron microscopic (TEM) images of endothelial cell activation (ECact). Panel (A) demonstrates a control BEC. Panel (B) depicts the thickened electron-lucent areas (red arrows) of ECact as compared to control models in Panel (A). Panel (C) depicts basement membrane (BM) thickening (red arrow) with increased vacuoles and vesicles (V). Panels (D,E) depict monocyte (D) and lymphocyte (E), platelet (F), and red blood cell (RBC) adhesion (G) to activated ECs (adhesions sites denoted by closed white arrows Panels (E,G); yellow arrows in Panel (F). Magnifications and scale bars vary. Panels (C–G) images are reproduced and modified with permission by CC 4.0 [25]. ACfp = astrocyte foot processes; Cl, capillary lumen; EC = endothelial cells, brain endothelial cells; MP = microparticle of the platelet.
References
- Aller, M.-A.; Arias, J.-I.; Arraez-Aybar, L.-A.; Gilsanz, C.; Arias, J. Wound healing reaction: A switch from gestation to senescence. World J. Exp. Med. 2014, 4, 16–26.
- Hayden, M.R.; Tyagi, S.C.; Kolb, L.; Sowers, J.R.; Khanna, R. Vascular ossification—Calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis—Calcific uremic arteriolopathy: The emerging role of sodium thiosulfate. Cardiovasc. Diabetol. 2005, 4, 4.
- Hayden, M.R.; Sowers, K.M.; Pulakat, L.; Joginpally, T.; Krueger, B.; Whaley-Connell, A.; Sowers, J.R. Possible Mechanisms of Local Tissue Renin-Angiotensin System Activation in the Cardiorenal Metabolic Syndrome and Type 2 Diabetes Mellitus. Cardiorenal Med. 2011, 1, 193–210.
- Hayden, M.R. Hypothesis: Neuroglia Activation Due to Increased Peripheral and CNS Proinflammatory Cytokines/Chemokines with Neuroinflammation May Result in Long COVID. Neuroglia 2021, 2, 7–35.
- Clark, R.A. Cutaneous tissue repair: Basic biologic considerations. J. Am. Acad. Dermatol. 1985, 13 Pt 1, 701–725.
- Stroncek, J.D.; Reichert, W.M. Overview of Wound Healing in Different Tissue Types. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2008; Chapter 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK3938/ (accessed on 31 May 2023).
- Shulyatnikova, T.; Hayden, M.R. Why Are Perivascular Spaces Important? Medicina 2023, 59, 917.
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial 557 Solutes, Including Amyloid β. Sci. Transl. Med. 2012, 4, 147ra111.
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838.
- Francis, F.; Ballerini, L.; Wardlaw, J.M. Perivascular spaces and their associations with risk factors, clinical disorders and neuroimaging features: A systematic review and meta-analysis. Int. J. Stroke 2019, 14, 359–371.
- Brown, R.; Benveniste, H.; Black, S.E.; Charpak, S.; Dichgans, M.; Joutel, A.; Nedergaard, M.; Smith, K.J.; Zlokovic, B.V.; Wardlaw, J.M. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc. Res. 2018, 114, 1462–1473.
- Arba, F.; Quinn, T.J.; Hankey, G.J.; Lees, K.R.; Wardlaw, J.M.; Ali, M.; Inzitari, D.; VISTA Collaboration. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. Int. J. Stroke 2016, 13, 47–56.
- Bokura, H.; Kobayashi, S.; Yamaguchi, S. Distinguishing silent lacunar infarction from enlarged Virchow-Robin spaces: A magnetic resonance imaging and pathological study. J. Neurol. 1998, 245, 116–122.
- Heier, L.A.; Bauer, C.J.; Schwartz, L.; Zimmerman, R.D.; Morgello, S.; Deck, M.D. Large Virchow-Robin spaces: MR-clinical correlation. Am. J. Neuroradiol. 1989, 10, 929–936.
- Doubal, F.N.; MacLullich, A.M.; Ferguson, K.J.; Dennis, M.S.; Wardlaw, J.M. Enlarged Perivascular Spaces on MRI Are a Feature of Cerebral Small Vessel Disease. Stroke 2010, 41, 450–454.
- Bown, C.W.; Carare, R.O.; Schrag, M.S.; Jefferson, A.L. Physiology and Clinical Relevance of Enlarged Perivascular Spaces in the Aging Brain. Neurology 2021, 98, 107–117.
- Troili, F.; Cipollini, V.; Moci, M.; Morena, E.; Palotai, M.; Rinaldi, V.; Romano, C.; Ristori, G.; Giubilei, F.; Salvetti, M.; et al. Perivascular Unit: This Must Be the Place. The Anatomical Crossroad Between the Immune, Vascular and Nervous System. Front. Neuroanat. 2020, 14, 17.
- Zhu, Y.-C.; Tzourio, C.; Soumaré, A.; Mazoyer, B.; Dufouil, C.; Chabriat, H. Severity of Dilated Virchow-Robin Spaces Is Associated with Age, Blood Pressure, and MRI Markers of Small Vessel Disease. Stroke 2010, 41, 2483–2490.
- Sweeney, M.D.; Montagne, A.; Sagare, A.P.; Nation, D.A.; Schneider, L.S.; Chui, H.C.; Harrington, M.G.; Pa, J.; Law, M.; Wang, D.J.J.; et al. Vascular dysfunction—The disregarded partner of Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 158–167.
- Loos, C.M.J.; Klarenbeek, P.; Van Oostenbrugge, R.J.; Staals, J. Association between Perivascular Spaces and Progression of White Matter Hyperintensities in Lacunar Stroke Patients. PLoS ONE 2015, 10, e0137323.
- Benjamin, P.; Trippier, S.; Lawrence, A.J.; Lambert, C.; Zeestraten, E.; Williams, O.; Patel, B.; Morris, R.G.; Barrick, T.R.; MacKinnon, A.D.; et al. Lacunar Infarcts, but Not Perivascular Spaces, Are Predictors of Cognitive Decline in Cerebral Small-Vessel Disease. Stroke 2018, 49, 586–593.
- Barisano, G.; Law, M.; Custer, R.M.; Toga, A.W.; Sepehrband, F. Perivascular Space Imaging at Ultrahigh Field MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2020, 29, 67–75.
- Yates, K.F.; Sweat, V.; Yau, P.L.; Turchiano, M.M.; Convit, A. Impact of Metabolic Syndrome on Cognition and Brain. Arter. Thromb. Vasc. Biol. 2012, 32, 2060–2067.
- Niazi, M.; Karaman, M.; Das, S.; Zhou, X.; Yushkevich, P.; Cai, K. Quantitative MRI of Perivascular Spaces at 3T for Early Diagnosis of Mild Cognitive Impairment. Am. J. Neuroradiol. 2018, 39, 1622–1628.
- Hayden, M.R. Overview and New Insights into the Metabolic Syndrome: Risk Factors and Emerging Variables in the Development of Type 2 Diabetes and Cerebrocardiovascular Disease. Medicina 2023, 59, 561.
- Gutierrez, J.; Rundek, T.; Ekind, M.; Sacco, R.; Wright, C. Perivascular Spaces Are Associated with Atherosclerosis: An Insight from the Northern Manhattan Study. Am. J. Neuroradiol. 2013, 34, 1711–1716.
- Passiak, B.S.; Liu, D.; Kresge, H.A.; Cambronero, F.E.; Pechman, K.R.; Osborn, K.E.; Gifford, K.A.; Hohman, T.J.; Schrag, M.S.; Davis, L.T.; et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology 2019, 92, e1309–e1321.
- Paradise, M.; Crawford, J.D.; Lam, B.C.; Wen, W.; Kochan, N.A.; Makkar, S.; Dawes, L.; Trollor, J.; Draper, B.; Brodaty, H.; et al. Association of Dilated Perivascular Spaces with Cognitive Decline and Incident Dementia. Neurology 2021, 96, e1501–e1511.
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438.
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497.
- Ali, S.; Malloci, M.; Safiedeen, Z.; Soleti, R.; Vergori, L.; Vidal-Gómez, X.; Besnard, C.; Dubois, S.; Le Lay, S.; Boursier, J.; et al. LPS-enriched small extracellular vesicles from metabolic syndrome patients trigger endothelial dysfunction by activation of TLR4. Metabolism 2021, 118, 154727.
- Qi, Y.; Lin, M.; Yang, Y.; Li, Y. Relationship of Visceral Adipose Tissue with Dilated Perivascular Spaces. Front. Neurosci. 2021, 14, 583557.
- Wu, D.; Yang, X.; Zhong, P.; Ye, X.; Li, C.; Liu, X. Insulin Resistance Is Independently Associated with Enlarged Perivascular Space in the Basal Ganglia in Nondiabetic Healthy Elderly Population. Am. J. Alzheimer’s Dis. Other Dementias. 2020, 35, 1533317520912126.
- Hayden, M.R. Brain Endothelial Cells Play a Central Role in the Development of Enlarged Perivascular Spaces in the Metabolic Syndrome. Medicina 2023, 59, 1124.
- United Nations Department of Economics and Social Affairs Populations Division Population Ageing and Sustainable Development. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 20 May 2023).
- Tucsek, Z.; Toth, P.; Tarantini, S.; Sosnowska, D.; Gautam, T.; Warrington, J.P.; Giles, C.B.; Wren, J.D.; Koller, A.; Ballabh, P.; et al. Aging Exacerbates Obesity-induced Cerebromicrovascular Rarefaction, Neurovascular Uncoupling, and Cognitive Decline in Mice. J. Gerontol. Ser. A 2014, 69, 1339–1352.
- Paavonsalo, S.; Hariharan, S.; Lackman, M.H.; Karaman, S. Capillary Rarefaction in Obesity and Metabolic Diseases—Organ-Specificity and Possible Mechanisms. Cells 2020, 9, 2683.
- Chantler, P.D.; Shrader, C.D.; Tabone, L.E.; D’Audiffret, A.C.; Huseynova, K.; Brooks, S.D.; Branyan, K.W.; Grogg, K.A.; Frisbee, J.C. Cerebral Cortical Microvascular Rarefaction in Metabolic Syndrome is Dependent on Insulin Resistance and Loss of Nitric Oxide Bioavailability. Microcirculation 2015, 22, 435–445.
- van Dinther, M.; Voorter, P.H.; Jansen, J.F.; Jones, E.A.; van Oostenbrugge, R.J.; Staals, J.; Backes, W.H. Assessment of microvascular rarefaction in human brain disorders using physiological magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2022, 42, 718–737.
- Bairamian, D.; Sha, S.; Rolhion, N.; Sokol, H.; Dorothée, G.; Lemere, C.A.; Krantic, S. Microbiota in neuroinflammation and synaptic dysfunction: A focus on Alzheimer’s disease. Mol. Neurodegener. 2022, 17, 19.
- Di Tommaso, N.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. The Gut–Vascular Barrier as a New Protagonist in Intestinal and Extraintestinal Diseases. Int. J. Mol. Sci. 2023, 24, 1470.
- Tilg, H.; Kaser, A. Gut microbiome, obesity, and metabolic dysfunction. J. Clin. Investig. 2011, 121, 2126–2132.
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783.
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412.
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541.
- Hayden, M.R. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J. Int. Med. Res. 2020, 48, 300060520939746.
- Christ, M.; Bauersachs, J.; Liebetrau, C.; Heck, M.; Günther, A.; Wehling, M. Glucose increases endothelial-dependent superoxide formation in coronary arteries by NAD (P) H oxidase activation: Attenuation by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor atorvastatin. Diabetes 2002, 51, 2648–2652.
More
Information
Subjects:
Neurosciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
669
Revisions:
3 times
(View History)
Update Date:
25 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




