Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sanaa Bardaweel | -- | 7447 | 2023-07-24 14:44:54 | | | |
| 2 | Camila Xu | Meta information modification | 7447 | 2023-07-25 03:31:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Almutairi, S.; Kalloush, H.M.; Manoon, N.A.; Bardaweel, S.K. Matrix Metalloproteinases Inhibitors in Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/47183 (accessed on 09 February 2026).
Almutairi S, Kalloush HM, Manoon NA, Bardaweel SK. Matrix Metalloproteinases Inhibitors in Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/47183. Accessed February 09, 2026.
Almutairi, Shriefa, Hanin Moh’d Kalloush, Nour A. Manoon, Sanaa K. Bardaweel. "Matrix Metalloproteinases Inhibitors in Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/47183 (accessed February 09, 2026).
Almutairi, S., Kalloush, H.M., Manoon, N.A., & Bardaweel, S.K. (2023, July 24). Matrix Metalloproteinases Inhibitors in Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/47183
Almutairi, Shriefa, et al. "Matrix Metalloproteinases Inhibitors in Cancer Treatment." Encyclopedia. Web. 24 July, 2023.
Copy Citation
Matrix metalloproteinases (MMPs) are a member of the enzyme group that is capable of protein degradation. MMPs are recognized as metalloproteinases because they require either zinc and calcium to perform their functions.
matrix metalloproteinases (MMPs)
inhibitors
anticancer activity
extra-cellular matrix remodeling
1. Introduction
Matrix metalloproteinases (MMPs) are a member of the enzyme group that is capable of protein degradation [1]. MMPs are recognized as metalloproteinases because they require either zinc and calcium to perform their functions [2]. The catalytic activity of MMPs was first identified in 1962 as collagen proteolytic enzymes [3]. Since that moment, the research field of MMPs has undergone extensive advancement. In humans, 23 structurally related MMPs are known. Members of this family belong to the metzincin superfamily of proteases that can mainly degrade or cleave many components of the extra-cellular matrix (ECM) during tissue remodeling [4]. The ECM is an intricate network of macromolecules that provides biochemical and structural assistance to neighboring cells by regulating physiological activities such as cell anchorage, migration, proliferation, differentiation, and metabolism [5][6]. ECM degradation is also associated with several pathological events, including cancer [7][8]. Proteoglycans, collagens, fibronectin, elastin, and laminins are examples of the major glycoproteins of ECM [9].
MMPs can be categorized into six groups based on substrate specificity and structure differences (Table 1): collagenases, gelatinases, stromelysins, matrilysins, metalloelastase, and membrane-type MMPs [10]. The MMPs’ substrates are different, in that some are considered constituents of the ECM, whereas others are constituents of the basement membrane [10][11]. Even though each MMP has its own set of substrates, substantial overlap in substrate specificities between MMPs is common (Table 1) [5]. For example, MMP-1, which belongs to the collagenase subcategory, is responsible for cleaving native fibrillar collagens and more efficiently cleaving collagen of type III [11], but it can degrade laminin as well, which is mainly cleaved by MMP-3 and MMP-7 [10]. In addition, the favored substrate of MMP-2 is gelatin, which can also be degraded by MMP-3, -7, -12, or -14 [10]. Moreover, MMP-12, -2, and -9 overlap in their substrate specificity and elastin degradation ability [12].
Table 1. Classification of the main members of the MMP family and their group of substrates [10].
| Traditional Classification (Common Name) |
Numerical Classification | Group of Substrates |
|---|---|---|
| Collagenases | ||
| Collagenase-1 | MMP-1 | Collagen (I, II, III, VII, VIII, X), casein, entactin, laminin, pro-MMP-1, -2, -9, and serpins |
| Collagenase-2 | MMP-8 | Collagen (I–III, V, VII, VIII, X), gelatin, aggrecan, fibronectin |
| Collagenase-3 | MMP-13 | Gelatin, collagen (IV–VI, X), elastin, fibronectin |
| Gelatinases | ||
| Gelatinase A | MMP-2 | Gelatin, collagens (IV, V, VII, X, XIV), elastin, fibrillin, osteonectin |
| Gelatinase B | MMP-9 | Gelatin, collagens (IV, V, VII, X, XIV), elastin, fibrillin, osteonectin |
| Stromelysins | ||
| Stromelysin-1 | MMP-3 | Laminin, aggrecan, gelatin, fibronectin |
| Stromelysin-2 | MMP-10 | Collagens (III–V), gelatin, casein, aggrecan, elastin, MMP-1,8 |
| Stromelysin-3 | MMP-11 | Fibronectin, laminin, aggrecan, gelatin |
| Matrilysins | ||
| Matrilysin-1 | MMP-7 | Collagen (IV–X), fibronectin, laminin, gelatin, aggrecan, pro-MMP-9 |
| Matrilysin-2 | MMP-26 | Gelatin, collagen IV, pro-MMP-9 |
| Macrophage Metalloelastase | ||
| Macrophage Metalloelastase | MMP-12 | Elastin, gelatin, collagen I, IV, fibronectin, laminin, vitronectin, proteoglycan |
| Membrane-type MMPs (MT-MM) | ||
| MT-MMP-1 | MMP-14 | Collagen (I, II, III), gelatin, fibronectin, laminin aggrecan, tenascin |
| MT-MMP-2 | MMP-15 | Fibronectin, laminin, aggrecan, perlecan |
| MT-MMP-3 | MMP-16 | Collagen III, gelatin, casein |
| MT-MMP-4 | MMP-17 | Fibrinogen, TNF precursor |
| MT-MMP-5 | MMP-24 | Proteoglycans |
MMPs are secreted by multiple connective tissues and pro-inflammatory cells such as fibroblasts, osteoblasts, endothelial cells, and macrophages [13]. Major sources of MMPs are dermal fibroblasts and leukocytes, especially for MMP-2, whereas MMP-1, -3, and -14 are highly expressed by platelets [13][14]. Also, MMP-1, -2, -3, and -7 are localized in endothelial cells and vascular smooth muscle cells (VSMCs), and MMP-12 is localized in fibroblasts of the human great saphenous vein, in addition to VSMCs and inflammatory macrophages [13][15].
MMPs are mostly expressed in an inactive form (pro-enzyme), which is subsequently activated by other proteolytic enzymes. MMPs are naturally regulated at any of the following three known levels: the transcription level (mRNA), the pro-enzyme activation level, and inhibition of the active forms by various tissue inhibitors of MMPs (TIMPs). In normal physiological conditions, MMPs form a 1:1 complex with endogenous tissue inhibitors of MMPs (TIMPs), which selectively modulate MMPs’ function [16]. On the other hand, under pathological conditions, this equilibrium is shifted towards increased MMP activity, leading to abnormal ECM metabolism and disease conditions [5].
Despite the functional differences between MMPs, they are highly comparable in their structural domain [17]. The basic MMP structure consists of four main domains: an N-terminal pro-peptide domain, a catalytic domain, a linker region, and a C-terminal hemopexin-like domain (Figure 1) [18][19]. An exception to this highly conserved structure is the MMP-7 domain structure, which does not include the hinge region or hemopexin domain [10]. The pro-peptide domain is composed of around 80 amino acids in length and contains a cysteine switch motif that is responsible for the maintenance of enzyme latency. Meanwhile, the hemopexin-like domain has a C-terminal that is composed of approximately 200 amino acids and is connected to the catalytic region by a flexible proline-rich hinge region. The structure of the hemopexin-like domain has a fundamental role in several activities involving substrate specificity and activation, inhibition, anchoring, and dimerization [20][21].
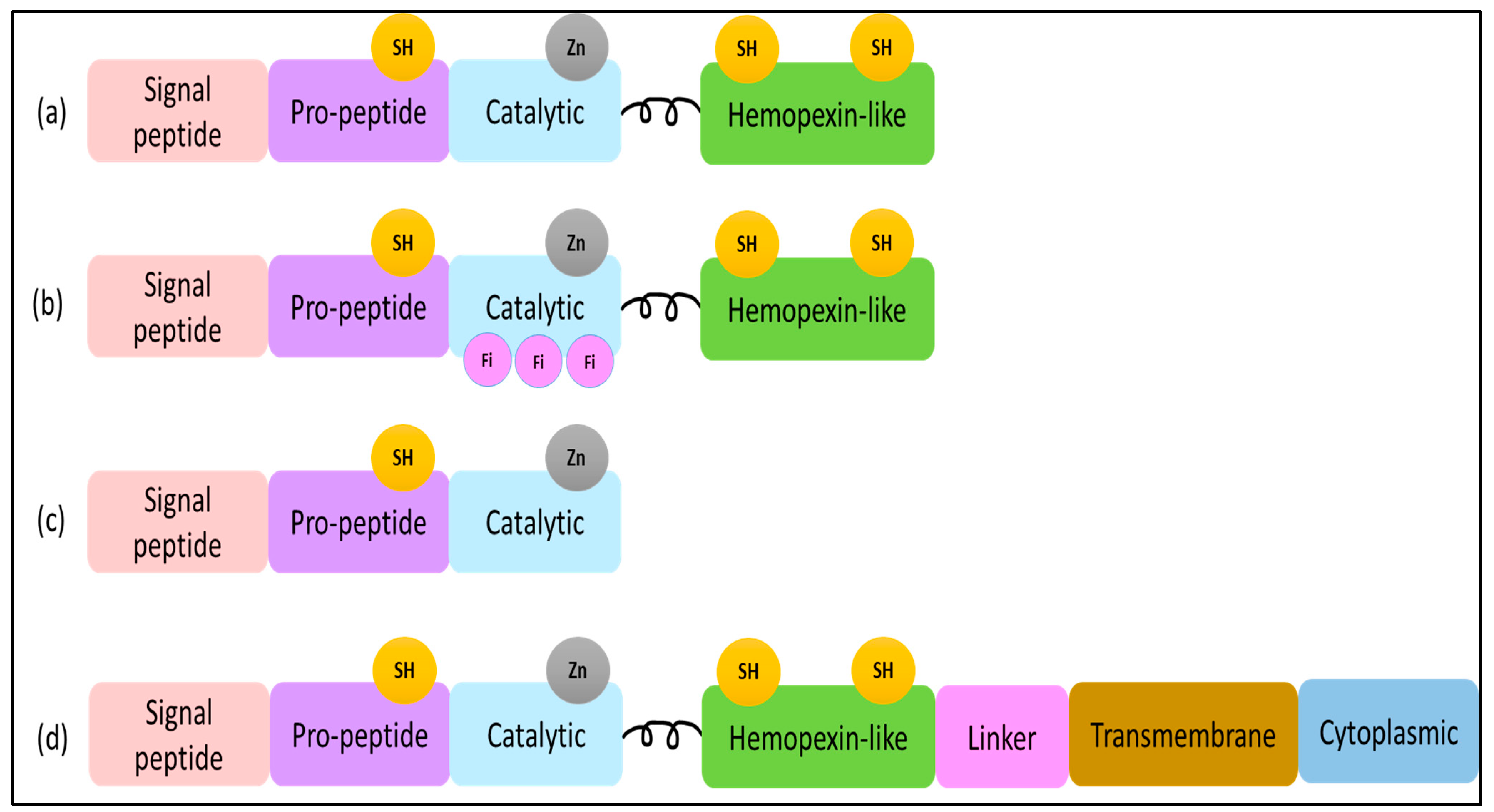
Figure 1. (a) MMP-1, -3, -12 structure domains. The minimal domains of MMPs are an amino-terminal signal peptide (SP), a pro-peptide, and a catalytic domain. The SP domain shows a role in guiding MMP-12 to the endoplasmic reticulum, and the pro-protein domain contains the thiol (SH) group that maintains MMP as inactive zymogens by interacting with zinc. (b) MMP-2 structure domains contain three fibronectins within the catalytic domain. (c) MMP-7 structure domains. (d) MMP-14 structure domains contain a linker, cytoplasmic domain, and transmembrane domain.
The catalytic domain of MMPs is composed of around 170 amino acids and includes a zinc-binding site and methionine residue or “Met-turn”, which chemically supports zinc-binding activity, as well as a loop called a Ω-loop that surrounds an S1′ pocket region [19][22][23]. The protease’s catalytic part is compromised of three α-helices as well as a profoundly twisted five-stranded β-sheet. Three His residues, His199, His203, and His209, and a molecule of water in the active site cleft bind zinc in the amino acid sequence HELGHXXGXXH [24]. The active site of MMPs involves two discrete regions: a groove in the protein surface and an S1′ pocket [17]. The active site is known to be highly conserved among MMPs except for the S1′ pocket region, which varies slightly among MMPs in sequence, shape, and depth [17][25]. The S1′ pocket is accessed via a tunnel formed by different amino acids and is bounded by the Ω-loop, which is also diverse among MMPs in length, flexibility, and amino acid residues [17]. An additional feature of the S1′ pocket is the diversity of certain residues in the pocket. For example, Arg 218 in MMP-1 is replaced with Leu in MMP-2, -3, -12, and -14 and with Tyr in MMP-7 [17]. The S1′ pockets’ differences among MMPs have been relied upon to determine the selectivity of inhibitors.
As agents capable of remodeling ECM, MMPs have been linked to a wide variety of biological processes, such as cell transformation and carcinogenesis. It is now well-known that each MMP has a different role in a specific disease, and the role may change from unfavorable to favorable during the progression of disease stages. Interestingly, their role in the development of cancer may include various mechanisms. Originally, MMPs were thought to only associate with metastasis facilitation by ECM remodeling, thereby allowing for the invasion of tumor cells into blood and lymphatic vessels [18]. However, it is now confirmed that they support most tumorigenic processes, including proliferation, invasion, angiogenesis, and metastasis [10]. Furthermore, MMPs were reported to be overexpressed in various types of solid tumors, such as breast, prostate, liver, and lung cancers [18]. Likewise, several reports have shown the apparent expression of MMPs in non-solid tumors [18]. Nonetheless, MMPs’ expression and their functional significance in solid and non-solid tumors are yet to be fully clarified [26].
Considering their important roles in virtually all major stages of tumor progression, various MMPs have become attractive targets for diagnosis and therapeutics. The first generation of MMP inhibitors (MMPIs) was peptide-based. It was designed by employing MMP substrates as a pharmacophore model [5]. However, due to their molecular flexibility and poor bioavailability, the potency of these small peptides as drugs was substantially limited. Modifications of the natural peptide resulted in an ideal substitute termed “peptidomimetics” [27], which was further modified to hydroxamate-based peptidomimetics [5]. These compounds inhibit MMPs by coordinating with the catalytic Zn 2+ of MMPs via the zinc-binding group (ZBG). Batimastat and Marimastat represent pioneering hydroxamate-based MMPIs, displaying excellent anticancer activity in preclinical studies [5][28]. However, the results of clinical trials were not adequate. Subsequently, a series of hydroxamate derivatives was developed by applying structural studies such as X-ray crystallography and computer simulation analysis, which aid in improving their selectivity as MMPIs [5].
The second generation of MMPIs has different ZBG within both peptidomimetic and non-peptidomimetic motifs and include thiol-based, carboxylic-acid-based, pyrimidine-based, and hydroxypyrone-based MMPIs [5]. Although these inhibitors have considerable in vitro antitumor activity, early clinical trials demonstrated that such compounds have low oral bioavailability in addition to safety concerns [5]. Provided that these small molecular drugs cannot target specific active sites, the failure of their clinical trials was foreseeable.
The principal idea of MMPI design works via targeting the Zn2+ of the enzyme active site that is highly conserved and shares common features among most MMPs [17]. Accordingly, the non-specificity of MMPIs has been a fundamental reason for the failure of their clinical use [17]. With a further understanding of specific MMPs’ function and role in different tumors, substantial work has been put into inhibiting MMPs’ activity more selectively by targeting the catalytic site in addition to the molecular structures surrounding it [17].
Thanks to the evolution of X-ray crystallography, NMR analysis, and homology modeling studies, it has been possible to distinguish the active site of various MMPs and, consequently, to develop more selective, second-generation MMP inhibitors [11]. MMP inhibitors, often designed to address and accommodate the S1′ pocket flexibility [29], have the required selectivity and lack high toxicity [30].
Thus, a series of MMPIs with more selectivity has been generated [29][31]. Even with the accessibility of structural information, poor selectivity remains a challenge for the accomplishment of MMP inhibitors in clinical trials. Likewise, the intrinsic and ligand-induced flexibility of the active site makes its analysis more challenging. Here discusses the recent development of MMP inhibitors and their evaluation as potential anticancer agents, particularly MMP-1, -2, -3, -7, -12, and -14, comprising one MMP from each subfamily. Databases were searched for the MMP inhibitors that were designed and synthesized in the period between 2013 and 2023.
2. Matrix Metalloprotease-1 (MMP-1)
2.1. Function and Localization
MMP-1 is an identifiable member of the MMP family. MMP-1 is responsible for breaking down interstitial collagen (types I, II, III, VII, and X); hence, it is also referred to as collagenase 1 [10][32]. In addition, it breaks down other substrates, such as gelatin, laminin, complement (C1), insulin-growth-factor-binding proteins, interleukin (IL-1), and tumor necrosis factor (TNF) [33][34][35][36]. MMP-1 also activates other MMPs, including MMP-9 and MMP-2 [37]. It plays a vital role in various biological processes, including angiogenesis, embryogenesis, morphogenesis, and the repair of wounds [38].
The gene encoding MMP-1 is localized to chromosome 11q22.3 [39]. MMP-1 is produced and released as an inactive proenzyme with 469 amino acids and a molecular weight of approximately 55 kilodaltons [40]. MMP-1 is released by various cell types and tissues, including endothelium, macrophages, fibroblasts, and platelets [40][41][42]. Furthermore, MMP-1 is found intracellularly, and evidence suggests that it might mediate signaling events that determine the cell’s function and phenotype [43]. MMP-1 has also been shown to be intracellular in several cells, including the vascular endothelium [44].
2.2. Role in Cancer
MMP-1 was linked to several diseases, including lung emphysema, arthritis, and especially cancer [3][4][5]. Recent studies suggest that MMP-1 promotes cancer cell invasion and migration and significantly correlates with malignancies’ unfavorable outcomes [45][46]. Increased MMP-1 expression has been observed in oral, bladder, gastric, and breast cancers and was linked to a poor prognosis for these diseases [47][48][49][50]. Research findings suggest that MMP-1 encourages the proliferation and invasion of breast cancer cells by cleaving the same Arg-Ser bond as thrombin do [51]. This eventually leads to activating the protease-activated receptor (PAR), which assists in the proliferation and invasion of breast cancer cells [51]. Moreover, Wang et al. found that MMP-1 has been linked to the promotion of malignant behavior in colorectal cancer via epithelial–mesenchymal transition EMT and the Akt signaling pathway [52]. Another study showed that increased MMP-1 and vascular endothelial growth factor-C (VEGF-C) expression was associated with an advanced tumor stage and a poor prognosis in patients with esophageal squamous cell carcinoma [53]. In addition, overexpression of MMP-1 was associated with the invasiveness of primary nodular melanoma [54]. Therefore, these findings indicate that a drug modifying MMP expression or activity might be used in cancer treatment.
2.3. Structure of the Catalytic Domain
The primary structural difference between MMP-1 and other MMP enzymes is the size and shape of S1’s pocket. The S1′ pocket of MMP-1 is short and narrow compared to other enzymes in the MMPs family [17]. MMP-1 inhibitors were developed by taking advantage of the presence of a metal ion inside the binding pocket, aside from the unique characteristics of the S1′ pocket [17][38]. Another study conducted by Gimeno et al. suggested that some inhibitors occupy the S1’ pocket interacting with the Ω-loop by protein–ligand docking of MMP-1 inhibitors of various sizes [17].
2.4. MMP-1 Inhibitors
Several synthetic chemical inhibitors have been created utilizing combinatorial chemistry and structure-based design [38]. The earliest designed compounds were broad-spectrum inhibitors targeting a wide range of MMPs. Thus, advanced clinical trials with them were unsuccessful due to their limited oral bioavailability, diminished in vivo efficacy, and adverse musculoskeletal effects [38]. Musculoskeletal syndrome (MSS) was the most prominent side effect with an unknown exact origin [55]. However, MMP-1 inhibition was first thought to be a contributing factor. As a result, MMP inhibitors that spare MMP-1 have gained more attention [55].
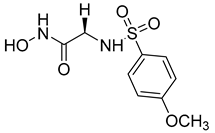
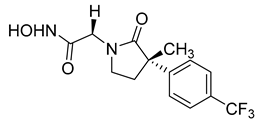
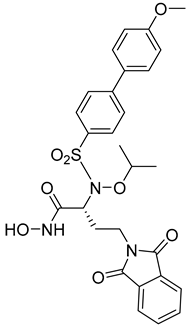
Over the past decade, new MMP-1 inhibitors have been developed using structure-based design techniques such as methyl rosmarinate derivatives. A series of methyl rosmarinate derivatives was assessed as selective MMP-1 inhibitors, and compound 1 was the most potent candidate with an IC50 of 0.4 µM (Table 2) [56]. Moreover, Umedera et al. have investigated new MMP-1 inhibitors using a structure–activity relationship (SAR) transfer method based on kinesin-like protein 11 (KIF11) analogs [57]. Among the candidate compounds generated, compound 2 was the most selective inhibitor with an IC50 of 0.034 µM (Table 2) [57]. Additionally, a series of thiazole derivatives was examined as MMP anti-neoplastic agents, and compound 3 was the most promising agent. It exhibited multiple MMP inhibition (MMP-1, MMP-8, and MMP-9). with an MMP-1 inhibitory activity of 10% ((at 1.3 µM concentration) (Table 2)). Moreover, it was the most effective and selective anticancer agent against the MCF-7 cell line, with an IC50 of 20 µg/mL [58]. An integrated structure-based approach was also used to design a new potent inhibitor utilizing an arylsulfonamide scaffold. Nevertheless, all the designed compounds were non-selective, and the most potent MMP-1 inhibitor was compound 4, which exhibited a ki value of 77 nM (Table 2) [59]. A SAR matrix approach was utilized by Asawa et al. to develop new inhibitory compounds against MMP-1. They also carried out an experimental validation for the predicted inhibitory compounds, and the most potent inhibitor was compound 5, displaying an IC50 of 0.18 µM (Table 2) [60].
Table 2. Chemical structures and potency of synthetic MMP-1 inhibitors.
3. Matrix Metalloprotease-2 (MMP-2)
3.1. Function and Localization
The 72KDa MMP-2 enzyme is quite remarkable because it can act on a wide variety of substances including gelatin, laminin, fibronectin, elastin, and collagen IV [61]. Because of this, it was previously classified as part of the collagenase IV subfamily [61]. Once MMP-5 was discovered, scientists decided to remove its numbering, because they found it was identical to MMP-2 in terms of its genetic composition [62][63].
MMP-2 plays a major role in the proteolytic decomposition of ECM components [64] and basement membrane. Therefore, MMP-2 inhibition tends to be a target for tissue repair and cancer metastasis [65][66][67][68]. The gene encoded MMP-2, which is localized on chromosome 16q13 [69].
3.2. Role in Cancer
Tumor cells can produce MMP-2 or stimulate its production in surrounding cells. This production creates an environment that helps tumor cells grow and spread [70]. Also, MMP-2’s ability to degrade the ECM components enhances cancer cells’ migration through tissues [70]. Moreover, MMP-2 can affect signaling pathways in cancer cells by promoting tumor cell growth, enhancing resistance to apoptosis, and improving angiogenesis [71]. MMP-2 is overexpressed in many types of cancer, including bladder [72], breast [73], bronchopulmonary [74], cervical [75], colon, glioma [76], laryngeal [77], lung, melanoma, myeloma, esophagus, ovary [78], pancreas, prostate, skin, and stomach cancer [64][65][71].
3.3. Structure of the Catalytic Domain
MMP-2 stands out from other MMPs in that it has three head-to-tail repeats within the catalytic domain [71] and contiguous fibronectin type-II-like (FN-II) motifs [64][79]. Those motifs are bound to the extra 175 amino acid residues [64] and are involved in destroying and remodeling ECM components [64][79]. Scientists believe that targeting both the active site and the collagen-binding FN-II domains will show promising effects in inhibiting the MMP-2 role in cancer [80].
Studies have revealed the presence of two hydrophobic domains designated as the S1′ and S1 pockets, in addition to the zinc2+ ion pocket [64]. Notably, the S1′ pocket is considered the predominant pocket of MMP-2 and is characterized by its relatively narrow and deep shape compared to other MMP subtypes [17]. Current research efforts have led to the identification of various structural features that are common to most MMP-2 inhibitors, including a zinc-binding group, a non-zinc-binding group, a hydrogen-bond-forming functional group, and one or more hydrophobic side chains that interact with the S1′ and S1 pockets [64]. Moreover, the hemopexin-like domain (HDM) of MMP-2 has a four-bladed propeller around a central cavity occupied by a Ca2+ ion [79]. HDM is linked to the catalytic domain through a flexible hinge region. According to a study [81], HDM plays a role in allowing MMP-2 to interact with integrin αVβ3, a protein, in melanoma cells and endothelial cells, even without the presence of the Arg-Gly-Asp (RGD) motif that is typically involved in such interactions [82].
MMP-2 is stimulated as a reaction to tissue damage, wound healing, and immune response [83][84]. Its production is regulated by numerous factors, such as growth factors, cytokines, and hormones [85]. Moreover, it can be activated in response to different physiological and pathological conditions [33]. However, excessive activation of MMP-2 has been linked to various health problems such as cancer and arthritis, whereby it promotes the growth and spread of tumors and contributes to tissue destruction and joint damage [86].
3.4. MMP-2 Inhibitors
In recent years, computational chemistry techniques along with machine-learning and artificial intelligence approaches [87] have been extensively explored for designing effective MMP-2 inhibitors [65][66][67][88][89][90][91] with particular binding patterns. This has enabled the development of numerous small-molecule MMP-2 inhibitors that have shown promising preclinical results [25][68][88][92][93][94][95].
Different synthesis techniques have executed a major impact on the development of MMP-2 inhibitors through the modification and optimization of lead compounds [65][66][67][88][96]. The synergy between computational chemistry and synthesis techniques has allowed researchers in the last decade to develop new small-molecule MMP-2 inhibitors, as shown in Table 3, specifically designed to target MMP-2 [25][29][65][66][88][96]. These inhibitors have been demonstrated to exhibit antitumor effects in both in vitro and in vivo [65][66][88][96].
About 72 molecules with different scaffolds were virtually screened (compound 6) using Regression-dependent quantitative structure–activity relationship (QSAR) strategies. The aim was to determine the structural features that are needed to find a suitable MMP-2 inhibitor [89]. Turra et al. utilized 4D-QSAR and pharmacophore modeling to study a group of 40 different chemical β-N-biaryl ether sulfonamide hydroxamate derivatives (compound 7). Their approach was used to predict the inhibitory activity of the compounds against MMP-2 [97].
A scaffold modification technique was used by Qiu et al. to design and synthesize a new set of MMP-2 inhibitors. The synthesized compounds were evaluated against several cancer cell lines, including A549, HepG2, MCF-7, and Hela cells. Among them, compound 8 showed the highest potency against MMP-2. Also, compound 8 shows almost no cytotoxicity against 293T kidney cells [66].
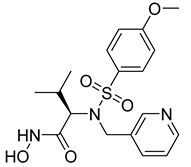
A series of sulfonamide’s derivatives, dihydropyrazole sulfonamide derivatives that contain 2- hydroxy phenyl moiety (compound 9), were synthesized by Wang et al. as MMP-2 inhibitors with anti-cancer activity. Bioactive assays on four cancer cell lines, A549, MCF-7, Hela, and HepG2 cells, were conducted, revealing a potential antagonistic effect on MMP-2. Also, molecular docking SARs were performed on the derivatives and demonstrated that compound 10 exhibits the most potent inhibition potentials [96].
The same team reported the synthesis of a new series of sulfonamide derivatives (compound 11) that contain dihydropyrazole moieties [98]. The reported compounds were found to have a dual effect on MMP-2 and -9. After testing on the same cell lines, the most active compound was compound 11 compared to the control positive compound [98].
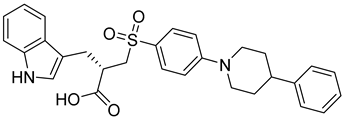
Halder et al. reported the design and the biological evaluation of compound 12, which acts on dual targets and shows selective activity toward MMP-2/HDAC-8 among their subtypes over A549 cell line lung carcinoma [99]. In addition to this, Ammazzalors et al. reported the synthesis as well as the molecular and biological evaluation of simple derivatives of hydroxyquinoline and hydroxynaphtyridine (compound 13) to be selective inhibitors toward MMP-2 and -13. These compounds are reported to have much fewer side effects because of the zinc-binding group in their structures [91]. Another reported compound (compound 14), which contains salicylaldehyde, was synthesized. This compound was found to exhibit dual inhibitory activity against MMP-2 and -8 [100]. The synthesis was achieved by cross-condensation reactions, and the biological evaluation was performed against MCF-7, Hela, and HepG2 cell lines [100].
Another dual inhibitory effect of the gelatinase enzymes was reported by Chen. et al., who designed and synthesized 8-hydroxyquinoline derivatives (compound 15) [65]. The most active compounds showed a good inhibitory effect toward MMP-2 and -9 and possessed potent anti-proliferative activity against different cancer cell lines, including MCF-7, PC-3, HL-60, K562, KG1, and A549 [65]. In addition to this, the compounds also had anti-invasive and anti-angiogenesis activity on A549 lung cancer cells [65].
Compound 16 was designed and synthesized with microwave assistance, mimicking the non-hydroxamate inhibitors [67]. The synthesized compounds were reported by Albelwi et al. to possess antagonistic activity toward MMP-2/9 [67].
The microwave-assisted technique was also used in triazoles as reported by Aouad et al. via various sulfonamide bridges in compound 17 [101]. The synthesized compounds were reported to have dual action on other targets including MMP-2 [101].
According to Kreituss et al., a series of (aryl triazolyl) methyl aziridines compounds was synthesized and evaluated for their selective inhibition of MMP-2 [102]. Compound 18 is made up of a hydrophilic aziridine, a lipophilic part, and a triazole fragment that connects the two [102]. Among all the synthesized inhibitors that were tested against melanoma and PT-67 fibroblast cell lines, compounds 19 and 20 showed the best inhibition of MMP-2, with a concentration of 20 and 10 µM, respectively [102].
Laghezza et al. reported a new non-zinc-binding MMP-2 inhibitor [103]. This was achieved by checking previously known compounds using virtual screening along with SAR for benzimidazole structure, resulting in compound 21. Simple molecules were synthesized, and their activity was measured [103]. Molecular dynamics were checked to confirm the design of more selective MMP-2 inhibitors [103].
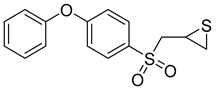
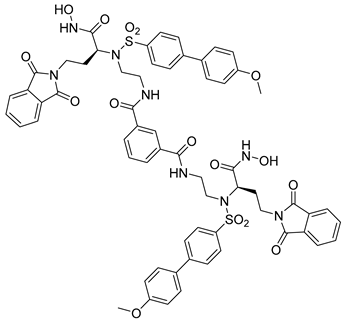
Researchers have attempted to develop gelatinase inhibitors that can cross the blood–brain barrier. Iproteos company researchers in Spain developed new gelatinase inhibitors, compounds 22 and 23, that were capable of inhibiting MMP-2 and MMP-9 with high potency and selectivity among different MMPs [11][104].
Table 3. Chemical structures and potencies of synthetic MMP-2 inhibitors.
| Compound | Potency | Chemical Structure | Reference |
|---|---|---|---|
| 6 | - |  |
[89] |
| 7 | pIC50 (−logIC50) = 8.60“IC50, nM” | 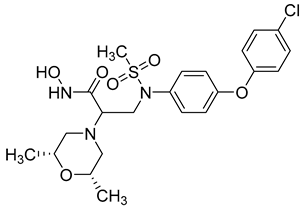 |
[97] |
| 8 | IC50 = 0.38 µM | 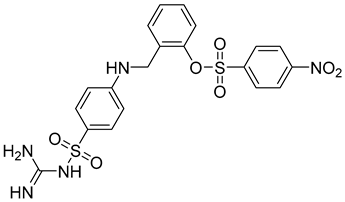 |
[66] |
| 9 | IC50 < 5 µM | 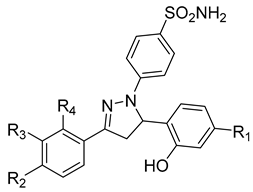 |
[96] |
| 10 | IC50 = 0.33 µM | 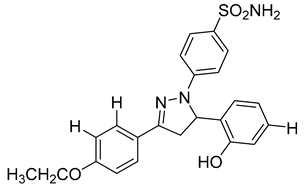 |
[96] |
| 11 | IC50 = 0.21 µM | 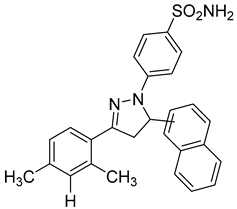 |
[98] |
| 12 | IC50 = 6.40 µM |  R1 = 4Br/R2= CH2C6H4 4-Nitrobenzyl |
[99] |
| 13 | IC50 = 7.4 ± 0.8 µM |  |
[91] |
| 14 | IC50 = 2.80 µM | 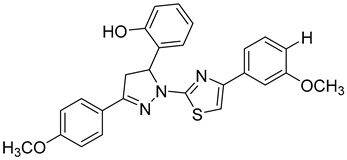 |
[100] |
| 15 | IC50 = 0.70 ± 0.02 µM | 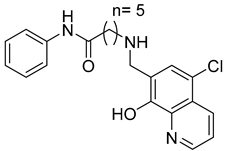 |
[65] |
| 16 | IC50 = 0.376 µM | 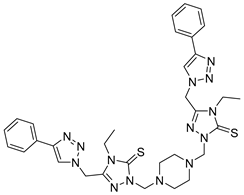 |
[67] |
| 17 | IC50 = 5.6 nM | 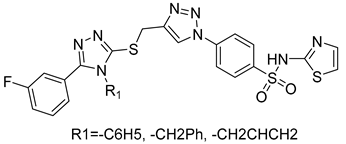 |
[101] |
| 18 | - | 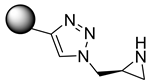 |
[102] |
| 19 | Percent of inhibition = 73.3% | 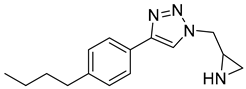 |
[102] |
| 20 | Percent of inhibition = 75.2% | 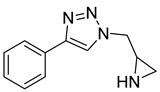 |
[102] |
| 21 | IC50 = 31 ± 5 µM | 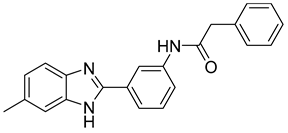 |
[103] |
| 22 | IC50 = 21 nM | 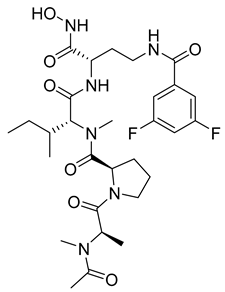 |
[104] |
| 23 | IC50 < 1nM | 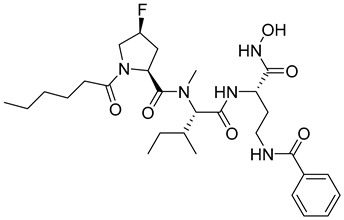 |
[104] |
4. Matrix Metalloprotease-3 (MMP-3)
4.1. Function and Localization
MMP-3 is a recognized endopeptidase that belongs to the stromelysins subfamily [105]. The MMP-3 protein comprises 475–478 amino acids in mammals with an approximated molecular weight of 57 kilodaltons [40][106]. The MMP-3 gene is localized in chromosome 11q22.3 [107]. Regarding MMP-3 subcellular localization, MMP-3 has been found in the nucleus and might be involved in the transcriptional or apoptosis processes in some cells, such as hepatocellular carcinoma, chondrocytes, and myofibroblast [108][109][110]. Furthermore, it is an exocrine protein typically secreted via the exocytosis process and extracellular vesicles [111]. Numerous cell types produce MMP-3, including chondrocytes, endothelial cells, macrophages, and fibroblasts [112][113][114].
MMP-3 is termed stromelysin-1 owing to its hydrolytic ability and stromal origin [105]. MMP-3 has distinct structural features that facilitate its role in hydrolyzing numerous extracellular matrix proteins (EMP), including collagen types II and III, laminin, fibronectin, and proteoglycans [111]. Further, it can dissolve adherent junctions through its activity on a cell-surface protein called E-cadherin. MMP-3 also activates tissue-remodeling MMPs, namely, pro-MMP-1 and pro-MMP-9 [115][116]. Additionally, MMP-3 is an efficient plasminogen activator via its interaction with tPA (tissue-type plasminogen activator) [117]. The broad substrate specificity of MMP-3 facilitates its contribution to a range of pathological disorders, including malignant, neurodegenerative (e.g., Alzheimer’s), and inflammatory joint diseases (e.g., rheumatoid arthritis) [118][119].
4.2. Role in Cancer
Concerning its role in malignancy, studies have suggested that MMP-3 has both tumor-promoting and tumor-inhibiting properties, depending on the substrates it interacts with [120]. For instance, MMP-3 catalyzes the formation of angiogenesis-inhibiting factors through the breakdown of plasminogen and type VIII collagen [121][122]. These factors will restrict tumor progression; MMP-3 demonstrates tumor suppression properties in such a case. While modulating growth factors (e.g., transforming growth factor), MMP-3 stimulates the proliferation of cancer cells and tumor progression [120][123]. Nevertheless, MMP-3 is more commonly involved in promoting tumor development than suppressing it [120].
MMP-3 is considered a prognostic factor in several types of cancers. Camacho et al. [117] reported an increased expression of MMP-3 in breast cancer tissue compared to normal breast tissue, which might be associated with breast cancer development. Interestingly, a study by Cai et al. found an upregulated expression of MMP-3 in oral squamous cell carcinoma, the most common oral cancer [124]. Moreover, recent research implies that MMP-3 has a role in prostate cancer progression to bone metastasis [125]. Chen et al. have also demonstrated the crucial role of ubiquitin-specific peptidase 15 and MMP3 in non-small-cell lung cancer development and prognosis [126]. Therefore, MMP-3 is a promising target for the generation of new antineoplastic drugs.
4.3. Structure of the Catalytic Domain
The hydrophobic S1′ pocket of MMP-3 has a tunnel-like shape, as revealed by X-ray crystallography [127]. Compared to other MMPs, this MMP3’s pocket is classified as deep and large, likely due to the length of the Ω-loop sequence that affects MMP-3 conformations [17]. Leu229’s side chain and the side chains of Leu226 and Thr227 in specific conformations restrict substrates’ entry into the bottom of the S1′ pocket of MMP-3 [17]. These unique characteristics of the S1′ pocket account for the distinct substrate specificity of this enzyme [37].
4.4. MMP-3 Inhibitors
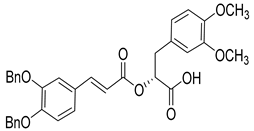
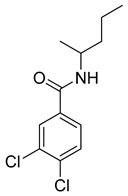
Several anticancer agents have been designed to target the catalytic hydrophobic pocket of MMP-3. One of the earliest MMP-3 inhibitors features the hydroxamate-based zinc-binding scaffold 24 with a Ki of 43 nM (Table 4). Unfortunately, this scaffold failed in clinical trials due to insufficient oral bioavailability and cartilage matrix adverse effects [38]. However, such inhibitors were utilized as a model for developing selective second-generation hydroxamate-based inhibitors [38]. On the other hand, Pavlovsky et al. have designed MMP-3 inhibitors with a carboxylic acid moiety that displays a low Ki value of 19 nM for the S enantiomer 25. Another study was conducted by Brown et al., which disclosed a potent thiirene-containing covalent-bond-forming inhibitor 26 with a Ki of 15 nM [38]. A series of 2-phthalimidinoglutaric-acid-based analogs were designed in silico as MMPs inhibitors. Among the designed inhibitors, compound 27 demonstrated auspicious predicted activity against MMP-3, with an approximated IC50 of 1 nM [128]. Recently, research findings have shed light on a series of sulfonamide-containing dehydroabietic-acid (DHAA)-based derivatives as MMPs inhibitors. Surprisingly, compound 28 exhibited selectivity towards MMP-3, with an IC50 of 0.4 µM [129].
Table 4. Chemical structures and potencies of synthetic MMP-3 inhibitors.
5. Matrix Metalloprotease-7 (MMP-7)
5.1. Function and Localization
MMP-7 (matrilysin-1) is a small secreted proteolytic enzyme that functions as zinc and calcium endopeptidase [130]. The gene encoding MMP-7 belongs to the gene cluster on chromosome 11 at q21 and q22 [131][132]. The complete coding sequence of MMP-7 is 1094 bp long and encodes 267 amino acids [133].
MMP-7 is produced as a pro-enzyme with a molecular weight of 28-kDa [134]. The full length of MMP-7 consists of two structural domains: the propeptide domain and the catalytic domain [135]. The activation process of pro-MMP-7 involves a proteolytic removal of the propeptide domain (9-kDa) that is responsible for the latency of the enzyme [136]. MMP-7 is the smallest member of the MMP family, because it lacks a further functional region called the hemopexin-like domain [137]. The hemopexin-like domain is responsible for substrate specificity [1][137]. Thus, the absence of this domain conforms with MMP-7 activities against a wide range of ECM components such as collagen (IV–X), fibronectin, laminin, and gelatin [138].
In addition, MMP-7 takes a part in the degradation of some non-ECM proteins such as pro-α-defensin, pro-tumor necrosis factor (TNF)-α, and E-cadherin [21]. In addition, MMP-7 also activates other MMPs, namely, pro-MMP-1, -2, -9, and -8 [139]. Under normal circumstances, MMP-7 is secreted by epithelial cells, normal endometrial, bronchial, ductal, skin glandular urogenital, and gastrointestinal tissues, and some macrophages [132][134][135]. Thus, it plays a critical role in the management of several processes, including inflammatory processes, aging, and bone growth as well as remodeling and signaling pathways that control cell growth, inflammation, and angiogenesis [132][140][141][142].
5.2. Role in Cancer
A substantial body of evidence indicates that overexpression of MMP-7 plays an integral role in tumor pathogenesis and progression [134][135][143][144]. Tumor pathogenesis is a sequential process implying cell growth, invasion, metastasis, and angiogenesis [134]. Abnormally high expression of the gene encoding MMP-7 can promote tumor progression by inhibiting cancer cells’ apoptosis [145], reducing cell adhesion [134][146], and inducing angiogenesis [147].
Like other MMPs, MMP7 promotes cancer invasion by the proteolytic degradation of ECM proteins [134][135]. Also, MMP-7 activation of pro-MMP-2 and -9 can facilitate tumor invasion. Additionally, MMP-7 promotes tumor invasion through its role in the regulation of non-ECM components. MMP-7 breaks down β4 integrin and E-cadherin, which function as positive regulators of cell adhesion [134][146]. Thus, the degradation of these components promotes cancer migration and invasion [134]. Additionally, MMP-7 has an anti-apoptotic effect by degrading insulin-like growth factor 3 (IGFBP-3). IGFBP-3 is a biologically active growth factor that has a role in promoting tumor cell proliferation [148]. Moreover, MMP-7 was confirmed to directly accelerate angiogenesis by activating the proliferation of vascular endothelial cells [149].
MMP-7 is abundantly expressed in many types of cancer tumors, including breast [150], colon [151], oesophageal [152], pancreatic [153], and lung cancer [143]. In addition, MMP-7 is associated with an aggressive cancer phenotype and poor prognosis in patients with gastric cancer [154]. MMP-7 is also linked to clinicopathological aspects such as tumor stage by predicting the histological grade of the tumor [155]. Also, MMP-7 can act as a potential molecular marker for cancer diagnosis [150].
5.3. Structure of the Catalytic Domain
The catalytic domains of MMPs share a subsequent similarity, where the percentage of similarity ranges between 33% and 86% [1]. The catalytic domain of MMP-7 has a ball-like structure with three α-helices, five β-sheets, and multiple loops [156]. It also contains two Zn2+ binding sites. The first binding site is a structural site with Zn2+ coordinated by three conserved histidine and aspartic acid molecules. The second binding site is a catalytic site where Zn2+ is coordinated by three conserved histidine and 1–2 solvent molecules [156].
In MMP-7, the residue equivalent to Leu218 of MMP-13 is Tyr215. With this particular residue, the S1′ pocket of MMP-7 is classified as shallow and small [29]. In MMP-7, Tyr215 adopts a conformation that prevents the binding between ligands and the S1′ pocket. Hence, this residue creates a steric hindrance for those ligands that interact with the Ω-loop and explains the selectivity of ligands that perform these types of interactions. This is illustrated by the docking on MMP-7 of several MMP inhibitors that show selectivity over MMP-7. Therefore, the size and shape of the S1′ pocket are crucial determining factors in the selectivity of many MMP inhibitors.
5.4. MMP-7 Inhibitors
MMP-7 has been identified as a validated drug target and anti-target for cancer therapy [157]. When downregulation of MMP-7 restores the normal state of the cell and tissue, it is considered a drug target. In cancer, such drugs result in cancer cell death or slow disease progression. On the other hand, downregulation of MMP-7 may result in clinically unacceptable side effects, the initiation of cancer, or deleterious alterations in disease progression. In this case, MMP-7 is considered a drug anti-target [157]. Hence, improving selective inhibitors for MMP-7 could induce the evolution of cancer management [158]. As explained earlier, S1′ is the key determinant for MMP-inhibitor selectivity. However, because both MMP-7 and MMP-1 share similar S1′ features, it remains an ongoing challenge to determine the selectivity of their inhibitors [29].
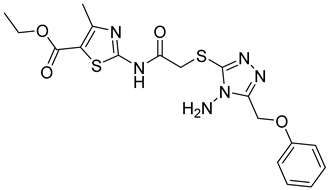
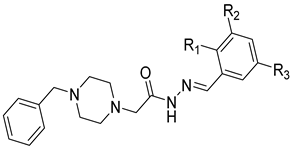
Li et al. have introduced a nitro group as a ZBG of MMP inhibitors [159]. They demonstrated that reasonable modification of the P3′ side chains of the nitro-based MMP inhibitors has enhanced the selectivity of inhibition for MMP-7 over MMP-1 [159]. A series of compounds were synthesized and evaluated as MMP inhibitors by molecular docking [159]. Five compounds presented the best positions based on the distance of their nitro group from the catalytic ZN2+ and the binding free energies [159]. However, one compound (compound 29) demonstrated a higher selectivity of MMP-7 over MMP-1 [159]. The inhibitory constant (Ki) of compound 29 to MMP-7 was 3.7 µM [159]. The structure of the compound scaffold is shown in Table 5.
Table 5. Chemical structures and potencies of synthetic MMP-7 inhibitors.
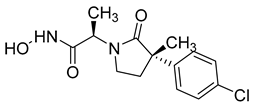
Fischer and Riedl aimed to improve the inhibition activity against MMP-7 by modifying a non-hydroxamate selective inhibitor previously identified as MMP-13 [158]. A series of modifications was accomplished, and the synthesized compounds were examined in vitro to predict their potency against MMP-7 [158]. Compound 30 was identified as the most potent inhibitor against MMP-7, with an IC-50 of 2.2 µM (Table 5). The authors noted that these modifications not only improve the selectivity against MMP-7 but also decrease the potency of the original compound toward MMP-13 [158].
6. Matrix Metalloprotease-12 (MMP-12)
6.1. Function and Localization
MMP-12 is an elastolytic protease that is primarily produced by inflammatory macrophages. Thus, it is also known as macrophage metalloelastase or macrophage elastase [15]. The significant role of MMP-12 in inflammation is demonstrated by its capability to degrade the basement membrane, which leads to macrophages’ penetration into injured tissue [160]. MMP-12 can degrade several extracellular matrix (ECM) structures, such as type IV collagen, fibronectin, fibrillin-1, laminin, vitronectin, chondroitin sulfate, and heparin sulfate proteoglycans. Furthermore, it can break down elastin, which is widely distributed in the lung [19][160].
MMP-12 is localized in the cytosol and nuclei of various cells [161]. The MMP-12 gene, like other MMPS genes, is positioned on human chromosome 11, at 11q22.3 [162]. MMP-12 is produced as a 54 kDa pro-form enzyme that goes through self-activation by autolytic processing, resulting in the generation of 45 kDa and 22 kDa active forms [19][160]. Alongside its autolytic processing activity, MMP-12 activates other MMPs such as pro-MMP-2 and pro-MMP-3, which, in turn, activate pro-MMP-1 and pro-MMP-9. This might be the reason behind MMP-12’s ability to amplify a cascade of proteolysis that leads to the degradation of a wide variety of ECM proteins.
The activity and expression levels of MMP-12 are controlled by multifaceted mechanisms involving different pathways at transcription, post-transcription, translation, and post-translation levels [18]. Of note is these mechanisms can impact the proteolytic and non-proteolytic functions of MMP-12 [18]. The transcriptional regulation of MMP-12 activity involves MMP-12 gene expression, transcript stability, promoter polymorphisms, and epigenetic alterations [18]. The post-transcriptional mechanism incorporates the regulatory effect of microRNAs (miRNAs) on the expression of one or multiple target genes. An example is the regulatory effect of miRNA-452 by targeting MMP-12 encoding [163][164]. Numerous studies have shown the importance of miRNAs in the development of cancer, specifically lung cancer [165][166][167][168][169].
Moreover, MMP-12 expression is upregulated at both mRNA and protein levels in visceral and subcutaneous white adipose tissue from obese mice and humans [170]. In addition, MMP-12 upregulation is subject to cellular differentiation status, which was proven by its non-existence in monocytes, the cells from which macrophages originate [19].
The location (compartmentalization) of the enzyme is another important factor regulating MMP-12 activity [171]. Localization stands for the congregation of MMPs around potential substrates or restricted availability of their endogenous inhibitors [171]. Secreted MMPs are found to bind to cell-surface membranes and therefore trigger intracellular cascade pathways [171]. Interestingly, extensive work has been undertaken to explain the transcriptional regulation of IkBα by MMP-12 in counteracting the protective anti-viral immune role of interferon-alpha (IFN α) [172][173].
6.2. Role in Cancer
Studies have shown that MMP-12 is involved in chronic obstructive pulmonary disease (COPD), emphysema, asthma, and arthritis [174]. Abdool et al. showed that MMP-12 degrades elastin in the lungs of smokers; it functions as a chemokine to enroll a pro-inflammatory immune response [4]. Moreover, MMP-12 is an identified mediator of arterial stiffening in both acute and chronic situations via the elastolytic effect of MMP-12 [4]. As a result, it may be involved in some vascular and neurological diseases such as atherosclerosis and aneurysms, spinal cord injury (SCI), multiple sclerosis (MS), Theiler murine encephalomyelitis, intracerebral haemorrhage (ICH), and ischemic stroke [19][175][176].
Additionally, MMP-12 is also implicated in the pathogenesis of different types of cancer; however, the risk of cancer susceptibility remains controversial [177][178]. Studies have documented that MMP-12 has antitumor activity against specific types of cancer, such as ovarian cancer [179] and colorectal cancer [180][181]. In contrast, researchers have reported that overexpression and functional polymorphism of MMP-12 is correlated with the occurrence and progression of colon cancer [182] and ovarian cancer [183]. Also, MMP-12 overexpression is a negative prognostic factor of hepatocellular carcinoma [184] breast cancer [185][186], oesophageal adenocarcinoma [187][188], skin cancer [189], and pancreatic cancer [186].
In addition, MMP-12 polymorphism relates to a higher risk of lung cancer dissemination, which has been documented by several studies [190][191][192]. Functionally, a defect in myeloid cells due to MMP-12 overexpression leads to abnormal myelopoiesis, immune suppression, and eventually the development of lung adenocarcinoma [193]. Furthermore, a meta-analysis that aimed to study the association between MMP-12 polymorphism and cancer concluded that the G allele of the MMP-12 82 A/G polymorphism may significantly increase the risk of epithelial ovarian cancer [177].
These studies confirm that MMP12 may be a significant regulator in the occurrence and growth of cancers.
6.3. Structure of Catalytic Domain
Structurally, MMP-12 is closely related to other MMPs, and it shares 49% sequence similarity with MMP-3 and MMP-1 [160]. MMP-12 is classified as having a medium-sized S1′ pocket that is characterized mostly by its hydrophobicity compared to other MMPs’ loop regions [17][194]. This hydrophobic environment is due to the presence of a series of residues (i.e., Ala234, Val235, Phe237, Lys241, Val243, and Phe248). Morales et al. have demonstrated the importance of hydrophobic interactions between S1′ pockets’ residues and inhibitors by developing a crystal structure of MMP-12 catalytic domain complex with non-zinc chelating inhibitors [194].
6.4. MMP-12 Inhibitors
Provided that MMP-12 is implicated in the development of a range of diseases, MMP-12 inhibitors are of interest in numerous areas of clinical therapy [19]. The number of MMP-12 inhibitors developed during the past decade is an indication of the significance of MMP-12 as a fundamental target in these diseases [19][195][196][197][198][199]. However, there are, to date, no MM-12 inhibitors documented in the literature that have effectively completed pre-clinical or clinical studies as a cancer therapy. As explained earlier, controlling the main central approaches of MMP-12 levels and activity have been pursued to develop compounds against MMP-12 activity [18]. Through the application of these strategies, various inhibitors of MMP-12 enzymatic activity have been developed in several diseases, though not in malignancies [22][200][201][202][203].
However, due to the extremely similar structures of the MMP family, nonselective MMP-12 inhibitors may interact with other MMPs, resulting in side effects [29]. Hence, the application of the rationale design of MMP-12 inhibitors would have a great effect on minimizing these undesirable effects by enhancing the selectivity of MMP-12 inhibitors. The rational design of selective MMP-12 inhibitors, based on the structure and specificity of MMP-12, comprises the introduction of a zinc-binding group (ZBG) to chelate the active site Zn(II) ion, an H-bond receptor or donor to interact with the amino acid backbone through hydrogen bonding, and a hydrophobic framework to fit into the S1′ pocket via hydrophobic interactions [17].
7. Matrix Metalloprotease-14 (MMP-14)
7.1. Function and Localization
MMP-14, previously known as MT1-MMP [204], was identified in 1994 and is classified as a membrane-type metalloproteinase [11]. MMP-14 is a protein that has been extensively studied and found in many different types of cells [205]. It plays an important role in various processes in our bodies. For example, it works on collagen I, as well as collagen II and III to some degree [206]. Also, it helps in the activation of the pro-MMP-2 protein, which is involved in cancer cell invasion [205].
Furthermore, MMP-14 works by forming a pair with itself on the cell surface and with MMP-2 and TIMP-2 to activate MMP-2 [207]. In addition to this, it is responsible for activating MMP-8 and -13 [204]. Moreover, MMP-14 has an important role in skeletal development, wound healing, inflammation, and many other functions [205]. Interesting to note is that MMP-14 can promote cell migration and invasion by breaking down the surrounding matrix [205]. Additionally, it acts as an enzyme that cleaves CD44, a hyaluronan receptor [207]. This cleavage enhances the growth and motility of cells through different mechanisms [205]. Beyond its enzymatic activity, MMP-14 interacts with different proteins and regulates processes like the immune response, bone development, and cell fusion [205]. MMP-14 is positioned at the forefront of moving cells, specifically on chromosome 14q11-q12 [62][204][208], and it is expressed at the cell surface [205].
7.2. Role in Cancer
In cases of human cancer, there is an overproduction and activation of MMP-14, which has been associated with the invasion and metastasis of cancer cells [209]. It plays a crucial role in enabling endothelial cells to invade and break down the surrounding tissue, ultimately facilitating the creation of new blood vessels [205]. This process is important in the development and progression of cancer [210][211]. The presence of MMP-14 has been linked to unfavorable outcomes in patients suffering from a variety of cancers [212], including melanoma [213]; advanced neuroblastoma [214]; mesothelioma [215]; lung cancer [216]; tongue, head, and neck carcinoma [217][218]; and bladder [219], breast [220], ovarian [221], pancreatic [222] and colorectal cancers [71][223]. Studies in animals have demonstrated that MMP-14 plays an important role in the spread of cancer cells to other parts of the body. Recent reviews have also suggested that MMP-14 can be used as an indicator of a patient’s prognosis for cancer [224].
7.3. Structure of the Catalytic Domain
The MMP-14 catalytic domain included an 8 amino acid sequence, PYAYIREG, that can affect the shape of the active site [204]. The S1′ pocket is shallow, since it contains leucine rather than arginine or tyrosine [1]. The hemopexin-like domain of MMP-14 plays a vital role in promoting the invasion of cells and enables it to interact with CD44. Also, it has a special site in its structure called a furin-like pro-protein convertase recognition site [1]. This site has a specific sequence of amino acids (RX[R/K]R) located at the end of the pro-domain of the protein. This special site allows for the protein to be activated through a process called proteolysis, in which the pro-domain is removed by specific enzymes [1]. Initially, the enzyme is produced as an inactive form, and it later becomes activated, removing the pro-peptide [225]. During secretion, the activation process takes place, and the activated enzyme is expressed on the cell surface [226].
7.4. MMP-14 Inhibitors
Computational chemistry techniques have been used to design and synthesize MMP-14 inhibitors for cancer treatment. These inhibitors work by targeting MMP-14 and preventing its ability to cleave substrates, which could potentially slow down or halt cancer progression. Virtual screening methods were also used to develop MMP-14 inhibitors [227]. Another way to discover inhibitors of MMP-14 is the high-throughput screening method, which involves testing large numbers of small molecules as potential MMP-14 inhibitors [228].
Researchers in the last decade have developed several small molecules of MMP-14 inhibitors (Table 6). Nuti and his team worked on improving the inhibitory activity of some compounds towards MMP-2, -9, and -14. They designed and tested a new series of N-isopropoxy-arylsulfonamide hydroxamates compounds. Of these, compound 31 was further analyzed for its binding mode to MMP-9 and-14 using X-ray crystallographic and docking studies [229]. Also, Cuffaro et al. tried to develop a dimeric compound (compound 32) to reduce MMP-14-dependent activity on pro-MMP-2 activation, collagen degradation, and collagen invasion [230]. Their results showed that compound 32 had better dose-dependent results than its monomeric counterpart (compound 1). Also, a virtual screening method was used to identify compound 33, which is a hemopexin-targeting small molecule (NSC405020) [227]. In addition, Sijoli and his coworkers docked eight PAC-1 (compound 34) and five isatin derivatives (compound 35) into MMP-9 and -14. Then, they synthesized, purified, and tested these compounds to see whether they could inhibit the activity of MMP-9 and -14. Though the compounds did bind to the active sites of the enzymes, they were not strong inhibitors. Even when tested at high concentrations, the compounds did not significantly affect the activity of the enzymes [231]. Scientists at the University of Notre Dame Du Lac have developed special inhibitors for MMP-2, -9, and -14, which are involved in various neurological disorders. These inhibitors are water-soluble and can cross the blood–brain barrier to reach therapeutic concentrations in the brain. They are also designed to be cleared without causing harmful side effects in the central nervous system. One of the inhibitors (compound 36), called ND-336, can inhibit all three enzymes simultaneously. The researchers believe that ND-336 and related compounds could be used to treat cancer [11].
References
- Woessner, J.F. The Matrix Metalloproteinase Family. In Biology of Extracellular Matrix, Matrix Metalloproteinases; Parks, W.C., Mecham, R.P., Eds.; Academic Press: Cambridge, MA, USA, 1998; pp. 1–14. ISBN 9780125450904.
- Nosrati, R.; Kheirouri, S.; Ghodsi, R.; Ojaghi, H. The effects of zinc treatment on matrix metalloproteinases: A systematic review. J. Trace Elem. Med. Biol. 2019, 56, 107–115.
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022.
- Abdool, A.Y.; Abbas, L.; Sebaei, T.; Schmitt, E.; Sikora, A. How can we design an inhibitor with an enhanced binding affinity that is selective for MMP12? In Protein Modeling Reports 4; NSUWorks: Fort Lauderdale, FL, USA, 2021.
- Shi, Y.; Ma, X.; Fang, G.; Tian, X.; Ge, C. Matrix metalloproteinase inhibitors (MMPIs) as attractive therapeutic targets: Recent progress and current challenges. NanoImpact 2021, 21, 100293.
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912.
- DeClerck, Y.A.; Mercurio, A.M.; Stack, M.S.; Chapman, H.A.; Zutter, M.M.; Muschel, R.J.; Raz, A.; Matrisian, L.M.; Sloane, B.F.; Noel, A. Proteases, extracellular matrix, and cancer: A workshop of the path B study section. Am. J. Pathol. 2004, 164, 1131–1139.
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801.
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27.
- Jablonska-Trypuc, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. S1), 177–183.
- Lenci, E.; Cosottini, L.; Trabocchi, A. Novel matrix metalloproteinase inhibitors: An updated patent review (2014–2020). Expert Opin. Ther. Pat. 2021, 31, 509–523.
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Butler, G.S.; Overall, C.M. Updated Biological Roles for Matrix Metalloproteinases and New “Intracellular” Substrates Revealed by Degradomics. Biochemistry 2009, 48, 10830–10845.
- Seizer, P.; May, A.E. Platelets and matrix metalloproteinases. Thromb. Haemost. 2013, 110, 903–909.
- Noël, A.; Perveen, Z.; Xiao, R.; Hammond, H.; Le Donne, V.; Legendre, K.; Gartia, M.R.; Sahu, S.; Paulsen, D.B.; Penn, A.L. Mmp12 Is Upregulated by in utero Second-Hand Smoke Exposures and Is a Key Factor Contributing to Aggravated Lung Responses in Adult Emphysema, Asthma, and Lung Cancer Mouse Models. Front. Physiol. 2021, 12, 704401.
- Kontogiorgis, C.A.; Papaioannou, P.; Hadjipavlou-Litina, D.J. Matrix metalloproteinase inhibitors: A review on pharmacophore mapping and (Q) SARs results. Curr. Med. Chem. 2005, 12, 339–355.
- Gimeno, A.; Beltrán-Debón, R.; Mulero, M.; Pujadas, G.; Garcia-Vallvé, S. Understanding the variability of the S1′ pocket to improve matrix metalloproteinase inhibitor selectivity profiles. Drug Discov. Today 2020, 25, 38–57.
- Alaseem, A.; Alhazzani, K.; Dondapati, P.; Alobid, S.; Bishayee, A.; Rathinavelu, A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Semin. Cancer Biol. 2019, 56, 100–115.
- Chelluboina, B.; Nalamolu, K.R.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. MMP-12, a promising therapeutic target for neurological diseases. Mol. Neurobiol. 2018, 55, 1405–1409.
- Amălinei, C.; Căruntu, I.-D.; Bălan, R.A. Biology of metalloproteinases. Rom. J. Morphol. Embryol. 2007, 48, 323–334.
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839.
- Ti, H.; Zhou, Y.; Liang, X.; Li, R.; Ding, K.; Zhao, X. Targeted treatments for chronic obstructive pulmonary disease (COPD) using low-molecular-weight drugs (LMWDs). J. Med. Chem. 2019, 62, 5944–5978.
- Ramezani, M.; Shamsara, J. An integrated structure-and pharmacophore-based MMP-12 virtual screening. Mol. Divers. 2018, 22, 383–395.
- Iyer, S.; Visse, R.; Nagase, H.; Acharya, K.R. Crystal structure of an active form of human MMP-1. J. Mol. Biol. 2006, 362, 78–88.
- Baidya, S.K.; Banerjee, S.; Adhikari, N.; Jha, T. Selective Inhibitors of Medium-Size S1′ Pocket Matrix Metalloproteinases: A Stepping Stone of Future Drug Discovery. J. Med. Chem. 2022, 65, 10709–10754.
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051.
- Kumar, R.; Gupta, Y.K.; Singh, S.; Arunraja, S. Picrorhiza kurroa inhibits experimental arthritis through inhibition of pro-inflammatory cytokines, angiogenesis and MMPs. Phytother. Res. 2016, 30, 112–119.
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future SuccessesMatrix Metalloproteinase Inhibitors in Cancer Therapy. Mol. Cancer Ther. 2018, 17, 1147–1155.
- Fabre, B.; Ramos, A.; de Pascual-Teresa, B. Targeting matrix metalloproteinases: Exploring the dynamics of the S1′ pocket in the design of selective, small molecule inhibitors: Miniperspective. J. Med. Chem. 2014, 57, 10205–10219.
- Devy, L.; Dransfield, D.T. New strategies for the next generation of matrix-metalloproteinase inhibitors: Selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem. Res. Int. 2011, 2011, 191670.
- Mahasenan, K.V.; Bastian, M.; Gao, M.; Frost, E.; Ding, D.; Zorina-Lichtenwalter, K.; Jacobs, J.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R. Exploitation of conformational dynamics in imparting selective inhibition for related matrix metalloproteinases. ACS Med. Chem. Lett. 2017, 8, 654–659.
- Hoseok, I.; Cho, J.-Y. Chapter Three—Lung Cancer Biomarkers. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 72, pp. 107–170.
- Ma, Y.; Iyer, R.P.; de Castro Brás, L.E.; Toba, H.; Yabluchanskiy, A.; Deleon-Pennell, K.Y.; Hall, M.E.; Lange, R.A.; Lindsey, M.L. Chapter 4—Cross Talk Between Inflammation and Extracellular Matrix Following Myocardial Infarction. In Inflammation in Heart Failure; Blankesteijn, W.M., Altara, R., Eds.; Academic Press: Boston, MA, USA, 2015.
- Lemaître, V.; D’Armiento, J. Matrix metalloproteinases in development and disease. Birth Defects Res. Part C Embryo Today Rev. 2006, 78, 1–10.
- Hatfield, K.J.; Reikvam, H.; Bruserud, Ø. The crosstalk between the matrix metalloprotease system and the chemokine network in acute myeloid leukemia. Curr. Med. Chem. 2010, 17, 4448–4461.
- Herrera, I.; Cisneros, J.; Maldonado, M.; Ramírez, R.; Ortiz-Quintero, B.; Anso, E.; Chandel, N.S.; Selman, M.; Pardo, A. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. J. Biol. Chem. 2013, 288, 25964–25975.
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73.
- Fischer, T.; Senn, N.; Riedl, R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry 2019, 25, 7960–7980.
- Overall, C.M.; López-Otín, C. Strategies for MMP inhibition in cancer: Innovations for the post-trial era. Nat. Rev. Cancer 2002, 2, 657–672.
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330.
- Chen, P.; Parks, W.C. Role of matrix metalloproteinases in epithelial migration. J. Cell. Biochem. 2009, 108, 1233–1243.
- Limb, G.A.; Matter, K.; Murphy, G.; Cambrey, A.D.; Bishop, P.N.; Morris, G.E.; Khaw, P.T. Matrix metalloproteinase-1 associates with intracellular organelles and confers resistance to lamin A/C degradation during apoptosis. Am. J. Pathol. 2005, 166, 1555–1563.
- Galt, S.W.; Lindemann, S.; Allen, L.; Medd, D.J.; Falk, J.M.; McIntyre, T.M.; Prescott, S.M.; Kraiss, L.W.; Zimmerman, G.A.; Weyrich, A.S. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ. Res. 2002, 90, 1093–1099.
- Naito, S.; Shimizu, S.; Matsuu, M.; Nakashima, M.; Nakayama, T.; Yamashita, S.; Sekine, I. Ets-1 Upregulates Matrix Metalloproteinase-1 Expression through Extracellular Matrix Adhesion in Vascular Endothelial Cells. Biochem. Biophys. Res. Commun. 2002, 291, 130–138.
- Said, A.H.; Hu, S.; Abutaleb, A.; Watkins, T.; Cheng, K.; Chahdi, A.; Kuppusamy, P.; Saxena, N.; Xie, G.; Raufman, J.P. Interacting post-muscarinic receptor signaling pathways potentiate matrix metalloproteinase-1 expression and invasion of human colon cancer cells. Biochem. J. 2017, 474, 647–665.
- Kumar, J.D.; Steele, I.; Moore, A.R.; Murugesan, S.V.; Rakonczay, Z.; Venglovecz, V.; Pritchard, D.M.; Dimaline, R.; Tiszlavicz, L.; Varro, A.; et al. Gastrin stimulates MMP-1 expression in gastric epithelial cells: Putative role in gastric epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G78–G86.
- Shin, D.H.; Dier, U.; Melendez, J.A.; Hempel, N. Regulation of MMP-1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells. Biochim. Biophys. Acta 2015, 1852, 2593–2602.
- Bostrom, P.; Soderstrom, M.; Vahlberg, T.; Soderstrom, K.O.; Roberts, P.J.; Carpen, O.; Hirsimaki, P. MMP-1 expression has an independent prognostic value in breast cancer. BMC Cancer 2011, 11, 348.
- Cai, Q.W.; Li, J.; Li, X.Q.; Wang, J.Q.; Huang, Y. Expression of STAT3, MMP-1 and TIMP-1 in gastric cancer and correlation with pathological features. Mol. Med. Rep. 2012, 5, 1438–1442.
- Shimizu, Y.; Kondo, S.; Shirai, A.; Furukawa, M.; Yoshizaki, T. A single nucleotide polymorphism in the matrix metalloproteinase-1 and interleukin-8 gene promoter predicts poor prognosis in tongue cancer. Auris Nasus Larynx 2008, 35, 381–389.
- Trivedi, V.; Boire, A.; Tchernychev, B.; Kaneider, N.C.; Leger, A.J.; O’Callaghan, K.; Covic, L.; Kuliopulos, A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell 2009, 137, 332–343.
- Wang, K.; Zheng, J.; Yu, J.; Wu, Y.; Guo, J.; Xu, Z.; Sun, X. Knockdown of MMP-1 inhibits the progression of colorectal cancer by suppressing the PI3K/Akt/c-myc signaling pathway and EMT. Oncol. Rep. 2020, 43, 1103–1112.
- Tao, Y.S.; Ma, X.Y.; Chai, D.M.; Ma, L.; Feng, Z.Z.; Cheng, Z.N.; Lai, M.D. Overexpression of MMP-1 and VEGF-C is associated with a less favorable prognosis in esophageal squamous cell carcinoma. Onkologie 2012, 35, 651–656.
- Zamolo, G.; Grahovac, M.; Žauhar, G.; Vučinić, D.; Kovač, L.; Brajenić, N.; Grahovac, B. Matrix metalloproteinases MMP-1, MMP-2, and MMP-13 are overexpressed in primary nodular melanoma. J. Cutan. Pathol. 2020, 47, 139–145.
- Becker, D.P.; Barta, T.E.; Bedell, L.J.; Boehm, T.L.; Bond, B.R.; Carroll, J.; Carron, C.P.; DeCrescenzo, G.A.; Easton, A.M.; Freskos, J.N.; et al. Orally Active MMP-1 Sparing α-Tetrahydropyranyl and α-Piperidinyl Sulfone Matrix Metalloproteinase (MMP) Inhibitors with Efficacy in Cancer, Arthritis, and Cardiovascular Disease. J. Med. Chem. 2010, 53, 6653–6680.
- Yuan, H.; Lu, W.; Wang, L.; Shan, L.; Li, H.; Huang, J.; Sun, Q.; Zhang, W. Synthesis of derivatives of methyl rosmarinate and their inhibitory activities against matrix metalloproteinase-1 (MMP-1). Eur. J. Med. Chem. 2013, 62, 148–157.
- Umedera, K.; Yoshimori, A.; Bajorath, J.; Nakamura, H. Design of MMP-1 inhibitors via SAR transfer and experimental validation. Sci. Rep. 2022, 12, 20915.
- Kaplancikli, Z.A.; Altintop, M.D.; Atli, O.; Sever, B.; Baysal, M.; Temel, H.E.; Demirci, F.; Ozdemir, A. Synthesis and Evaluation of A New Series of Thiazole Derivatives as Potential Antitumor Agents and MMP Inhibitors. Anticancer Agents Med. Chem. 2017, 17, 674–681.
- Mori, M.; Massaro, A.; Calderone, V.; Fragai, M.; Luchinat, C.; Mordini, A. Discovery of a New Class of Potent MMP Inhibitors by Structure-Based Optimization of the Arylsulfonamide Scaffold. ACS Med. Chem. Lett. 2013, 4, 565–569.
- Asawa, Y.; Yoshimori, A.; Bajorath, J.; Nakamura, H. Prediction of an MMP-1 inhibitor activity cliff using the SAR matrix approach and its experimental validation. Sci. Rep. 2020, 10, 14710.
- Hrabec, E.; Naduk, J.; Strek, M.; Hrabec, Z. . Postep. Biochem. 2007, 53, 37–45.
- Skiles, J.W.; Gonnella, N.C.; Jeng, A.Y. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr. Med. Chem. 2001, 8, 425–474.
- Nagase, H.; Murphy, G. Metalloproteinases, Matrix. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 90–97.
- Baidya, S.K.; Amin, S.A.; Jha, T. Outline of gelatinase inhibitors as anti-cancer agents: A patent mini-review for 2010-present. Eur. J. Med. Chem. 2021, 213, 113044.
- Chen, C.; Yang, X.; Fang, H.; Hou, X. Design, synthesis and preliminary bioactivity evaluations of 8-hydroxyquinoline derivatives as matrix metalloproteinase (MMP) inhibitors. Eur. J. Med. Chem. 2019, 181, 111563.
- Qiu, H.-Y.; Wang, Z.-C.; Wang, P.-F.; Yan, X.-Q.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. Design, synthesis, evaluation and 3D-QSAR analysis of benzosulfonamide benzenesulfonates as potent and selective inhibitors of MMP-2. RSC Adv. 2014, 4, 39214–39225.
- Albelwi, F.F.; Teleb, M.; Abu-Serie, M.M.; Moaty, M.N.A.A.; Alsubaie, M.S.; Zakaria, M.A.; El Kilany, Y.; Aouad, M.R.; Hagar, M.; Rezki, N. Halting tumor progression via novel non-hydroxamate triazole-based mannich bases MMP-2/9 inhibitors; design, microwave-assisted synthesis, and biological evaluation. Int. J. Mol. Sci. 2021, 22, 10324.
- Das, S.; Amin, S.A.; Jha, T. Inhibitors of gelatinases (MMP-2 and MMP-9) for the management of hematological malignancies. Eur. J. Med. Chem. 2021, 223, 113623.
- Dofara, S.G.; Chang, S.-L.; Diorio, C. Gene Polymorphisms and Circulating Levels of MMP-2 and MMP-9: A Review of Their Role in Breast Cancer Risk. Anticancer Res. 2020, 40, 3619–3631.
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol./Hematol. 2019, 137, 57–83.
- Zucker, S.; Vacirca, J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 101–117.
- Rodriguez Faba, O.; Palou-Redorta, J.; Fernández-Gómez, J.M.; Algaba, F.; Eiró, N.; Villavicencio, H.; Vizoso, F.J. Matrix Metalloproteinases and Bladder Cancer: What is New? ISRN Urol. 2012, 2012, 581539.
- Jezierska, A.; Motyl, T. Matrix metalloproteinase-2 involvement in breast cancer progression: A mini-review. Med. Sci. Monit. 2009, 15, Ra32–Ra40.
- Guo, C.B.; Wang, S.; Deng, C.; Zhang, D.L.; Wang, F.L.; Jin, X.Q. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol. Diagn. Ther. 2007, 11, 183–192.
- Chen, W.; Huang, S.; Shi, K.; Yi, L.; Liu, Y.; Liu, W. Prognostic Role of Matrix Metalloproteinases in Cervical Cancer: A Meta-Analysis. Cancer Control 2021, 28, 10732748211033743.
- Zhang, H.; Ma, Y.; Wang, H.; Xu, L.; Yu, Y. MMP-2 expression and correlation with pathology and MRI of glioma. Oncol. Lett. 2019, 17, 1826–1832.
- Liu, R.R.; Li, M.D.; Li, T.; Tan, Y.; Zhang, M.; Chen, J.C. Matrix metalloproteinase 2 (MMP2) protein expression and laryngeal cancer prognosis: A meta analysis. Int. J. Clin. Exp. Med. 2015, 8, 2261–2266.
- Al-Alem, L.; Curry, T.E., Jr. Ovarian cancer: Involvement of the matrix metalloproteinases. Reproduction 2015, 150, R55–R64.
- Morgunova, E.; Tuuttila, A.; Bergmann, U.; Isupov, M.; Lindqvist, Y.; Schneider, G.; Tryggvason, K. Structure of Human Pro-Matrix Metalloproteinase-2: Activation Mechanism Revealed. Science 1999, 284, 1667–1670.
- Jani, M.; Tordai, H.; Trexler, M.; Bányai, L.; Patthy, L. Hydroxamate-based peptide inhibitors of matrix metalloprotease 2. Biochimie 2005, 87, 385–392.
- Brooks, P.C.; Silletti, S.; von Schalscha, T.L.; Friedlander, M.; Cheresh, D.A. Disruption of Angiogenesis by PEX, a Noncatalytic Metalloproteinase Fragment with Integrin Binding Activity. Cell 1998, 92, 391–400.
- Choi, W.S.; Jeon, O.H.; Kim, H.H.; Kim, D.S. MMP-2 regulates human platelet activation by interacting with integrin αIIbβ3. J. Thromb. Haemost. 2008, 6, 517–523.
- Kandhwal, M.; Behl, T.; Singh, S.; Sharma, N.; Arora, S.; Bhatia, S.; Al-Harrasi, A.; Sachdeva, M.; Bungau, S. Role of matrix metalloproteinase in wound healing. Am. J. Transl. Res. 2022, 14, 4391–4405.
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546.
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739.
- Corcoran, M.L.; Hewitt, R.E.; Kleiner, D.E., Jr.; Stetler-Stevenson, W.G. MMP-2: Expression, activation and inhibition. Enzym. Protein 1996, 49, 7–19.
- Yang, R.; Zhao, G.; Cheng, B.; Yan, B. Identification of potential matrix metalloproteinase-2 inhibitors from natural products through advanced machine learning-based cheminformatics approaches. Mol. Divers. 2022, 27, 1053–1066.
- El-Hussieny, M.; Mansour, S.T.; Hashem, A.I.; Fouad, M.A.; Abd-El-Maksoud, M.A. Design, synthesis, and biological evaluation of new heterocycles bearing both silicon and phosphorus as potent MMP-2 inhibitors. J. Chin. Chem. Soc. 2022, 69, 1908–1923.
- Sanyal, S.; Amin, S.A.; Adhikari, N.; Jha, T. Ligand-based design of anticancer MMP2 inhibitors: A review. Future Med. Chem. 2021, 13, 1987–2013.
- Sanyal, S.; Amin, S.; Adhikari, N.; Jha, T. QSAR modelling on a series of arylsulfonamide-based hydroxamates as potent MMP-2 inhibitors. SAR QSAR Environ. Res. 2019, 30, 247–263.
- Ammazzalorso, A.; De Filippis, B.; Campestre, C.; Laghezza, A.; Marrone, A.; Amoroso, R.; Tortorella, P.; Agamennone, M. Seeking for non-zinc-binding MMP-2 inhibitors: Synthesis, biological evaluation and molecular modelling studies. Int. J. Mol. Sci. 2016, 17, 1768.
- Zhong, Y.; Lu, Y.-T.; Sun, Y.; Shi, Z.-H.; Li, N.-G.; Tang, Y.-P.; Duan, J.-A. Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer. Expert Opin. Drug Discov. 2018, 13, 75–87.
- Chien, M.-H.; Lin, C.-W.; Cheng, C.-W.; Wen, Y.-C.; Yang, S.-F. Matrix metalloproteinase-2 as a target for head and neck cancer therapy. Expert Opin. Ther. Targets 2013, 17, 203–216.
- Fingleton, B. Matrix metalloproteinase inhibitors for cancer therapy: The current situation and future prospects. Expert Opin. Ther. Targets 2003, 7, 385–397.
- Hoekstra, R.; Eskens, F.; Verweij, J. Matrix metalloproteinase inhibitors: Current developments and future perspectives. Oncologist 2001, 6, 415–427.
- Wang, P.F.; Qiu, H.Y.; Baloch, S.K.; Gong, H.B.; Wang, Z.C.; Zhu, H.L. Synthesis, Biological Evaluation, and Docking of Dihydropyrazole Sulfonamide Containing 2-hydroxyphenyl Moiety: A Series of Novel MMP-2 Inhibitors. Chem. Biol. Drug Des. 2015, 86, 1405–1410.
- Turra, K.M.; Rivelli, D.P.; de Moraes Barros, S.B.; Pasqualoto, K.F.M. Predicting Novel Antitumor Agents: 3D-Pharmacophore Mapping of β-N-biaryl Ether Sulfonamide-Based Hydroxamates as Potentially MMP-2 Inhibitors. Mol. Inform. 2014, 9, 573–587.
- Yan, X.-Q.; Wang, Z.-C.; Li, Z.; Wang, P.-F.; Qiu, H.-Y.; Chen, L.-W.; Lu, X.-Y.; Lv, P.-C.; Zhu, H.-L. Sulfonamide derivatives containing dihydropyrazole moieties selectively and potently inhibit MMP-2/MMP-9: Design, synthesis, inhibitory activity and 3D-QSAR analysis. Bioorg. Med. Chem. Lett. 2015, 25, 4664–4671.
- Halder, A.K.; Mallick, S.; Shikha, D.; Saha, A.; Saha, K.D.; Jha, T. Design of dual MMP-2/HDAC-8 inhibitors by pharmacophore mapping, molecular docking, synthesis and biological activity. RSC Adv. 2015, 5, 72373–72386.
- Wang, Z.-C.; Shen, F.-Q.; Yang, M.-R.; You, L.-X.; Chen, L.-Z.; Zhu, H.-L.; Lu, Y.-D.; Kong, F.-L.; Wang, M.-H. Dihydropyrazothiazole derivatives as potential MMP-2/MMP-8 inhibitors for cancer therapy. Bioorg. Med. Chem. Lett. 2018, 28, 3816–3821.
- Aouad, M.R.; Almehmadi, M.A.; Albelwi, F.F.; Teleb, M.; Tageldin, G.N.; Abu-Serie, M.M.; Hagar, M.; Rezki, N. Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation. Bioorg. Chem. 2022, 124, 105816.
- Kreituss, I.; Rozenberga, E.; Zemītis, J.; Trapencieris, P.; Romanchikova, N.; Turks, M. Discovery of aziridine-triazole conjugates as selective MMP-2 inhibitors. Chem. Heterocycl. Compd. 2013, 49, 1108–1117.
- Laghezza, A.; Luisi, G.; Caradonna, A.; Di Pizio, A.; Piemontese, L.; Loiodice, F.; Agamennone, M.; Tortorella, P. Virtual screening identification and chemical optimization of substituted 2-arylbenzimidazoles as new non-zinc-binding MMP-2 inhibitors. Bioorg. Med. Chem. 2020, 28, 115257.
- Bertran, A.; Khomiak, D.; Konopka, A.; Rejmak, E.; Bulska, E.; Seco, J.; Kaczmarek, L.; Tarragó, T.; Prades, R. Design and synthesis of selective and blood-brain barrier-permeable hydroxamate-based gelatinase inhibitors. Bioorg. Chem. 2020, 94, 103365.
- Mirastschijski, U.; Dinesh, N.; Baskaran, S.; Wedekind, D.; Gavrilovic, J.; Murray, M.Y.; Bevan, D.; Kelm, S. Novel specific human and mouse stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) antibodies for biochemical and immunohistochemical analyses. Wound Repair Regen. 2019, 27, 309–323.
- Adamcova, M.; Simko, F. Multiplex biomarker approach to cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1068–1072.
- Honsawek, S.; Malila, S.; Yuktanandana, P.; Tanavalee, A.; Deepaisarnsakul, B.; Parvizi, J. Association of MMP-3 (-1612 5A/6A) polymorphism with knee osteoarthritis in Thai population. Rheumatol. Int. 2013, 33, 435–439.
- Eguchi, T.; Kubota, S.; Kawata, K.; Mukudai, Y.; Uehara, J.; Ohgawara, T.; Ibaragi, S.; Sasaki, A.; Kuboki, T.; Takigawa, M. Novel transcription-factor-like function of human matrix metalloproteinase 3 regulating the CTGF/CCN2 gene. Mol. Cell. Biol. 2008, 28, 2391–2413.
- Si-Tayeb, K.; Monvoisin, A.; Mazzocco, C.; Lepreux, S.; Decossas, M.; Cubel, G.; Taras, D.; Blanc, J.F.; Robinson, D.R.; Rosenbaum, J. Matrix metalloproteinase 3 is present in the cell nucleus and is involved in apoptosis. Am. J. Pathol. 2006, 169, 1390–1401.
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864 Pt A, 2043–2055.
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978.
- Lee, J.Y.; Choi, H.Y.; Yune, T.Y. MMP-3 secreted from endothelial cells of blood vessels after spinal cord injury activates microglia, leading to oligodendrocyte cell death. Neurobiol. Dis. 2015, 82, 141–151.
- Lindsey, M.L.; Zamilpa, R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc. Ther. 2012, 30, 31–41.
- Chao, P.Z.; Hsieh, M.S.; Cheng, C.W.; Lin, Y.F.; Chen, C.H. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J. Biomed. Sci. 2011, 18, 86.
- Christensen, J.; Shastri, V.P. Matrix-metalloproteinase-9 is cleaved and activated by cathepsin K. BMC Res. Notes 2015, 8, 322.
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164.
- Argote Camacho, A.X.; Gonzalez Ramirez, A.R.; Perez Alonso, A.J.; Rejon Garcia, J.D.; Olivares Urbano, M.A.; Torne Poyatos, P.; Rios Arrabal, S.; Nunez, M.I. Metalloproteinases 1 and 3 as Potential Biomarkers in Breast Cancer Development. Int. J. Mol. Sci. 2021, 22, 9012.
- Nagase, H. Chapter 158: Matrix Metalloproteinase-3/Stromelysin-1. In Handbook of Proteolytic Enzymes; Rawlings, N.D.S.G., Ed.; Elsevier: London, UK, 2013; pp. 763–774.
- Wang, X.X.; Tan, M.S.; Yu, J.T.; Tan, L. Matrix metalloproteinases and their multiple roles in Alzheimer’s disease. BioMed Res. Int. 2014, 2014, 908636.
- Suhaimi, S.A.; Chan, S.C.; Rosli, R. Matrix Metallopeptidase 3 Polymorphisms: Emerging genetic Markers in Human Breast Cancer Metastasis. J. Breast Cancer 2020, 23, 1–9.
- Duffy, M.J.; Maguire, T.M.; Hill, A.; McDermott, E.; O’Higgins, N. Metalloproteinases: Role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res. 2000, 2, 252–257.
- Koujan, S.E.; Gargarib, B.P.; Khalili, M. Matrix Metalloproteinases and Breast Cancer. Thrita 2015, 4, e21959.
- Lynch, C.C.; Matrisian, L.M. Matrix metalloproteinases in tumor-host cell communication. Differentiation 2002, 70, 561–573.
- Cai, M.; Zheng, Z.; Bai, Z.; Ouyang, K.; Wu, Q.; Xu, S.; Huang, L.; Jiang, Y.; Wang, L.; Gao, J.; et al. Overexpression of angiogenic factors and matrix metalloproteinases in the saliva of oral squamous cell carcinoma patients: Potential non-invasive diagnostic and therapeutic biomarkers. BMC Cancer 2022, 22, 530.
- Frieling, J.S.; Li, T.; Tauro, M.; Lynch, C.C. Prostate cancer-derived MMP-3 controls intrinsic cell growth and extrinsic angiogenesis. Neoplasia 2020, 22, 511–521.
- Chen, W.; Ni, D.; Zhang, H.; Li, X.; Jiang, Y.; Wu, J.; Gu, Y.; Gao, M.; Shi, W.; Song, J.; et al. Over-expression of USP15/MMP3 predict poor prognosis and promote growth, migration in non-small cell lung cancer cells. Cancer Genet 2023, 272–273, 9–15.
- Park, H.I.; Jin, Y.; Hurst, D.R.; Monroe, C.A.; Lee, S.; Schwartz, M.A.; Sang, Q.X. The intermediate S1′ pocket of the endometase/matrilysin-2 active site revealed by enzyme inhibition kinetic studies, protein sequence analyses, and homology modeling. J. Biol. Chem. 2003, 278, 51646–51653.
- Amin, E.A.; Welsh, W.J. A preliminary in silico lead series of 2-phthalimidinoglutaric acid analogues designed as MMP-3 inhibitors. J. Chem. Inf. Model. 2006, 46, 2104–2109.
- Huang, R.Z.; Liang, G.B.; Huang, X.C.; Zhang, B.; Zhou, M.M.; Liao, Z.X.; Wang, H.S. Discovery of dehydroabietic acid sulfonamide based derivatives as selective matrix metalloproteinases inactivators that inhibit cell migration and proliferation. Eur. J. Med. Chem. 2017, 138, 979–992.
- Goyal, G.; Palaniappan, A.; Liedberg, B. Protease functional assay on membrane. Sens. Actuators B Chem. 2020, 305, 127442.
- Moskalenko, M.; Ponomarenko, I.; Reshetnikov, E.; Dvornyk, V.; Churnosov, M. Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia. Sci. Rep. 2021, 11, 5224.
- Liao, H.-Y.; Da, C.-M.; Liao, B.; Zhang, H.-H. Roles of matrix metalloproteinase-7 (MMP-7) in cancer. Clin. Biochem. 2021, 92, 9–18.
- Niu, B.; Liu, L.; Chen, Z.; Kou, M.; Yang, X.; Sun, Y.; Di, S.; Wang, X.; Cai, J.; Guo, D. Characterization, mRNA expression profile, subcellular distribution and association analysis with piglet diarrhea of porcine matrix metallopeptidase 7 (pMMP7). Gene 2022, 821, 146319.
- Ii, M.; Yamamoto, H.; Adachi, Y.; Maruyama, Y.; Shinomura, Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp. Biol. Med. 2006, 231, 20–27.
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Niczyporuk, M.; Ławicki, S. Matrilysins and stromelysins in pathogenesis and diagnostics of cancers. Cancer Manag. Res. 2020, 12, 10949.
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290.
- Dufour, A.; Sampson, N.S.; Zucker, S.; Cao, J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008, 217, 643–651.
- Luukkaa, H.; Klemi, P.; Hirsimäki, P.; Vahlberg, T.; Kivisaari, A.; Kähäri, V.-M.; Grénman, R. Matrix metalloproteinase (MMP)-7 in salivary gland cancer. Acta Oncol. 2010, 49, 85–90.
- Dozier, S.; Escobar, G.; Lindsey, M. Matrix metalloproteinase (MMP)-7 activates MMP-8 but not MMP-13. Med. Chem. 2006, 2, 523–526.
- Chen, P.; Abacherli, L.E.; Nadler, S.T.; Wang, Y.; Li, Q.; Parks, W.C. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting α2β1 integrin activation. PLoS ONE 2009, 4, e6565.
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868.
- Lwin, S.; Fowler, J.; Drake, M.; Edwards, J.; Lynch, C.; Edwards, C. A loss of host-derived MMP-7 promotes myeloma growth and osteolytic bone disease in vivo. Mol. Cancer 2017, 16, 49.
- Yamada, K.; Kadota, K.; Fujimoto, S.; Yoshida, C.; Ibuki, E.; Ishikawa, R.; Haba, R.; Yokomise, H.; Yajima, T. MMP-7 expression is associated with a higher rate of tumor spread through air spaces in resected lung adenocarcinomas. Lung Cancer 2023, 175, 125–130.
- Liao, C.-H.; Chang, W.-S.; Hsu, W.-L.; Hu, P.-S.; Wu, H.-C.; Hsu, S.-W.; Wang, B.-R.; Yueh, T.-C.; Chen, C.-H.; Hsia, T.-C. Association of Matrix Metalloproteinase-7 Genotypes With Prostate Cancer Risk. Anticancer Res. 2023, 43, 381–387.
- Wang, W.-S.; Chen, P.-M.; Wang, H.-S.; Liang, W.-Y.; Su, Y. Matrix metalloproteinase-7 increases resistance to Fas-mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis 2006, 27, 1113–1120.
- McCawley, L.J.; Matrisian, L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001, 13, 534–540.
- Nomden, M.; Beljaars, L.; Verkade, H.J.; Hulscher, J.B.; Olinga, P. Current concepts of biliary atresia and matrix metalloproteinase-7: A review of literature. Front. Med. 2020, 7, 617261.
- Nakamura, M.; Miyamoto, S.; Maeda, H.; Ishii, G.; Hasebe, T.; Chiba, T.; Asaka, M.; Ochiai, A. Matrix metalloproteinase-7 degrades all insulin-like growth factor binding proteins and facilitates insulin-like growth factor bioavailability. Biochem. Biophys. Res. Commun. 2005, 333, 1011–1016.
- Huo, N.; Ichikawa, Y.; Kamiyama, M.; Ishikawa, T.; Hamaguchi, Y.; Hasegawa, S.; Nagashima, Y.; Miyazaki, K.; Shimada, H. MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett. 2002, 177, 95–100.
- Piskór, B.M.; Przylipiak, A.; Dąbrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Ławicki, S. Plasma concentrations of matrilysins MMP-7 and MMP-26 as diagnostic biomarkers in breast cancer. J. Clin. Med. 2021, 10, 1436.
- Koskensalo, S.; Louhimo, J.; Nordling, S.; Hagström, J.; Haglund, C. MMP-7 as a prognostic marker in colorectal cancer. Tumor Biol. 2011, 32, 259–264.
- Palumbo, A., Jr.; Meireles Da Costa, N.; Pontes, B.; Leite de Oliveira, F.; Lohan Codeço, M.; Ribeiro Pinto, L.F.; Nasciutti, L.E. Esophageal cancer development: Crucial clues arising from the extracellular matrix. Cells 2020, 9, 455.
- Van Doren, S.R. MMP-7 marks severe pancreatic cancer and alters tumor cell signaling by proteolytic release of ectodomains. Biochem. Soc. Trans. 2022, 50, 839–851.
- Wattanawongdon, W.; Bartpho, T.S.; Tongtawee, T. Expression of Matrix Metalloproteinase-7 Predicts Poor Prognosis in Gastric Cancer. Biomed Res. Int. 2022, 2022, 2300979.
- Impola, U.; Uitto, V.-J.; Hietanen, J.; Hakkinen, L.; Zhang, L.; Larjava, H.; Isaka, K.; Saarialho-Kere, U. Differential expression of matrilysin-1 (MMP-7), 92 kD gelatinase (MMP-9), and metalloelastase (MMP-12) in oral verrucous and squamous cell cancer. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2004, 202, 14–22.
- Meng, F.; Yang, H.; Jack, C.; Zhang, H.; Moller, A.; Spivey, D.; Page, R.C.; Tierney, D.L.; Crowder, M.W. Biochemical characterization and zinc binding group (ZBGs) inhibition studies on the catalytic domain of MMP7 (cdMMP7). J. Inorg. Biochem. 2016, 165, 7–17.
- Overall, C.M.; Kleifeld, O. Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat. Rev. Cancer 2006, 6, 227–239.
- Fischer, T.; Riedl, R. Development of a non-hydroxamate dual matrix metalloproteinase (MMP)-7/-13 inhibitor. Molecules 2017, 22, 1548.
- Matt, C.; Hess, T.; Benlian, A. Digital transformation strategies. Bus. Inf. Syst. Eng. 2015, 57, 339–343.
- Hitaoka, S.; Chuman, H.; Yoshizawa, K. A QSAR study on the inhibition mechanism of matrix metalloproteinase-12 by arylsulfone analogs based on molecular orbital calculations. Org. Biomol. Chem. 2015, 13, 793–806.
- Bassiouni, W.; Ali, M.A.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182.
- Zhang, G.; Li, W.; Guo, Y.; Li, D.; Liu, Y.; Xu, S. MMP gene polymorphisms, MMP-1-1607 1G/2G,-519 A/G, and MMP-12-82 A/G, and ischemic stroke: A meta-analysis. J. Stroke Cerebrovasc. Dis. 2018, 27, 140–152.
- Graff, J.W.; Powers, L.S.; Dickson, A.M.; Kim, J.; Reisetter, A.C.; Hassan, I.H.; Kremens, K.; Gross, T.J.; Wilson, M.E.; Monick, M.M. Cigarette smoking decreases global microRNA expression in human alveolar macrophages. PLoS ONE 2012, 7, e44066.
- Li, L.; Li, H. Role of microRNA-mediated MMP regulation in the treatment and diagnosis of malignant tumors. Cancer Biol. Ther. 2013, 14, 796–805.
- Ren, X.-S.; Yin, M.-H.; Zhang, X.; Wang, Z.; Feng, S.-P.; Wang, G.-X.; Luo, Y.-J.; Liang, P.-Z.; Yang, X.-Q.; He, J.-X. Tumor-suppressive microRNA-449a induces growth arrest and senescence by targeting E2F3 in human lung cancer cells. Cancer Lett. 2014, 344, 195–203.
- Kong, Q.; Shu, N.; Li, J.; Xu, N. miR-641 functions as a tumor suppressor by targeting MDM2 in human lung cancer. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2018, 26, 735–741.
- Fortunato, O.; Boeri, M.; Moro, M.; Verri, C.; Mensah, M.; Conte, D.; Caleca, L.; Roz, L.; Pastorino, U.; Sozzi, G. Mir-660 is downregulated in lung cancer patients and its replacement inhibits lung tumorigenesis by targeting MDM2-p53 interaction. Cell Death Dis. 2014, 5, e1564.
- Li, J.; Lei, H.; Xu, Y.; Tao, Z.-Z. miR-512-5p suppresses tumor growth by targeting hTERT in telomerase positive head and neck squamous cell carcinoma in vitro and in vivo. PLoS ONE 2015, 10, e0135265.
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810.
- Amor, M.; Moreno-Viedma, V.; Sarabi, A.; Grün, N.G.; Itariu, B.; Leitner, L.; Steiner, I.; Bilban, M.; Kodama, K.; Butte, A.J. Identification of matrix metalloproteinase-12 as a candidate molecule for prevention and treatment of cardiometabolic disease. Mol. Med. 2016, 22, 487–496.
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.O. Regulation of matrix metalloproteinase activity in health and disease. FEBS J. 2011, 278, 28–45.
- Marchant, D.J.; Bellac, C.L.; Moraes, T.J.; Wadsworth, S.J.; Dufour, A.; Butler, G.S.; Bilawchuk, L.M.; Hendry, R.G.; Robertson, A.G.; Cheung, C.T. A new transcriptional role for matrix metalloproteinase-12 in antiviral immunity. Nat. Med. 2014, 20, 493–502.
- Siddhartha, R.; Garg, M. Molecular and clinical insights of matrix metalloproteinases into cancer spread and potential therapeutic interventions. Toxicol. Appl. Pharmacol. 2021, 426, 115593.
- Itoh, Y. Metalloproteinases in rheumatoid arthritis: Potential therapeutic targets to improve current therapies. Prog. Mol. Biol. Transl. Sci. 2017, 148, 327–338.
- Chelluboina, B.; Warhekar, A.; Dillard, M.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci. Rep. 2015, 5, srep09504.
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix metalloproteinase-12 induces blood–brain barrier damage after focal cerebral ischemia. Stroke 2015, 46, 3523–3531.
- Chen, S.-S.; Song, J.; Tu, X.-Y.; Zhao, J.-H.; Ye, X.-Q. The association between MMP-12 82 A/G polymorphism and susceptibility to various malignant tumors: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 10845.
- Ng, K.T.-P.; Qi, X.; Kong, K.-L.; Cheung, B.Y.-Y.; Lo, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Man, K. Overexpression of matrix metalloproteinase-12 (MMP-12) correlates with poor prognosis of hepatocellular carcinoma. Eur. J. Cancer 2011, 47, 2299–2305.
- Zeng, L.; Qian, J.; Zhu, F.; Wu, F.; Zhao, H.; Zhu, H. The prognostic values of matrix metalloproteinases in ovarian cancer. J. Int. Med. Res. 2020, 48, 0300060519825983.
- Yang, W.; Arii, S.; Gorrin-Rivas, M.J.; Mori, A.; Onodera, H.; Imamura, M. Human macrophage metalloelastase gene expression in colorectal carcinoma and its clinicopathologic significance. Cancer 2001, 91, 1277–1283.
- Cheng, P.; Jiang, F.H.; Zhao, L.M.; Dai, Q.; Yang, W.Y.; Zhu, L.M.; Wang, B.J.; Xu, C.; Bao, Y.J.; Zhang, Y.J. Human macrophage metalloelastase correlates with angiogenesis and prognosis of gastric carcinoma. Dig. Dis. Sci. 2010, 55, 3138–3146.
- Klupp, F.; Neumann, L.; Kahlert, C.; Diers, J.; Halama, N.; Franz, C.; Schmidt, T.; Koch, M.; Weitz, J.; Schneider, M. Serum MMP7, MMP10 and MMP12 level as negative prognostic markers in colon cancer patients. BMC Cancer 2016, 16, 494.
- Li, Y.; Jia, J.-H.; Kang, S.; Zhang, X.-J.; Zhao, J.; Wang, N.; Zhou, R.-M.; Sun, D.-L.; Duan, Y.-N.; Wang, D.-J. The functional polymorphisms on promoter region of matrix metalloproteinase-12,-13 genes may alter the risk of epithelial ovarian carcinoma in Chinese. Int. J. Gynecol. Cancer 2009, 19, 129–133.
- He, M.K.; Le, Y.; Zhang, Y.F.; Ouyang, H.Y.; Jian, P.E.; Yu, Z.S.; Wang, L.J.; Shi, M. Matrix metalloproteinase 12 expression is associated with tumor FOXP3(+) regulatory T cell infiltration and poor prognosis in hepatocellular carcinoma. Oncol. Lett. 2018, 16, 475–482.
- Shin, A.; Cai, Q.; Shu, X.-O.; Gao, Y.-T.; Zheng, W. Genetic polymorphisms in the matrix metalloproteinase 12 gene (MMP12) and breast cancer risk and survival: The Shanghai Breast Cancer Study. Breast Cancer Res. 2005, 7, R506–R512.
- Balaz, P.; Friess, H.; Kondo, Y.; Zhu, Z.; Zimmermann, A.; Büchler, M.W. Human macrophage metalloelastase worsens the prognosis of pancreatic cancer. Ann. Surg. 2002, 235, 519.
- Bradbury, P.A.; Zhai, R.; Hopkins, J.; Kulke, M.H.; Heist, R.S.; Singh, S.; Zhou, W.; Ma, C.; Xu, W.; Asomaning, K. Matrix metalloproteinase 1, 3 and 12 polymorphisms and esophageal adenocarcinoma risk and prognosis. Carcinogenesis 2009, 30, 793–798.
- Kerkelä, E.; Ala-aho, R.; Klemi, P.; Grénman, S.; Shapiro, S.D.; Kähäri, V.M.; Saarialho-Kere, U. Metalloelastase (MMP-12) expression by tumour cells in squamous cell carcinoma of the vulva correlates with invasiveness, while that by macrophages predicts better outcome. J. Pathol. 2002, 198, 258–269.
- Kerkelä, E.; Ala-aho, R.; Jeskanen, L.; Rechardt, O.; Grénman, R.; Shapiro, S.D.; KaÈhaÈri, V.-M.; Saarialho-Kere, U. Expression of human macrophage metalloelastase (MMP-12) by tumor cells in skin cancer. J. Investig. Dermatol. 2000, 114, 1113–1119.
- Lv, F.; Wang, J.; Wu, Y.; Chen, H.; Shen, X. Knockdown of MMP12 inhibits the growth and invasion of lung adenocarcinoma cells. Int. J. Immunopathol. Pharmacol. 2015, 28, 77–84.
- Cho, N.H.; Hong, K.P.; Hong, S.H.; Kang, S.; Chung, K.Y.; Cho, S.H. MMP expression profiling in recurred stage IB lung cancer. Oncogene 2004, 23, 845–851.
- Hofmann, H.-S.; Hansen, G.; Richter, G.; Taege, C.; Simm, A.; Silber, R.-E.; Burdach, S. Matrix metalloproteinase-12 expression correlates with local recurrence and metastatic disease in non–small cell lung cancer patients. Clin. Cancer Res. 2005, 11, 1086–1092.
- Qu, P.; Yan, C.; Du, H. Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood J. Am. Soc. Hematol. 2011, 117, 4476–4489.
- Morales, R.; Perrier, S.; Florent, J.-M.; Beltra, J.; Dufour, S.; De Mendez, I.; Manceau, P.; Tertre, A.; Moreau, F.; Compere, D. Crystal structures of novel non-peptidic, non-zinc chelating inhibitors bound to MMP-12. J. Mol. Biol. 2004, 341, 1063–1076.
- Pickett, S.D.; Green, D.V.; Hunt, D.L.; Pardoe, D.A.; Hughes, I. Automated lead optimization of MMP-12 inhibitors using a genetic algorithm. ACS Med. Chem. Lett. 2011, 2, 28–33.
- Gona, K.; Toczek, J.; Ye, Y.; Sanzida, N.; Golbazi, A.; Boodagh, P.; Salarian, M.; Jung, J.-J.; Rajendran, S.; Kukreja, G. Hydroxamate-based selective macrophage elastase (MMP-12) inhibitors and radiotracers for molecular imaging. J. Med. Chem. 2020, 63, 15037–15049.
- Butsch, V.; Börgel, F.; Galla, F.; Schwegmann, K.; Hermann, S.; Schäfers, M.; Riemann, B.; Wünsch, B.; Wagner, S. Design, (radio) synthesis, and in vitro and in vivo evaluation of highly selective and potent matrix metalloproteinase 12 (MMP-12) inhibitors as radiotracers for positron emission tomography. J. Med. Chem. 2018, 61, 4115–4134.
- Baggio, C.; Velazquez, J.V.; Fragai, M.; Nordgren, T.M.; Pellecchia, M. Therapeutic targeting of MMP-12 for the treatment of chronic obstructive pulmonary disease. J. Med. Chem. 2020, 63, 12911–12920.
- Krarup, P.-M.; Eld, M.; Heinemeier, K.; Jorgensen, L.N.; Hansen, M.B.; Ågren, M.S. Expression and inhibition of matrix metalloproteinase (MMP)-8, MMP-9 and MMP-12 in early colonic anastomotic repair. Int. J. Color. Dis. 2013, 28, 1151–1159.
- Le Quement, C.; Guenon, I.; Gillon, J.Y.; Valenca, S.; Cayron-Elizondo, V.; Lagente, V.; Boichot, E. The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br. J. Pharmacol. 2008, 154, 1206–1215.
- Nuti, E.; Panelli, L.; Casalini, F.; Avramova, S.I.; Orlandini, E.; Santamaria, S.; Nencetti, S.; Tuccinardi, T.; Martinelli, A.; Cercignani, G. Design, synthesis, biological evaluation, and NMR studies of a new series of arylsulfones as selective and potent matrix metalloproteinase-12 inhibitors. J. Med. Chem. 2009, 52, 6347–6361.
- Li, W.; Li, J.; Wu, Y.; Wu, J.; Hotchandani, R.; Cunningham, K.; McFadyen, I.; Bard, J.; Morgan, P.; Schlerman, F. A selective matrix metalloprotease 12 inhibitor for potential treatment of chronic obstructive pulmonary disease (COPD): Discovery of (S)-2-(8-(methoxycarbonylamino) dibenzo furan-3-sulfonamido)-3-methylbutanoic acid (MMP408). J. Med. Chem. 2009, 52, 1799–1802.
- Wu, Y.; Li, J.; Wu, J.; Morgan, P.; Xu, X.; Rancati, F.; Vallese, S.; Raveglia, L.; Hotchandani, R.; Fuller, N. Discovery of potent and selective matrix metalloprotease 12 inhibitors for the potential treatment of chronic obstructive pulmonary disease (COPD). Bioorg. Med. Chem. Lett. 2012, 22, 138–143.
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076.
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223.
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864 Pt A, 1940–1951.
- Vos, M.C.; van der Wurff, A.A.M.; van Kuppevelt, T.H.; Massuger, L. The role of MMP-14 in ovarian cancer: A systematic review. J. Ovarian Res. 2021, 14, 101.
- Mignon, C.; Okada, A.; Mattei, M.G.; Basset, P. Assignment of the human membrane-type matrix metalloproteinase (MMP14) gene to 14q11-q12 by in situ hybridization. Genomics 1995, 28, 360–361.
- Li, M.; Li, S.; Zhou, L.; Yang, L.; Wu, X.; Tang, B.; Xie, S.; Fang, L.; Zheng, S.; Hong, T. Immune Infiltration of MMP14 in Pan Cancer and Its Prognostic Effect on Tumors. Front. Oncol. 2021, 11, 717606.
- Zarrabi, K.; Dufour, A.; Li, J.; Kuscu, C.; Pulkoski-Gross, A.; Zhi, J.; Hu, Y.; Sampson, N.S.; Zucker, S.; Cao, J. Inhibition of matrix metalloproteinase 14 (MMP-14)-mediated cancer cell migration. J. Biol. Chem. 2011, 286, 33167–33177.
- Spencer, H.L.M.; Shnyder, S.D.; Loadman, P.M.; Falconer, R.A. The role of MT1-MMP in the progression and metastasis of osteosarcoma. J. Cancer Metastasis Treat. 2022, 8, 2.
- Knapinska, A.M.; Fields, G.B. The expanding role of MT1-MMP in cancer progression. Pharmaceuticals 2019, 12, 77.
- Thakur, V.; Bedogni, B. The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis. Pharmacol. Res. 2016, 111, 17–22.
- Nyalendo, C.; Sartelet, H.; Barrette, S.; Ohta, S.; Gingras, D.; Béliveau, R. Identification of membrane-type 1 matrix metalloproteinase tyrosine phosphorylation in association with neuroblastoma progression. BMC Cancer 2009, 9, 422.
- Doi, T.; Maniwa, Y.; Tanaka, Y.; Tane, S.; Hashimoto, S.; Ohno, Y.; Nishio, W.; Nishimura, Y.; Ohbayashi, C.; Okita, Y. MT1-MMP plays an important role in an invasive activity of malignant pleural mesothelioma cell. Exp. Mol. Pathol. 2011, 90, 91–96.
- Liao, H.; Wang, Z.; Deng, Z.; Ren, H.; Li, X. Curcumin inhibits lung cancer invasion and metastasis by attenuating GLUT1/MT1-MMP/MMP2 pathway. Int. J. Clin. Exp. Med. 2015, 8, 8948.
- Markovic, D.; Vinnakota, K.; Chirasani, S.; Synowitz, M.; Raguet, H.; Stock, K.; Sliwa, M.; Lehmann, S.; Kälin, R.; Van Rooijen, N. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 12530–12535.
- Rosenthal, E.L.; Matrisian, L.M. Matrix metalloproteases in head and neck cancer. Head Neck J. Sci. Spec. Head Neck 2006, 28, 639–648.
- Kanayama, H.o.; Yokota, K.y.; Kurokawa, Y.; Murakami, Y.; Nishitani, M.; Kagawa, S. Prognostic values of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 expression in bladder cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1998, 82, 1359–1366.
- Mimori, K.; Ueo, H.; Shirasaka, C.; Mori, M. Clinical significance of MT1-MMP mRNA expression in breast cancer. Oncol. Rep. 2001, 8, 401–403.
- Sakata, K.; Shigemasa, K.; Nagai, N.; Ohama, K. Expression of matrix metalloproteinases (MMP-2, MMP-9, MT1-MMP) and their inhibitors (TIMP-1, TIMP-2) in common epithelial tumors of the ovary. Int. J. Oncol. 2000, 17, 673–754.
- Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Büchler, M. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 2000, 85, 14–20.
- Leeman, M.F.; Curran, S.; Murray, G.I. New insights into the roles of matrix metalloproteinases in colorectal cancer development and progression. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2003, 201, 528–534.
- Gonzalez-Molina, J.; Gramolelli, S.; Liao, Z.; Carlson, J.W.; Ojala, P.M.; Lehti, K. MMP14 in Sarcoma: A Regulator of Tumor Microenvironment Communication in Connective Tissues. Cells 2019, 8, 991.
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596.
- Pietraszek-Gremplewicz, K.; Karamanou, K.; Niang, A.; Dauchez, M.; Belloy, N.; Maquart, F.-X.; Baud, S.; Brézillon, S. Small leucine-rich proteoglycans and matrix metalloproteinase-14: Key partners? Matrix Biol. 2017, 75, 271–285.
- Remacle, A.G.; Golubkov, V.S.; Shiryaev, S.A.; Dahl, R.; Stebbins, J.L.; Chernov, A.V.; Cheltsov, A.V.; Pellecchia, M.; Strongin, A.Y. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012, 72, 2339–2349.
- Lee, H.; Youn, I.; Demissie, R.; Vaid, T.M.; Che, C.T.; Azar, D.T.; Han, K.Y. Identification of small molecule inhibitors against MMP-14 via High-Throughput screening. Bioorg. Med. Chem. 2023, 85, 117289.
- Nuti, E.; Cantelmo, A.R.; Gallo, C.; Bruno, A.; Bassani, B.; Camodeca, C.; Tuccinardi, T.; Vera, L.; Orlandini, E.; Nencetti, S.; et al. N-O-Isopropyl Sulfonamido-Based Hydroxamates as Matrix Metalloproteinase Inhibitors: Hit Selection and in Vivo Antiangiogenic Activity. J. Med. Chem. 2015, 58, 7224–7240.
- Cuffaro, D.; Nuti, E.; Gifford, V.; Ito, N.; Camodeca, C.; Tuccinardi, T.; Nencetti, S.; Orlandini, E.; Itoh, Y.; Rossello, A. Design, synthesis and biological evaluation of bifunctional inhibitors of membrane type 1 matrix metalloproteinase (MT1-MMP). Bioorg. Med. Chem. 2019, 27, 196–207.
- Sjøli, S.; Solli, A.I.; Akselsen, Ø.; Jiang, Y.; Berg, E.; Hansen, T.V.; Sylte, I.; Winberg, J.-O. PAC-1 and isatin derivatives are weak matrix metalloproteinase inhibitors. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 3162–3169.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
827
Revisions:
2 times
(View History)
Update Date:
25 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No