Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kevin Yang Wu | -- | 6271 | 2023-07-24 05:57:33 | | | |
| 2 | Camila Xu | Meta information modification | 6271 | 2023-07-24 07:12:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wu, K.Y.; Tan, K.; Akbar, D.; Choulakian, M.Y.; Jong, A.; Tran, S.D. Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions. Encyclopedia. Available online: https://encyclopedia.pub/entry/47161 (accessed on 15 January 2026).
Wu KY, Tan K, Akbar D, Choulakian MY, Jong A, Tran SD. Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions. Encyclopedia. Available at: https://encyclopedia.pub/entry/47161. Accessed January 15, 2026.
Wu, Kevin Y., Kenneth Tan, Dania Akbar, Mazen Y. Choulakian, Ashley Jong, Simon D. Tran. "Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions" Encyclopedia, https://encyclopedia.pub/entry/47161 (accessed January 15, 2026).
Wu, K.Y., Tan, K., Akbar, D., Choulakian, M.Y., Jong, A., & Tran, S.D. (2023, July 24). Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions. In Encyclopedia. https://encyclopedia.pub/entry/47161
Wu, Kevin Y., et al. "Drug Delivery Systems for Uveitis and Neuro-Ophthalmologic Conditions." Encyclopedia. Web. 24 July, 2023.
Copy Citation
To circumvent these constraints, the development of biodegradable nano-based drug delivery systems (DDS) has gained prominence. These systems promise extended residence time in ocular tissues, improved penetration through ocular barriers, and are composed of nanosized, biodegradable polymers, thereby diminishing the risk of toxicity and adverse reactions.
uveitis
neuro-ophthalmology
drug-delivery systems (DDS)
polymeric nano-based DDS
1. Introduction
Managing uveitis and neuro-ophthalmologic conditions presents a significant challenge due to the eye’s complex anatomy, which restricts effective medication delivery. Conventional therapies such as topical eye drops and intravitreal injections face limitations due to their poor bioavailability, short residence time, and the need for frequent dosing.
To circumvent these constraints, the development of biodegradable nano-based drug delivery systems (DDS) has gained prominence. These systems promise extended residence time in ocular tissues, improved penetration through ocular barriers, and are composed of nanosized, biodegradable polymers, thereby diminishing the risk of toxicity and adverse reactions.
2. Overview of the Biodegradable Nano-Based Drug Delivery System (DDS)
2.1. Enhancing Drug Delivery with Biodegradable Nanocarriers
Biodegradable nanocarriers enhance drug delivery by improving bioavailability and reducing frequent dosages, increasing patient compliance. Polymers, like cellulose derivatives, extend drug retention time, and mucoadhesive polymers limit lacrimal clearance. The use of targeting moieties directs nanocarriers to specific ocular sites, and disease-responsive designs prevent undesired drug release. These systems also enhance drug stability by modulating interactions with tear proteins and adjusting to varying eye pH levels [1].
2.2. Ideal Properties of Nanocarriers
Nanocarriers for drug delivery should have the ideal characteristics highlighted in Figure 1. The use of multiple polymers to form nano-assemblies, especially copolymers, provides a flexible platform for designing effective drug delivery systems with optimal properties like charge, solubility, and aggregation, and diverse shapes such as spheres, rods, or cylinders [1].
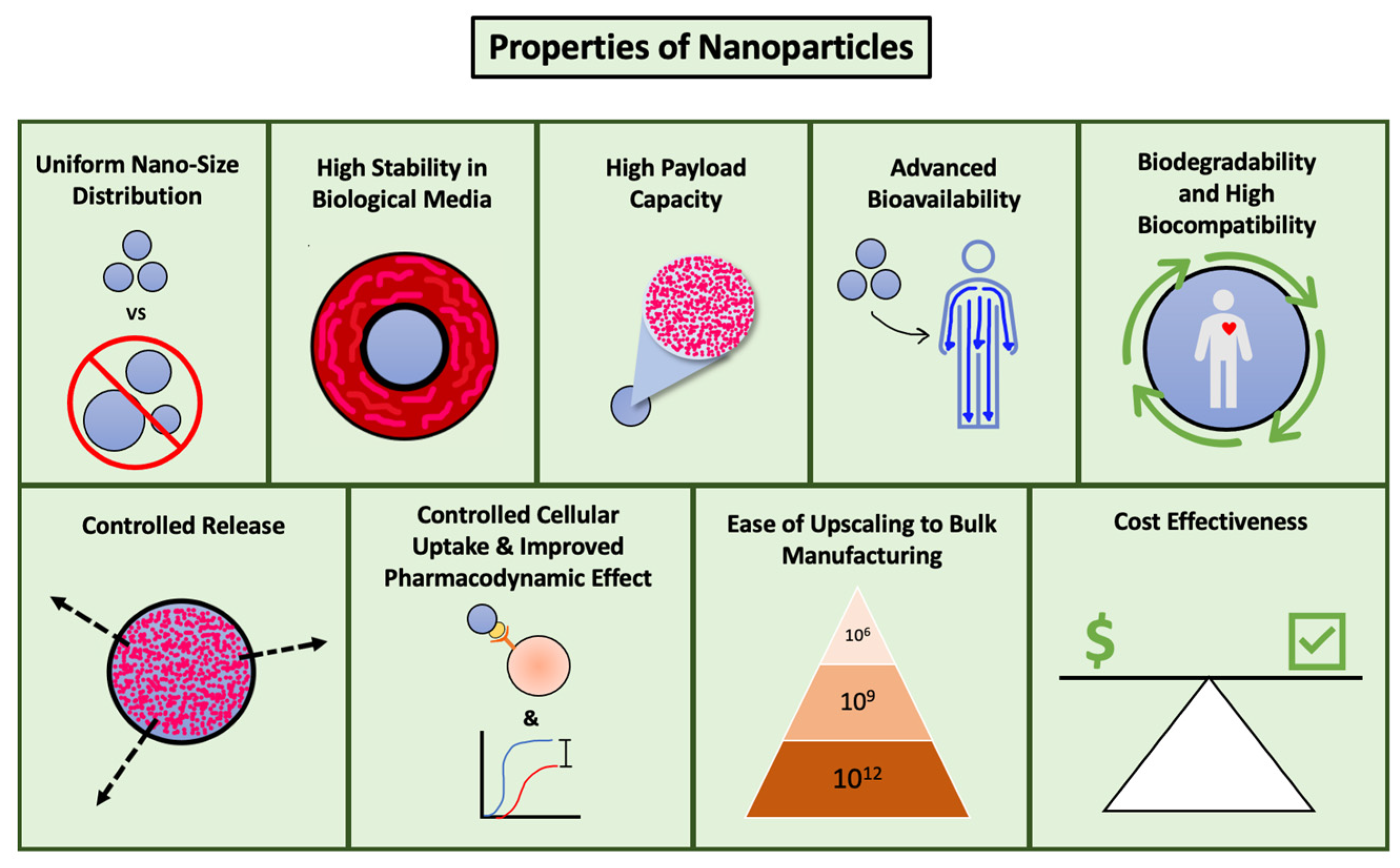
Figure 1. Ideal properties of nanocarriers.
2.3. Exploring Various Biodegradable Polymers and Their Advantages in Ocular Drug Delivery
Ensuring the biodegradability and morphological appropriateness of polymers in nanocarriers is essential for safe and efficient drug delivery, as these characteristics allow for their natural breakdown into harmless metabolites [2]. The application of biocompatible and biodegradable polymers in ocular drug delivery systems has gained significant traction in recent years. The surface properties of these polymers, including size and charge, can influence their binding affinity and vary across different regions of the eye. Table 1 summarizes some of the most commonly used biopolymers in ocular drug delivery and highlights their unique advantages.
Table 1. Overview of the characteristics and advantages of biopolymers.
| Biopolymer | Characteristics | Advantages |
|---|---|---|
| Hyaluronic Acid | Anionic polymer and high water-retention capacity. |
|
| Cellulose | Can self-assemble into nanorods, nanospheres, nanosponges, and nanorods upon functionalization with a copolymer, allowing for ease of bulk manufacturing. |
|
| Chitosan | Requires chemical modification, is mucoadhesive, and has unique in situ gelling properties. |
|
| Alginate | Anionic copolymer that can exert cell immobilization and can be used in copolymeric nanoparticles with chitosan derivatives. |
|
| PLGA | Commonly used, subject to abundant modifications, can be enhanced in size and surface potential, and can be modified with PEG. |
|
| Poloxamers | Biodegradable, mucomimetic, and non-ionic surfactants. |
|
| Cyclodextrins | Cyclic oligosaccharides can form hydrophobic cavities with externally hydrophilic surfaces. |
|
FDA: Food and Drug Administration. PEG: Poly(ethylene glycol). PLGA: Poly(lactide-co-glycolide).
Hyaluronic acid, due to its negative charge and water retention capabilities, excels in enhancing the mechanical strength and drug release of hydrogels and liposome coatings [2]. Cellulose nanocrystals offer improved viscosity and varied structures for ocular drug delivery, with derivatives like carboxymethylcellulose and hydroxypropyl methylcellulose contributing to dry eye treatments and mucoadhesive enhancements, respectively [3][4][5][6]. Chitosan, with its distinctive mucoadhesive properties and permeability enhancement, can be chemically modified for additional antibacterial activity [7][8][9]. Alginate, characterized by its reversible gelation properties and functional groups, is instrumental in efficient drug encapsulation in copolymeric nanoparticles and hydrogels [10][11]. PLGA stands out for its entrapment efficiency and trans-ocular permeation, courtesy of its tunable size and surface potential [12][13]. Poloxamer 407, an FDA-approved biodegradable surfactant, is versatile in various ocular formulations [10]. Finally, cyclodextrins’ unique chemistry significantly boosts the bioavailability of numerous molecules, making them a preferred choice for ocular drug delivery [14].
2.4. Categories of Nano-Based DDS: Features and Improvements
Nanocarriers are tailored to deliver medicine effectively to specific eye regions by utilizing the interplay of different biodegradable polymers. This customization considers the drug’s properties and the microenvironment of the target ocular tissue. The key features are briefly summarized in Table 2 [15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35].
Table 2. Comparative summary of drug delivery systems for ocular applications: key characteristics and benefits.
| Drug Delivery System | Characteristics | Advantages |
|---|---|---|
| Nanomicelles | Spherical structures made up of surfactant molecules that self-assemble in water or polar solvents. |
|
| Liposomes | Vesicles composed of one or more phospholipid bilayers. |
|
| Dispersed nanoparticles | Self-assembling supramolecular assemblies. |
|
| Dendrimers | Repeating multibranched polymers with high-density functional groups. |
|
| Hydrogels | Highly absorbent polymer networks. |
|
| Nanosuspensions and nanoemulsions | Aqueous dispersions of insoluble drug particles or droplets of one liquid in another liquid. |
|
| Microneedles | Small, needle-like structures. |
|
Hydrogels are garnering interest in ocular drug delivery due to their customizable pH-responsive release and target specificity, reducing the need for frequent injections [15][16]. Dendrimers, with their unique structure, efficiently encapsulate drugs and allow tunable release rates. They can be used to create other systems like dendrimer hydrogels, nanogels, and liposomes [17][18]. Liposomes, known for their vesicle structure, exhibit improved pre-corneal and conjunctival penetration. Coatings like PAMAM further enhance their permeability and bioactivity [18][19][20].
Nanomicelles, surfactant assemblies, are adept at enclosing hydrophobic compounds, facilitating corneal penetration. They are enhanced by mucin-targeting moieties and stability-enhancing cross-linking techniques [21][22][23][24][25][26]. Dispersed nanoparticles, with their self-assembling nature, offer an effective platform for drug delivery. Their size and ability to accumulate selectively in tissue make them highly suitable for ocular applications [27][28][29][30].
Nanosuspensions and nanoemulsions, used for delivering poorly soluble and permeable drugs, have shown effectiveness. Nanoemulsions boost drug solubility, while nanosuspensions, stabilized by various polymers, are ideal for high-molecular-weight drugs [31][32][33][34]. Finally, microneedles, a newer technique, offer improved accuracy, self-administration, and fewer complications from injections [35].
3. Biodegradable Nano-Based DDS for Uveitis
In general, non-infectious anterior uveitis is treated by applying topical glucocorticoid steroids hourly, which are then gradually tapered over several weeks once the anterior chamber inflammation is resolved. While this treatment approach is successful for the majority of patients, a subgroup does not respond favorably due to factors such as increased intraocular pressure (i.e., steroid responders), flare-ups during the tapering period, non-compliance with the treatment (such as abruptly discontinuing the steroid treatment), and ocular irritation from the frequent application of topical drugs [3].
The treatment of intermediate, posterior, and panuveitis tends to be more complex. These typically necessitate intravitreal injections because the eye’s anatomical barriers restrict the topical steroids’ bioavailability to the eye’s posterior segment. However, intravitreal injections of triamcinolone are not risk-free; they can lead to rare but severe complications like endophthalmitis and retinal detachment, as well as the inevitable adverse effects such as cataract formation and ocular hypertension if performed routinely. Additionally, they necessitate frequent follow-up office visits for re-injection, relying heavily on patients’ compliance. Intravitreal implants offering sustained corticosteroid release have been explored, but limitations such as no substantial long-term benefits, potential anterior migration (which leads to corneal decompensation), and exceedingly high rates of cataracts and ocular hypertension exist. These often necessitate cataract surgery and intraocular pressure-lowering agents [3].
In cases of bilateral disease, oral corticosteroids are an option. However, these are often associated with numerous systemic adverse effects, including fatigue, muscle weakness, weight gain, insomnia, and an increased risk of infection. For patients experiencing chronic recurrent uveitis, such as that associated with juvenile idiopathic arthritis, where topical steroid treatments cannot be successfully tapered down, alternative oral, subcutaneous, or intravenous therapies may be utilized. These include oral immunosuppressants, disease-modifying agents, and biologics. However, it should be noted that these systemic medications carry their own risks and potential systemic adverse effects, such as an increased risk of infection and malignancy [3].
Given these existing challenges and limitations in uveitis treatment, biodegradable nano-based drug delivery systems (DDS) present a promising alternative. Their enhanced penetration and superior bioavailability could reduce dosing frequency, thereby improving compliance and minimizing ocular irritation. Furthermore, their potential for controlled release and tissue-specific targeting could mitigate the risk of adverse intraocular pressure increases and cataract formation. Additionally, they may have the capacity to penetrate deeper into the posterior eye segment, possibly eliminating the need for intravitreal injections and their associated risks for the management of posterior segment inflammation.
3.1. Biodegradable Nano-Based DDS for Experimentally Induced Uveitis
In a study conducted by Kasper et al. (2018), they tested the topical treatment of cyclosporin A loaded methoxy-poly(ethylene-glycol)-hexyl substituted poly- (lactic acid) (mPEGhexPLA) nanocarriers (ApidSOL) for effectiveness and tissue distribution in a mouse model of experimental autoimmune uveitis (EAU). The ointment was found to be well tolerated both locally and systemically, and non-toxic when applied to one eye five times a day for nine consecutive days. This resulted in drug accumulation predominantly in the cornea, sclera-choroidal tissue, and lymph nodes of the treated eye, as well as a significantly reduced severity of EAU compared to the untreated counterparts. Furthermore, the treatment regimen was associated with proximally located immunosuppression due to reduced T-cell counts, T-cell proliferation, and IL-2 secretion in the treated eye’s lymph nodes. Overall, the use of cyclosporin A-containing nanocarriers applied topically was found to be an effective treatment for EAU [4].
Other authors have investigated the use of topical treatment, specifically PLGA nanocapsules as eye drops loaded with tacrolimus, to treat ocular inflammation. Rebibo et al. (2021) obtained rats and rabbits to assess the efficacy of a treatment in an EAU model. These nanocapsules were found to be suitable for use in the eye and were able to increase drug retention in the cornea. This also enabled the nanocapsules to penetrate deeper into the eye structures in both porcine ex vivo and rabbit and rat in vivo models, showing improved anti-inflammatory effects compared to the drug in oil solution [5]. With similar success as eyedrops, Chen et al. (2021) reported hydrogel eye drops made from low-deacetylated chitosan and beta-glycerophosphate to deliver adalimumab. The researchers found that the hydrogel eye drops were more effective in treating and delivering the medication than solely using the medication on its own [6].
Another approach to treating uveitis was the use of biodegradable poly(lactic-co-glycolic acid) (PLGA)-based nanoparticles. Luo et al. 2019 conducted a study using biodegradable nanoparticles made of carboxyl-terminated PLGA and divalent zinc ions (DSP-Zn-NP) to encapsulate dexamethasone sodium phosphate. These nanoparticles had high drug content, a small average diameter, and a neutral surface charge. When injected subconjunctivally into the eye, DSP-Zn-NP was deemed to be effective in reducing inflammation in a rat model of autoimmune uveitis for at least 3 weeks. This also decreased the expression of inflammatory cytokines while preserving retinal structure and function and reducing microglial cell density in the retina. Regarding its safety, it was found that four weekly injections of DSP-Zn-NP had similar effects on retinal structure and function as saline injections. Subconjunctival injections of DSP-Zn-NP, importantly, did not result in any retinal toxicity in rats, as assessed by histology and electroretinography, suggesting that DSP-Zn-NP may be a promising and safe treatment option for autoimmune uveitis [7]. During that same year, in 2019, Guo et al. created a nanoparticle-based drug delivery system that was also promising. Specifically, they employed mPEG-PLGA nanoparticles that contained triamcinolone acetonide (TA) using a modified double emulsification method, characterized the loaded nanoparticles, and examined their effects on rats with EAU. They found that the nanoparticles had a spherical shape and were approximately 82 nm in size, and they were also capable of releasing TA for at least 45 days. The TA tended to be incorporated into the hydrophobic PLGA domain of the nanoparticles, while a smaller amount was present in the hydrophilic PEG domain. When tested on the rats, the nanoparticles had stronger anti-inflammatory effects than TA alone, as shown by histopathological examination and changes in the levels of interleukin-17 and IL-10 in the aqueous humor and serum. It should be noted that, given that this is a polymer-based biodegradable drug delivery system, the release of TA from the mPEG-PLGA nanoparticles is primarily influenced by diffusion, swelling, and erosion processes. In this case, the release of TA occurred through diffusion and surface erosion as the mPEG-PLGA polymers degraded. The PLGA copolymer was found to degrade through hydrolysis or biodegradation, breaking down into oligomeric and monomeric constituents [8].
Another study that investigated the use of nanoparticles was by Huang et al. in 2018. They created nanoparticles with a high drug payload for the topical treatment of ocular inflammation. The nanoparticles were created by mixing a succinated triamcinolone acetonide (TA-SA) supramolecular hydrogel with a poly (ethylene glycol)-poly (ɛ-caprolactone)-poly (ethylene glycol) (PECE) aqueous solution. After applying the TA-SA/PECE nanoparticles topically, it was found that they had good ocular biocompatibility and did not cause any irritation or harmful changes. During in vivo rabbit model studies, the nanoparticles also showed better effectiveness at reducing neutrophil infiltration and the quantity of fibrinous exudate in the anterior chamber of the eye compared to treatment with TA alone [9]. Moreover, Xing et al. in 2021 assessed the use of triamcinolone acetonide prepared in poly(D,L-lactide-co-glycolide) (PLGA)-chitosan (PLC) nanoparticles. This formulation was characterized and found to have controlled drug release for 100 h and was biocompatible. In cell and animal studies, the TA-loaded PLC nanoparticles had excellent anti-inflammatory activity and significantly reduced inflammation compared to a control treatment. They also had a longer-lasting pharmacokinetic profile in rabbit eyes compared to control treatments, highlighting the concept that the chitosan coating of this drug delivery system was critical for prolonged drug release [10].
While there have been limited studies on the use of extracellular vesicles (sEVs) for ocular drug delivery, a recent study by Li et al. (2022) examined the effectiveness of using sEVs from mesenchymal stem cells as a drug delivery system for the treatment of EAU using rapamycin as a model drug. The sEVs were loaded with rapamycin and administered to the eye via subconjunctival injection. The study found that the sEVs were able to reach the targeted area in the eye and that the combination of sEVs and rapamycin was more effective at reducing inflammation and cell infiltration than either treatment alone. Additionally, sEVs have the advantage of being naturally occurring, found in all bodily fluids, and able to protect the drugs they engulf from degradation due to their lipid bilayers while overcoming various biological barriers, such as the blood-ocular barrier. Despite the positive remarks about these results, additional research must be performed to thoroughly examine their drug release kinetics and the potential toxic effects of Rapa-sEVs. Still, these results suggest that mesenchymal stem cell-derived sEVs have potential as a drug delivery system for the treatment of EAU and could be an alternative to steroid-based therapies [11]. On a similar note, with respect to sEVs, a study by Garg et al. in 2021 investigated the use of a modified lipid vesicle called a proglycosome nanovesicle from lipid vesicles to improve the effectiveness of tacrolimus in treating EIU in rabbits. This delivery system was found to be deformable and able to sustain the release of tacrolimus for 12 h. The results showed that treatment with these nanovesicles can significantly reduce clinical symptoms of EIU and prevent the breakdown of the blood-aqueous barrier [12].
Another method for the treatment of uveitis is the use of cubosomes. Cubosomes have potential as a new type of ocular drug delivery system due to several advantages. They are made from cost-effective biodegradable lipids using a straightforward and scalable method and are able to incorporate a variety of different types of drugs, including those that are hydrophilic, hydrophobic, and amphiphilic. In another study published in 2020 by Gaballa et al., they proposed the use of cubosomal gels for the delivery of beclomethasone dipropionate for uveitis management while increasing the ocular bioavailability of the drug and increasing its residence time. The results showed that the cubosomes were of a suitable size, had high encapsulation efficiency, and that their use significantly improved transcorneal permeation compared to the control formulation of beclomethasone dipropionate suspension. The optimized cubosomes were incorporated into a gel, which had desirable rheological properties and good ocular tolerability. Importantly, the gel also displayed superior anti-inflammatory properties in the treatment of uveitis in the rabbit model compared to the controls. These findings indicate that cubosomes and the resulting gel may be a promising ocular delivery system for effectively treating uveitis [13].
Antioxidant enzymes, such as SOD1, have the potential to effectively remove reactive oxygen species. However, delivering these enzymes to the eye can be challenging due to their limited penetration. In a recent study by Vaneev et al. published in 2021, they tested a novel treatment option in the form of multilayer nanoparticles made from SOD1, which were deemed to have good stability and a strong therapeutic effect without causing side effects like irritation, acute or chronic toxicity, allergenicity, immunogenicity, or mutagenicity. The researchers tested the nano-SOD1 formulation in a uveitis rabbit model to reduce inflammation. Their results showed that applying nano-SOD1 topically was more effective at decreasing uveitis symptoms, including edema in the cornea and conjunctiva, hyperemia in the iris, and fibrin clots, when compared to the free enzyme. Furthermore, another advantage is that the nano-SOD1 was able to stay on the surface of the cornea better than the native enzyme, which allowed a longer-lasting enzyme and more effective penetration into the interior structures of the eye. Consequently, this not only reduced inflammation but also restored the antioxidant properties of the eye tissue. Therefore, nano-SOD1 may be a promising treatment option for ocular inflammatory disorders [14].
Research by Wu et al. in 2017 developed a dexamethasone sodium phosphate (Dex) supramolecular hydrogel and tested its effectiveness in controlling ocular inflammation. It was found to have thixotropic properties that were influenced by the concentration of calcium ions and Dex. Additionally, the drug release rate from the hydrogel was also dependent on the concentration of calcium ions. When the DDS was administered as a single intravitreal injection in an EAU model in rats, the method was well tolerated with minimal risk of inducing lens opacity and fundus blood vessel tortuosity and showed similar anti-inflammatory effects to the native Dex solution. The hydrogel was found to be effective in reducing inflammatory responses in both the anterior and posterior chambers of the eye while downregulating Th1 and Th17 effector cells. Although the exact molecular processes behind uveitis are not fully understood, there is increasing evidence to suggest that T cells play a role in the development of uveitis [15].
Safwat et al. published an article in 2020 where they looked at using micelles made from a combination of triamcinolone acetonide (TA) and either poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL) or poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PLA) as a potential treatment for ocular inflammation. Both types of micelles had high drug loading and encapsulation efficiencies, but the PEG2-b-PLA1 micelles had the highest capacity. To extend the drug’s residence time in the eye, the highest-capacity micelles were suspended in chitosan hydrogel. This slowed the drug’s release rate, with only approximately 42% being released after one week, compared to 95% in just 8 h when the drug was suspended on its own. When tested in a rabbit model of ocular inflammation, the PEG2-b-PLA1 micelles suspended in chitosan hydrogel were effective in reducing inflammation and restoring normal corneal tissue [16].
In a study by Tiwari et al. in 2017, they described an ocular self-microemulsifying drug delivery system (SMEDDS) of prednisolone to improve the treatment of experimental uveitis in rabbits. The developed SMEDDS was formulated with linoleic acid, Cremophore RH 40, and propylene glycol, which showed acceptable physicochemical properties, stability, and sustained drug release. When tested topically in a rabbit eye model, it was deemed tolerable without any signs of irritation while showcasing significant improvements in anti-inflammatory activity compared to a marketed formulation. The conclusions of the study suggest that these SMEDDS could potentially be a viable alternative to eye drop treatments due to their ability to increase bioavailability [17]. In a study by Yu et al. published in 2018, they created a hydrogel composed of a GFFY peptide linked to ibuprofen via an ester bond for the treatment of ocular inflammation. The hydrogel demonstrated negligible cytotoxicity and sustained ibuprofen release when activated, showcasing an enzymatically controlled drug delivery method with a higher anti-inflammatory effect when compared to just administering ibuprofen on its own in RAW264.7 macrophages. When this formulation was tested in vivo through a rabbit model, they found that the hydrogel had similar anti-inflammatory therapeutic effects compared to the current treatment, which was sodium diclofenac eyedrops. This was the first study to demonstrate an effective method of supramolecular assemblies in reducing ophthalmic inflammation [18]. However, the biodegradability of the DDS in the two previous studies is uncertain. There is limited evidence on the biodegradability of the self-microemulsifying drug delivery system (SMEDDS), made up of ingredients such as oil and surfactants, used as ophthalmic drug delivery with the peptide supramolecular hydrogel composition.
A noteworthy study utilized a biodegradable intravitreal implant. In a recent research article by Paiva et al. in 2021, they examined the effects of a biodegradable sirolimus-loaded PGLA intravitreal implant for treating uveitis in rabbits. Through clinical and histopathological exams after 35 days, the treated eyes with implants revealed less severe inflammation and reduced damage in cell infiltration in the anterior and posterior portions of the eye while preserving the integrity of blood-ocular barriers. Based on these findings and due to the lack of signs of cataracts, hemorrhage, or other signs of toxicity, the SRL-PLGA implant was deemed to be a safe and promising future treatment for non-infectious uveitis [19].
Finally, Alshmsan et al. evaluated the topical treatment of uveitis through PolyGel™, a poly(α-carboxylate-co-α-benzylcarboxylate-ε-caprolactone)-block-poly(ethylene glycol)-block-poly(α-carboxylate-co-α-benzylcarboxylate-ε-caprolactone) as an in situ gel system for delivery of Cyclosporine A compared to Restasis, and PEO-b-PCL, which is a non-gelling micelle formulation. The irritation studies showed that while PEO-b-PCL and PolyGel are tolerable for use in the eye, the highest ocular bioavailability of cyclosporine A was found in Restasis®. The PolyGel allowed for the most prolonged penetration of the drug into the eye, but the amount of the drug that permeated into the eye was initially higher with Restasis® and lower in the other two. Despite this, both CyA-PolyGel™ and Restasis® were effective at reducing inflammation in the eyes of rabbits, making them potentially suitable for treating uveitis [20].
Despite the encouraging results that have been achieved, the full extent of the long-term effects of nano-based biodegradable DDS is still unclear. The studies mentioned above have only used animal models with artificially induced uveitis and have not taken into consideration the effects of DDS on animal models with pre-existing conditions that can cause uveitis. It is crucial that before moving forward with clinical trials, further research is conducted using animal models that have underlying diseases that lead to uveitis, rather than relying solely on those with experimentally induced uveitis. This will provide a clearer understanding of the impact of these DDS on the natural progression of the condition and the potential for any long-term adverse effects.
3.2. Biodegradable Nano-Based DDS for Anterior Uveitis
In a study by Wong et al. in 2018, the effectiveness of liposomal triamcinolone acetonide phosphate and liposomal prednisolone phosphate as a treatment for anterior uveitis in rabbits was evaluated. Liposomes have been widely studied for various purposes due to their biocompatibility and biodegradability. In this research, liposomes were administered as a single subconjunctival injection, and the results showed that the liposomal treatment significantly reduced inflammatory scores in the rabbits compared to untreated controls on days 4 and 8 after the induction of uveitis. The liposomal treatment was also found to be more effective at reducing inflammation than topical prednisolone on day 8. Furthermore, the anti-inflammatory effect of the liposomal treatment persisted even after being challenged with the antigen on day 11. Through histology and immunostaining, the localization of the liposomes was observed to remain in the eye for at least one month. In addition, the researchers highlighted that this methodology poses little risk of globe injury compared to peribulbar injections and provides no risk of endophthalmitis, which is usually associated with intravitreal and intracameral injections. However, this study was not designed to assess the potential adverse effects of the treatment, and the follow-up period may not have been long enough to identify the development of long-term complications such as cataracts and increased intraocular pressure. This study was the first to compare the effectiveness of a single subconjunctival injection of liposomal steroids to a single injection of unencapsulated steroids and to the current standard treatment of intensive topically applied steroid eyedrops in treating anterior uveitis [21].
Another lipid carrier was assessed according to a study by Garg et al. published in 2021. Cationic nanostructured lipid carriers (NLCs) were prepared as a drug delivery system to increase the penetration and retention of corticosteroids in the eye. These NLCs, which contain the drug triamcinolone acetonide, were small in size (less than two hundred nanometers), contained a positive charge, and were made using a hot microemulsion method. They released the drug slowly and sustainably over a period of 24 h, and it was shown to be non-toxic, non-irritant, and effective in reducing inflammation in cells. The cationic NLCs also had a high level of drug entrapment efficiency (88%) and could be taken up by cells, remaining inside for a period of 24 h but penetrating deeper into eye layers within 2 h. These characteristics make cationic NLCs a promising option for improving the effectiveness of corticosteroid treatment for anterior uveitis and other ocular conditions [22].
In addition, the study by Alami-Milani et al. (2019) revealed that polycaprolactone-polyethylene glycol-polycaprolactone (PCL-PEG-PCL) micelles could be used to improve the anti-inflammatory effects of dexamethasone (DEX) in the treatment of anterior uveitis. They showed that the PCL-PEG-PCL micelles had good compatibility and uptake by cells, and they reduced the symptoms of uveitis after a lag time. However, the micelles were not significantly more effective than the marketed dexamethasone eye drop at 24 and 36 h after treatment. This suggests that the PCL-PEG-PCL micelles have potential as carriers for DEX in the treatment of uveitis, but more research is needed to fully understand their potential, such as including more trials and monitoring the prolonged anti-inflammatory impacts of sustained-release of the compound [23].
Moreover, an important development in the field of anterior uveitis treatment is the use of biodegradable hydrogels. In a study by Fang et al. in 2022, polypseudorotaxane hydrogels were created by mixing Soluplus micelles with a cyclodextrin solution. These hydrogels have the ability to thin under shear force and release their contents over an extended period of time. They also showed higher transcorneal permeability, increased precorneal retention, and intraocular bioavailability of flurbiprofen in animal studies. These hydrogels were also effective at reducing inflammation in a rabbit model of endotoxin-induced uveitis with fewer administrations and were shown to be safe in cytotoxicity and ocular irritation studies [24].
In another article by Yu et al. in 2019, they developed and tested dexamethasone-peptide conjugate, which is made up of a combination of dexamethasone and a peptide connected by a biodegradable ester bond. The formulation could form a high concentration of nanoparticles in aqueous solution to treat anterior uveitis, and topical instilled application was well tolerated as it did not cause any significant side effects such as alteration of the thickness of the cornea or intraocular pressure. This new formulation was found to be just as effective as aqueous solutions containing dexamethasone sodium phosphate [25].
3.3. Biodegradable Nano-Based DDS for Posterior Uveitis
Posterior uveitis is a difficult to manage condition due to its localized inflammation in the posterior segments of the eye and the presence of critical structures such as the macula, optic nerve, and retinal vessels. Irreversible vision loss and blindness can quickly occur if these critical structures are affected. Diagnosis is more challenging than anterior uveitis, as microbiological sampling is difficult and the condition is often associated with underlying infectious or systemic causes that require multimodal treatments. Invasive administration routes, such as periocular or intravitreal injection, are often necessary as topical eye drops cannot effectively penetrate the ocular barriers to reach the posterior segment. Systemic medications may also be used in bilateral cases but have poor penetration and bioavailability to ocular structures due to the blood-retinal barrier.
In the literature, it is known that both intravitreal and periocular injections of triamcinolone acetonide suspension maintain a high risk for negative effects such as high intraocular pressure and retinal toxicity, despite being a treatment option for non-infectious posterior uveitis. Therefore, in 2018, Xiong et al. reported the use of hydrogels as an alternative treatment. They generated an injectable glycosylated triamcinolone acetonide hydrogelator (TA-SA-Glu) hydrogel that is thermosensitive to ease uveitis. This novel biodegradable DDS was found to have minimal retinal toxicity when injected at a dosage of 69 nmol per eye in an in vivo rat study and was more effective in controlling non-infectious posterior uveitis than the conventional TA injection. Particularly, the TA-SA-glu hydrogel system aids the downregulation of pro-inflammatory effector responses in Th1 and Th17 effector cells [26].
In 2021, Mehra et al. proposed the use of a topical nanomicellar formulation using Soluplus, a copolymer of polyvinyl caprolactam-polyvinylalcohol-polyethyleneglycol (PVCL-PVA-PEG) to deliver everolimus to treat posterior uveitis using ex vivo goat cornea and in vitro methods. The nanomicelles were found to have a low critical micelle concentration, be 65.55 nm in size, and have a smooth surface, as well as high encapsulation efficiency and sustained release of everolimus. They also showed significantly higher permeation across the goat cornea compared to everolimus suspension and deeper permeation through the cornea, as confirmed by confocal laser scanning microscopy. The nanomicellar formulation also improved drug bioavailability while remaining in the circulatory system for a longer duration and seeming to have accumulated in the inflammatory site of interest. As this drug delivery system demonstrated adequate stability, there was no toxicity in the eye as found in the Hen’s egg test-chorioallatonic membrane assay, so it can be reassured that the prepared method is safe for ocular use [27].
Nanoparticles have also been researched for posterior uveitis. In a study by Badr et al. in 2022, they employed a mouse model of posterior EAU to assess its treatment with rapamycin in a nanoparticle-based eye drop. The compound called Molecular Envelope Technology-Rapamycin (MET-RAP) was successful in controlling the progression of the disease by reducing the level of a protein called RORγt, increasing the expression of Foxp3, and increasing the secretion of IL-10. These effects likely play a role in the mechanism that shifts the balance between T helper 17 cells and regulatory T cells, which in turn reduces the progression of EAU. Based on these results, MET-RAP eye drops may be a promising treatment for retinal inflammatory diseases [28].
3.4. Biodegradable Nano-Based DDS for Endophthalmitis
Endophthalmitis is an inflammation of the vitreous and aqueous fluids within the eye caused by a bacterial or fungal infection. It can occur due to various reasons, such as intraocular surgery, trauma, or corneal ulcers. The hallmark of endophthalmitis is the infiltration of the vitreous cavity and anterior chamber with inflammatory cells, leading to clinical signs of vitritis and hypoplasia. Treatment options can include eyedrops or intravitreal methods containing antibiotics or antifungals with multiple drug combinations, and pars plana vitrectomy (PPV). However, the use of intravitreal antibiotics can result in side effects such as retinal toxicity and corneal opacification. PPV is an invasive surgical procedure that comes with several risks and complications, including but not limited to retinal tears and detachments, increased intraocular pressure, re-infection, and permanent vision loss. Systemic antibiotics are usually not sufficient as monotherapy and are used in combination with intravitreal antibiotics [29].
A study by Coburn et al., published in 2019, found that biomimetic nanosponges, synthetic materials that mimic the properties of red blood cells, have been shown to neutralize pore-forming toxins and preserve retinal function. Their findings showed that nanosponge pretreatment reduced hemolytic activity in vitro, improved retinal function, and reduced ocular pathology in a murine model of endophthalmitis. Treatment with gatifloxacin and gatifloxacin-nanosponges also reduced intraocular bacterial burdens and decreased ocular pathology and inflammation. In the future, research should be conducted to determine the pharmacokinetics of red blood cell-derived nanosponges when used in combination with antibiotics to treat eye infections, as well as to optimize the concentrations and timing of administration. This will provide the necessary foundation for the use of nanosponges as a supplementary treatment for bacterial intraocular infections [30]. While nanosponges showcase promising data, unilamellar liposomes have also recently been investigated for periocular transscleral co-delivery of steroids and antibiotics, offering potential new possibilities for treating chronic ophthalmic infections [31].
Alternatively, nanoparticles can be useful for the management of endophthalmitis. An article by Mahaling et al. in 2021 created a nanoparticle delivery system made up of a hydrophobic polylactic acid (PLA) core and a hydrophilic chitosan (CHI) shell, which contained either the antibiotic azithromycin (AZM) or the corticosteroid triamcinolone acetonide (TCA). These nanoparticles were developed for the treatment of endophthalmitis and were administered as an eyedrop. The delivery system showed good compatibility with blood and tissues as well as sustained release of the drugs, making it suitable for long-term treatment of endophthalmitis. The combination of PLA-CHI-AZM and PLA-CHI-TCA nanoparticles was more effective in treating endophthalmitis than any of the individual components alone, due to their enhanced antibacterial and anti-inflammatory properties. This nanoparticle-based combinatorial drug delivery system administered as an eye drop could be a promising non-invasive therapy for endophthalmitis [32].
3.5. Biodegradable Nano-Based DDS for Postoperative Uveitis and Endophthalmitis
Receptor-mediated drug delivery has been recently explored for the use of postoperative uveitis treatment. In a recent study published in 2020, Ganugula et al. assessed the capacity of receptor-mediated delivery of curcumin to decrease inflammation in a model of lens-induced uveitis. The researchers successfully encapsulated curcumin in double-headed polyester nanoparticles using gambogic acid as a coupling agent and PLGA as the polymer. When administered orally to canine models with lens-induced uveitis, these PLGA-GA2-CUR nanoparticles resulted in significant levels of curcumin in the aqueous humor and produced comparable clinical effects to commonly used anti-inflammatory medications. This novel nanoparticle delivery system may enhance the bioavailability of curcumin while reducing the adverse effects associated with the use of topical corticosteroids or NSAIDs [33].
Another method that has been tested and proven successful is the use of a chitosan-based hydrogel, as reported by Cheng et al. in 2019, for the treatment and prevention of postoperative endophthalmitis. Their goal was to generate sustained drug release that could deliver levofloxacin safely and effectively without the need for injections or topical eyedrops. When tested in the laboratory, in vitro studies showed that the hydrogel was found to release the drug in a sustained manner and showed good antibacterial properties against certain bacteria through the observation of a significant inhibition zone of the bacteria and the long-term antibacterial property of the developed hydrogel. In addition, the hydrogel was found to be biocompatible with corneal epithelial cells [34]. The following year, an article by Cheng et al. 2021 created and studied a dual drug delivery system known as PAgel-LNPs, which is made up of a thermosensitive chitosan/gelatin-based hydrogel that is able to sustainably release two drugs, prednisolone acetate and levofloxacin-loaded nanoparticles (LNPs). They suggest that the optimal concentrations of these drugs for the treatment of corneal epithelial cells are 5 μg/mL and 50 μg/mL for LNP and prednisolone acetate, respectively. PAgel-LNPs contain a porous structure and have been shown to be biocompatible, thermosensitive, and capable of sustained drug release. In laboratory tests using damaged corneal epithelial cells and a rabbit model of S. aureus keratitis, the system demonstrated anti-inflammatory and anti-bacterial properties. This drug delivery system may have the potential to be used for the treatment and prevention of postoperative uveitis and may also improve patient compliance due to its ability to sustainably release both levofloxacin and prednisolone acetate [35].
Furthermore, unique nanoparticles composed of AuAgCu2O-bromfenac sodium (AuAgCu2O-BS NPs) have shown promising results. In an article by Ye et al. published in 2020, they formed AuAgCu2O-BS nanoparticles that were designed to combine anti-bacterial and anti-inflammatory effects to treat postoperative endophthalmitis after cataract surgery. The preliminary toxicity investigations of the nanosystem revealed its superior biocompatibility, with low levels of cytotoxicity and minimal impact on intraocular pressure and other major organs. This study provides a promising synergic therapeutic strategy for the treatment of post-cataract extraction endophthalmitis [36].
References
- Bodratti, A.M.; Alexandridis, P. Amphiphilic Block Copolymers in Drug Delivery: Advances in Formulation Structure and Performance. Expert Opin. Drug Deliv. 2018, 15, 1085–1104.
- Allyn, M.M.; Luo, R.H.; Hellwarth, E.B.; Swindle-Reilly, K.E. Considerations for Polymers Used in Ocular Drug Delivery. Front. Med. 2021, 8, 787644.
- Valdes, L.M.; Sobrin, L. Uveitis Therapy: The Corticosteroid Options. Drugs 2020, 80, 765–773.
- Kasper, M.; Gabriel, D.; Möller, M.; Bauer, D.; Wildschütz, L.; Courthion, H.; Rodriguez-Aller, M.; Busch, M.; Böhm, M.R.R.; Loser, K.; et al. Cyclosporine A-Loaded Nanocarriers for Topical Treatment of Murine Experimental Autoimmune Uveoretinitis. Mol. Pharm. 2018, 15, 2539–2547.
- Rebibo, L.; Tam, C.; Sun, Y.; Shoshani, E.; Badihi, A.; Nassar, T.; Benita, S. Topical Tacrolimus Nanocapsules Eye Drops for Therapeutic Effect Enhancement in Both Anterior and Posterior Ocular Inflammation Models. J. Control. Release Off. J. Control. Release Soc. 2021, 333, 283–297.
- Chen, Z.; Yang, M.; Wang, Q.; Bai, J.; McAlinden, C.; Skiadaresi, E.; Zhang, J.; Pan, L.; Mei, C.; Zeng, Z.; et al. Hydrogel Eye Drops as a Non-Invasive Drug Carrier for Topical Enhanced Adalimumab Permeation and Highly Efficient Uveitis Treatment. Carbohydr. Polym. 2021, 253, 117216.
- Luo, L.; Yang, J.; Oh, Y.; Hartsock, M.J.; Xia, S.; Kim, Y.-C.; Ding, Z.; Meng, T.; Eberhart, C.G.; Ensign, L.M.; et al. Controlled Release of Corticosteroid with Biodegradable Nanoparticles for Treating Experimental Autoimmune Uveitis. J. Control. Release Off. J. Control. Release Soc. 2019, 296, 68–80.
- Guo, D.; Li, Q.; Sun, Y.; Guo, J.; Zhao, Q.; Yin, X.; Wei, H.; Wu, S.; Bi, H. Evaluation of Controlled-Release Triamcinolone Acetonide-Loaded MPEG-PLGA Nanoparticles in Treating Experimental Autoimmune Uveitis. Nanotechnology 2019, 30, 165702.
- Huang, J.; Yu, X.; Zhou, Y.; Zhang, R.; Song, Q.; Wang, Q.; Li, X. Directing the Nanoparticle Formation by the Combination with Small Molecular Assembly and Polymeric Assembly for Topical Suppression of Ocular Inflammation. Int. J. Pharm. 2018, 551, 223–231.
- Xing, Y.; Zhu, L.; Zhang, K.; Li, T.; Huang, S. Nanodelivery of Triamcinolone Acetonide with PLGA-Chitosan Nanoparticles for the Treatment of Ocular Inflammation. Artif. Cells Nanomed. Biotechnol. 2021, 49, 308–316.
- Li, H.; Zhang, Z.; Li, Y.; Su, L.; Duan, Y.; Zhang, H.; An, J.; Ni, T.; Li, X.; Zhang, X. Therapeutic Effect of Rapamycin-Loaded Small Extracellular Vesicles Derived from Mesenchymal Stem Cells on Experimental Autoimmune Uveitis. Front. Immunol. 2022, 13, 864956.
- Garg, V.; Nirmal, J.; Riadi, Y.; Kesharwani, P.; Kohli, K.; Jain, G.K. Amelioration of Endotoxin-Induced Uveitis in Rabbit by Topical Administration of Tacrolimus Proglycosome Nano-Vesicles. J. Pharm. Sci. 2021, 110, 871–875.
- Gaballa, S.A.; El Garhy, O.H.; Moharram, H.; Abdelkader, H. Preparation and Evaluation of Cubosomes/Cubosomal Gels for Ocular Delivery of Beclomethasone Dipropionate for Management of Uveitis. Pharm. Res. 2020, 37, 198.
- Vaneev, A.N.; Kost, O.A.; Eremeev, N.L.; Beznos, O.V.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Chesnokova, N.B.; Kabanov, A.V.; Klyachko, N.L. Superoxide Dismutase 1 Nanoparticles (Nano-SOD1) as a Potential Drug for the Treatment of Inflammatory Eye Diseases. Biomedicines 2021, 9, 396.
- Wu, W.; Zhang, Z.; Xiong, T.; Zhao, W.; Jiang, R.; Chen, H.; Li, X. Calcium Ion Coordinated Dexamethasone Supramolecular Hydrogel as Therapeutic Alternative for Control of Non-Infectious Uveitis. Acta Biomater. 2017, 61, 157–168.
- Safwat, M.A.; Mansour, H.F.; Hussein, A.K.; Abdelwahab, S.; Soliman, G.M. Polymeric Micelles for the Ocular Delivery of Triamcinolone Acetonide: Preparation and in Vivo Evaluation in a Rabbit Ocular Inflammatory Model. Drug Deliv. 2020, 27, 1115–1124.
- Tiwari, R.; Dubey, V.; Kesavan, K. Ocular Self-Microemulsifying Drug Delivery System of Prednisolone Improves Therapeutic Effectiveness in the Treatment of Experimental Uveitis. Ocul. Immunol. Inflamm. 2019, 27, 303–311.
- Yu, X.; Zhang, Z.; Yu, J.; Chen, H.; Li, X. Self-Assembly of a Ibuprofen-Peptide Conjugate to Suppress Ocular Inflammation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 185–193.
- Paiva, M.R.B.D.; Vasconcelos-Santos, D.V.; Vieira, L.C.; Fialho, S.L.; Silva-Cunha, A. Sirolimus-Loaded Intravitreal Implant for Effective Treatment of Experimental Uveitis. AAPS PharmSciTech 2021, 22, 35.
- Alshamsan, A.; Abul Kalam, M.; Vakili, M.R.; Binkhathlan, Z.; Raish, M.; Ali, R.; Alturki, T.A.; Safaei Nikouei, N.; Lavasanifar, A. Treatment of Endotoxin-Induced Uveitis by Topical Application of Cyclosporine a-Loaded PolyGelTM in Rabbit Eyes. Int. J. Pharm. 2019, 569, 118573.
- Wong, C.W.; Czarny, B.; Metselaar, J.M.; Ho, C.; Ng, S.R.; Barathi, A.V.; Storm, G.; Wong, T.T. Evaluation of Subconjunctival Liposomal Steroids for the Treatment of Experimental Uveitis. Sci. Rep. 2018, 8, 6604.
- Nirbhavane, P.; Sharma, G.; Singh, B.; Begum, G.; Jones, M.-C.; Rauz, S.; Vincent, R.; Denniston, A.K.; Hill, L.J.; Katare, O.P. Triamcinolone Acetonide Loaded-Cationic Nano-Lipoidal Formulation for Uveitis: Evidences of Improved Biopharmaceutical Performance and Anti-Inflammatory Activity. Colloids Surf. B Biointerfaces 2020, 190, 110902.
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Sattari, S.; Salatin, S.; Jelvehgari, M. Evaluation of Anti-Inflammatory Impact of Dexamethasone-Loaded PCL-PEG-PCL Micelles on Endotoxin-Induced Uveitis in Rabbits. Pharm. Dev. Technol. 2019, 24, 680–688.
- Fang, G.; Wang, Q.; Yang, X.; Qian, Y.; Zhang, G.; Tang, B. γ-Cyclodextrin-Based Polypseudorotaxane Hydrogels for Ophthalmic Delivery of Flurbiprofen to Treat Anterior Uveitis. Carbohydr. Polym. 2022, 277, 118889.
- Yu, X.; Zhang, R.; Lei, L.; Song, Q.; Li, X. High Drug Payload Nanoparticles Formed from Dexamethasone-Peptide Conjugates for the Treatment of Endotoxin-Induced Uveitis in Rabbit. Int. J. Nanomed. 2019, 14, 591–603.
- Xiong, T.; Li, X.; Zhou, Y.; Song, Q.; Zhang, R.; Lei, L.; Li, X. Glycosylation-Enhanced Biocompatibility of the Supramolecular Hydrogel of an Anti-Inflammatory Drug for Topical Suppression of Inflammation. Acta Biomater. 2018, 73, 275–284.
- Mehra, N.; Aqil, M.; Sultana, Y. A Grafted Copolymer-Based Nanomicelles for Topical Ocular Delivery of Everolimus: Formulation, Characterization, Ex-Vivo Permeation, in-Vitro Ocular Toxicity, and Stability Study. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2021, 159, 105735.
- Badr, M.Y.; Halwani, A.A.; Odunze, U.; Eskandarpour, M.; Calder, V.L.; Schätzlein, A.G.; Uchegbu, I.F. The Topical Ocular Delivery of Rapamycin to Posterior Eye Tissues and the Suppression of Retinal Inflammatory Disease. Int. J. Pharm. 2022, 621, 121755.
- Baudin, F.; Benzenine, E.; Mariet, A.-S.; Ben Ghezala, I.; Bron, A.M.; Daien, V.; Korobelnik, J.F.; Quantin, C.; Creuzot-Garcher, C. Epidemiology of Acute Endophthalmitis after Intraocular Procedures: A National Database Study. Ophthalmol. Retin. 2022, 6, 442–449.
- Disarming Pore-Forming Toxins with Biomimetic Nanosponges in Intraocular Infections|Msphere. Available online: https://journals.asm.org/doi/10.1128/mSphere.00262-19 (accessed on 5 February 2023).
- Su, J.; Lu, W.; Guo, Y.; Liu, Z.; Wang, X.; Yan, H.; Zhang, R.X. Depot Unilamellar Liposomes to Sustain Transscleral Drug Co-Delivery for Ophthalmic Infection Therapy. J. Drug Deliv. Sci. Technol. 2023, 86, 104629.
- Mahaling, B.; Baruah, N.; Ahamad, N.; Maisha, N.; Lavik, E.; Katti, D.S. A Non-Invasive Nanoparticle-Based Sustained Dual-Drug Delivery System as an Eyedrop for Endophthalmitis. Int. J. Pharm. 2021, 606, 120900.
- Ganugula, R.; Arora, M.; Lepiz, M.A.; Niu, Y.; Mallick, B.K.; Pflugfelder, S.C.; Scott, E.M.; Kumar, M.N.V.R. Systemic Anti-Inflammatory Therapy Aided by Double-Headed Nanoparticles in a Canine Model of Acute Intraocular Inflammation. Sci. Adv. 2020, 6, eabb7878.
- Cheng, Y.-H.; Chang, Y.-F.; Ko, Y.-C.; Liu, C.J.-L. Sustained Release of Levofloxacin from Thermosensitive Chitosan-Based Hydrogel for the Treatment of Postoperative Endophthalmitis. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 8–13.
- Cheng, Y.-H.; Chang, Y.-F.; Ko, Y.-C.; Liu, C.J.-L. Development of a Dual Delivery of Levofloxacin and Prednisolone Acetate via PLGA Nanoparticles/ Thermosensitive Chitosan-Based Hydrogel for Postoperative Management: An in-Vitro and Ex-Vivo Study. Int. J. Biol. Macromol. 2021, 180, 365–374.
- Ye, Y.; He, J.; Qiao, Y.; Qi, Y.; Zhang, H.; Santos, H.A.; Zhong, D.; Li, W.; Hua, S.; Wang, W.; et al. Mild Temperature Photothermal Assisted Anti-Bacterial and Anti-Inflammatory Nanosystem for Synergistic Treatment of Post-Cataract Surgery Endophthalmitis. Theranostics 2020, 10, 8541–8557.
More
Information
Subjects:
Ophthalmology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
24 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




