You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Brandon Lucke-Wold | -- | 1642 | 2023-07-24 02:24:48 | | | |
| 2 | Wendy Huang | Meta information modification | 1642 | 2023-07-24 03:06:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nguyen, A.; Nguyen, A.; Dada, O.T.; Desai, P.D.; Ricci, J.C.; Godbole, N.B.; Pierre, K.; Lucke-Wold, B. Mimics of Leptomeningeal Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/47154 (accessed on 27 December 2025).
Nguyen A, Nguyen A, Dada OT, Desai PD, Ricci JC, Godbole NB, et al. Mimics of Leptomeningeal Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/47154. Accessed December 27, 2025.
Nguyen, Andrew, Alexander Nguyen, Oluwaferanmi T. Dada, Persis D. Desai, Jacob C. Ricci, Nikhil B. Godbole, Kevin Pierre, Brandon Lucke-Wold. "Mimics of Leptomeningeal Disease" Encyclopedia, https://encyclopedia.pub/entry/47154 (accessed December 27, 2025).
Nguyen, A., Nguyen, A., Dada, O.T., Desai, P.D., Ricci, J.C., Godbole, N.B., Pierre, K., & Lucke-Wold, B. (2023, July 24). Mimics of Leptomeningeal Disease. In Encyclopedia. https://encyclopedia.pub/entry/47154
Nguyen, Andrew, et al. "Mimics of Leptomeningeal Disease." Encyclopedia. Web. 24 July, 2023.
Copy Citation
Leptomeningeal disease (LMD) is commonly characterized as the metastatic involvement of various meningeal regions—the arachnoid mater, subarachnoid space, and pia mater—defined as the leptomeningeal layer. LMD can stem from non-cancerous pathologies, such as multiple sclerosis (MS) or meningitis with subsequent inflammation and infection, respectively. Clinically, the presentation of LMD includes non-specific, generalized neurological symptoms, such as headaches, confusion, seizures, and radiculopathy, among others.
leptomeningeal disease
central nervous system
MRI
CSF
sarcoidosis
mimic
1. Introduction
The Prevalence of LMD exists in 1–8% of all cancer patients, with a recorded incidence of 110,000 cases in the US annually [1]. Furthermore, the prevailing sites of origin include breast and lung cancer, melanoma, and lastly, primary central nervous system (CNS) cancers [1]. Additionally, hematologic origins, namely non-Hodgkin’s lymphoma (NHL), acute lymphoblastic leukemia (ALL), and multiple myeloma, are seldom negligible in occurrence [2]. Several mechanisms for the general onset of LMD have been purported: (1) direct invasion via surrounding structures, such as the dura mater, bone, or nerves; (2) hematogenous spread often by way of venous vasculature; and, lastly, (3) entry of the fenestrated pores of the choroid plexus typically permitting solute transport [1][3]. Within LMD-related cerebrospinal fluid (CSF), the presence of increased levels of complement component three protein (C3) has been observed. Postulations describe the role of C3 in its ability to interact with choroid plexus C3a receptors, thereby increasing the endothelial permeability of the normally intact barrier [4].
Clinically, the presentation of LMD includes non-specific, generalized neurological symptoms, such as headaches, confusion, seizures, and radiculopathy, among others. Though this substantially shifts the weight of efficacy to other modes of diagnosis, more refined schemas for focused diagnosis have been formally developed. For example, within cerebral localizations, symptoms may manifest as headache and confusion, while cranial nerve (CN) deficits may be attributed to involvement of the posterior fossa; the anatomical afflictions and their corresponding presentations are described more extensively in recent literature [3]. Regarding prognosis, the National Comprehensive Cancer Network (NCNN) has established technical criteria for stratification of prognosis [5]. Karnofsky Performance Scale (KPS) values < 60, systematic neurological presentation, and encephalopathy have all been associated with a higher risk of disease progression. Additionally, hematologic origins have displayed seemingly better outcomes [6]. To date, the clinical repertoire for guiding differential diagnoses has been through obtaining medical history and physical examination. It is important to note the overlap between COVID-19 neurological symptoms and findings with leptomeningeal metastasis. The two pathologies share both molecular and structural shifts. Namely, COVID-19 patients have been shown to present with CSF positive for LMD inflammatory cytokines and MRI involvement within LMD regions of interest. Similarities are also found in other disorders, such as Sturge-Weber syndrome (SWS), due to the presence of leptomeningeal angiomatosis. Overall, the median time from diagnosis of primary cancer to LMD has ranged from 1.2–2 years for solid tumors and 11 months for hematologic cancers [3].
On the matter of modality-based diagnosis, the gold standard comprises two modalities in the form of imaging and cytological analyses. T1-weighted magnetic resonance imaging (MRI) with gadolinium contrast is the imaging tool of choice. The observational patterns to hearken to include various morphologic enhancements of the cranial nerve, or linear/curvilinear enhancements, and nodular aberrancies [2][7]. These are often noted in select areas, such as the cerebral convexities, basal cisterns, and ventricular ependyma [4]. Figure 1 depicts an MRI scan of leptomeningeal involvement following breast cancer metastases. Within the spine, particularly in the cauda equina region, similar observations should raise suspicion for LMD [4]. Preliminary MRI is becoming an increasingly routine measure for early brain metastasis. As such, several structure densities along the brainstem, cranial nerves, meninges, and ventricles have been correlated with leptomeningeal metastasis. Of note, some findings, such as increased ventricle MRI density, can be a consequence of LMD-associated disorders, in this case hydrocephalus. Since negative MRI findings are not exclusionary, the second modality, CSF cytology, is an essential layer in the diagnosis of this pathology. Conventional cytology hinges on the presence of malignant cells, irrespective of their primary origin, as determined by a cytopathologist. However, cancer specific markers may aid in the determination of the origin, such as the presence of VEGF. In general, the sensitivity and specificity of LMD diagnosis following CSF cytology range from 50–60% and 75–80%, respectively, upon first and second aspirations of CSF [1][3]. Therefore, it is highly encouraged to conduct a second analysis of the CSF when possible, in order to increase the diagnostic capacity for LMD. As a soft requirement, a minimum of 10 CSF mL should be collected for sufficient analysis, with some studies reporting a minimum of 5–10 mL [1][3]. Additionally, it is recommended that the suspected region of affliction (based on several parameters, such as clinical presentation and imaging) be the area of aspiration; in cases where this is not feasible, the lumbar or cisternal regions should be considered as immediate alternatives [1]. The select biomarkers that are measured to supplement ruling in LMD include pleocytosis, hypoglycorrhachia, and hyperproteinorrachia (elevated CSF protein levels). These findings have been observed with a sensitivity of 50–70% [8]. More novel forms of investigation have arisen in the form of tumor-specific antigens, such as carcinoembryonic antigen (CEA) and alpha-fetoprotein. The latest of these endeavors has manifested itself by relying on cell-free DNA as a source of direction [3]. The sensitivity of this latter technique has been nascently explored, though its sensitivity has been reported at 94% with a specificity of 100% [9]. Indeed, in a recent meta-analysis of 668 patients with circulating tumor cells (CTC) and cell-free tumor DNA, sensitivities and specificities were reported at 87.0% and 97.9% (sensitivity, respectively) and 93.8% and 89.0% (specificity, respectively). This has been more robustly assessed in particular cancer-specific LMDs, such as breast cancer [10]. This includes optimization of isolating and purifying circulating tumor-specific DNA and subsequently identifying targetable mutations. Abnormal MRI and CSF findings, as previously mentioned, in the presence of generalized neurological symptoms can be cause for LMD suspicion.
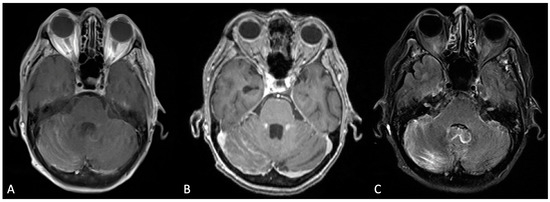
Figure 1. Bilateral cerebellar folia hyperenhancement, more prominent on the right, was observed in the post-contrast T1-weighted Turbo Spin Echo (TSE) sequence (A); the T1-weighted Multi-Planar Reconstruction (MPR) sequence (B); and the T2-weighted Turbo Inversion Recovery Magnitude Fat Suppressed (TIRM-FS) sequence (C).
2. Leptomeningeal Mimics
To note, leptomeningeal involvement need not be attributed to metastatic or malignant etiologies. Various diseases may present with leptomeningeal involvement, mimicking LMD. Currently, these have been sparsely described in the literature, primarily in the context of case reports, including rare instances of neuroborreliosis, giant cell arteritis, and sarcoidosis [11][12][13].
In particular, sarcoidosis appears to be the most widely encountered mimic of LMD in instances of CNS involvement. Sarcoidosis is well acknowledged as a systemic ailment characterized most aptly upon histological observation of non-caseating sarcoid granulomas [14]. Its prevalence ranges from 0.001% to 0.04% of individuals, more widely affecting females and those aged 20–40 [15][16]. To date, it carries an unexplainable etiology, shifting considerable diagnostic reliance on biopsy. The majority of sarcoidosis cases affect the respiratory system in nearly 90% of individuals, inadvertently lowering suspicion in the context of other organ involvement [14]. However, in the context of CNS involvement, it is particularly salient to acknowledge any form of systemic involvement prior to the onset of neurological symptoms. This can provide considerable guidance towards the diagnosis of sarcoidosis pertinent to the regions of the CNS—neurosarcoidosis [15][17].
Neurosarcoidosis can affect the brain, cranial nerves, meninges, and spinal cord [17]. Symptomatic presentation can manifest as generalized neurological symptoms including headache, diplopia, and vertigo, among others, providing low utility regarding neurosarcoidosis diagnosis, though the sequence of region involvement can provide more value in this; moreover, the number of systems involved has displayed a notable association [17]. For example, in a prospective study of 166 patients, systemic manifestation in 100 cases coincided with, in 55 cases preceded, and in only 10 cases followed CNS involvement chronologically [17]. Other pertinent biomarkers included MRI and CSF abnormalities, as well as LMD. This involved hyperproteinorrachia, hypoglycorrhachia, and elevated angiotensin converting enzyme (ACE) CSF levels [17]. MRI observations included regional enhancement on T1-weighted MRI scans following gadolinium administration [18]. Overall, it veers toward a rule-out approach, requiring sound exclusion for other etiologic explanations. Current treatment options are rarely definitive and exist primarily in the form of inflammatory control, as with other forms of sarcoidosis, including glucocorticoids and inflammatory modulators, such as tumor necrosis factor (TNF) alpha inhibitors [19].
Rarer instances of LMD mimics are relatively sparse, though these will be discussed. As elucidated, neuroborreliosis has been accounted for in the literature as a potential condition highly reminiscent of leptomeningeal involvement. Neuroborreliosis entails the neurological affiliation of infection by the genus Borrelia, colloquially acknowledged as Lyme disease. Such affiliation following CNS involvement occurs in up to 15% of borrelia infections, often manifesting as non-specific neurological symptoms including meningitis, cranial nerve deficits, and radiculopathy. The gold standard of diagnosis for LMD, MRI, has little distinctive capacity between neuroborreliosis and LMD, thereby producing complex cases in the current literature. Caretakers should then be highly suspicious of the former in the setting of potential signs of vector-borne infections, such as erythema and rash. Upon suspicion, further serological testing via antibody detection of borrelia has more definitively ruled out a diagnosis of neuroborreliosis. Treatment intuitively entails antibiotic therapy, which exclusively resolves neuroborreliosis as opposed to LMD.
Giant cell arteritis has likewise been scantily reported, though it has appeared as a potential mimic of LMD. Giant cell arteritis (GCA), synonymously termed temporal arteritis, is a vasculitis affecting medium- and large-sized vessels, quite often the external carotid artery and its branches, namely the temporal branch. A primary concern in GCA is that of ischemic optic neuropathy, which is understandably observed in the form of vision loss. Indeed, the case report detailing the leptomeningeal mimicking characteristics of giant cell arteritis was characterized by a similar presentation with progressive vision loss. Additionally, MRI may reveal nodular enhancement, more so localized to the optic nerve sheaths, which is also reminiscent of sarcoidosis. Temporal artery biopsy is the most conclusive modality for diagnosing GCA, though the path towards this diagnosis remains relatively difficult to land upon. Similar to sarcoidosis and other inflammatory disorders, steroid treatment has demonstrated efficacy.
References
- Nayar, G.; Ejikeme, T.; Chongsathidkiet, P.; Elsamadicy, A.A.; Blackwell, K.L.; Clarke, J.M.; Lad, S.P.; Fecci, P.E. Leptomeningeal disease: Current diagnostic and therapeutic strategies. Oncotarget 2017, 8, 73312–73328.
- Gleissner, B.; Chamberlain, M.C. Neoplastic meningitis. Lancet Neurol. 2006, 5, 443–452.
- Batool, A.; Kasi, A. Leptomeningeal Carcinomatosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023.
- Nguyen, T.K.; Nguyen, E.K.; Soliman, H. An overview of leptomeningeal disease. Ann. Palliat. Med. 2021, 10, 909–922.
- Horbinski, C.; Nabors, L.B.; Portnow, J.; Baehring, J.; Bhatia, A.; Bloch, O.; Brem, S.; Butowski, N.; Cannon, D.M.; Chao, S.; et al. NCCN Guidelines® Insights: Central Nervous System Cancers, Version 2.2022. J. Natl. Compr. Cancer Netw. 2023, 21, 12–20.
- Prabhu, R.S.; Turner, B.E.; Asher, A.L.; Marcrom, S.R.; Fiveash, J.B.; Foreman, P.M.; Press, R.H.; Patel, K.R.; Curran, W.J.; Breen, W.G.; et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro-Oncology 2019, 21, 1049–1059.
- Collie, D.; Brush, J.; Lammie, G.; Grant, R.; Kunkler, I.; Leonard, R.; Gregor, A.; Sellar, R. Imaging features of leptomeningeal metastases. Clin. Radiol. 1999, 54, 765–771.
- Pavlidis, N. The diagnostic and therapeutic management of leptomeningeal carcinomatosis. Ann. Oncol. 2004, 15 (Suppl. S4), iv285–iv291.
- van Bussel, M.T.J.; Pluim, D.; Milojkovic Kerklaan, B.; Bol, M.; Sikorska, K.; Linders, D.T.; Broek, D.V.D.; Beijnen, J.H.; Schellens, J.H.; Brandsma, D. Circulating epithelial tumor cell analysis in CSF in patients with leptomeningeal metastases. Neurology 2020, 94, e521–e528.
- Nayak, L.; Fleisher, M.; Gonzalez-Espinoza, R.; Lin, O.; Panageas, K.; Reiner, A.; Liu, C.-M.; DeAngelis, L.M.; Omuro, A. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology 2013, 80, 1598–1605.
- Kornberg, M.D.; Ratchford, J.N.; Subramaniam, R.M.; Probasco, J.C. Giant cell arteritis mimicking infiltrative leptomeningeal disease of the optic nerves. BMJ Case Rep. 2015, 2015, bcr2014209160.
- Fischer, S.; Weber, J.; Senn-Schönenberger, I.; Cerny, T.; Hundsberger, T. Neuroborreliosis Mimicking Leptomeningeal Carcinomatosis in a Patient With Breast Cancer: A Case Report. J. Investig. Med. High Impact Case Rep. 2014, 2, 2324709614529417.
- Saltijeral, S.N.; Grosu, H.B.; De La Garza, H.; O’brien, B.; Iliescu, G. Leptomeningeal Enhancement due to Neurosarcoidosis Mimicking Malignancy. Case Rep. Med. 2020, 2020, 9513576.
- Ungprasert, P.; Ryu, J.H.; Matteson, E.L. Clinical Manifestations, Diagnosis, and Treatment of Sarcoidosis. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 358–375.
- Ramos-Casals, M.; Pérez-Alvarez, R.; Kostov, B.; Gómez-De-La-Torre, R.; Feijoo-Massó, C.; Chara-Cervantes, J.; Pinilla, B.; González-García, A.; Garcia-Morillo, J.-S.; López-Dupla, M.; et al. Clinical characterization and outcomes of 85 patients with neurosarcoidosis. Sci. Rep. 2021, 11, 13735.
- Bergantini, L.; Nardelli, G.; D’alessandro, M.; Montuori, G.; Piccioli, C.; Rosi, E.; Gangi, S.; Cavallaro, D.; Cameli, P.; Bargagli, E. Combined Sarcoidosis and Idiopathic Pulmonary Fibrosis (CSIPF): A New Phenotype or a Fortuitous Overlap? Scoping Review and Case Series. J. Clin. Med. 2022, 11, 2065.
- Kidd, D.P. Sarcoidosis of the central nervous system: Clinical features, imaging, and CSF results. J. Neurol. 2018, 265, 1906–1915.
- Galnares-Olalde, J.A.; Berebichez-Fridman, R.; Gómez-Garza, G.; Mercado, M.; Moreno-Sánchez, F.; Alegría-Loyola, M.A. Not everything is as it seems: Neurosarcoidosis presenting as leptomeningitis. Clin. Case Rep. 2018, 6, 596–602.
- Voortman, M.; Drent, M.; Baughman, R.P. Management of neurosarcoidosis: A clinical challenge. Curr. Opin. Neurol. 2019, 32, 475–483.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
24 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




