These contents aim to provide a comprehensive understanding of the various architectures of AuNP-liposome nanocomposites and the factors influencing their formation. The objectives of these contents are to: 1. Define the term "architecture" concerning the spatial assembly of AuNP (gold nanoparticles) and liposomes, highlighting the various forms in which these assemblies can exist. 2. Explain how the architectures of AuNP-liposome nanocomposites depend on factors such as the size, shape, and surface chemistry of AuNP, as well as the characteristics of the hosting liposomes and the methods used to prepare the nanocomposites. 4. Discuss the different starting materials that can be used to create AuNP-liposome nanocomposites, including pre-prepared AuNP, gold ions that need to be reduced in situ, and ionic/molecular precursors of gold. 4. Describe the specific chemistry which can be employed to encapsulate hydrophilic and hydrophobic pre-prepared AuNP into liposomes, depending on the type of interactions (electrostatic or covalent) provided and the size of the AuNP.
1. Unveiling the Architectures of AuNP-Liposome Nanocomposites
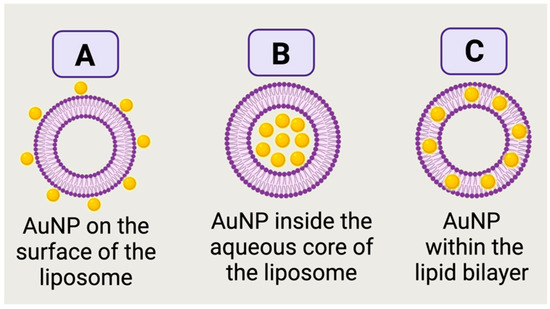
The term “architecture” herein as the spatial assembly of AuNP and liposomes, which can take various forms: AuNP in the aqueous core of the liposome, AuNP on the outermost shell of the liposome, AuNP in the bilayer or even mixed assemblies. These architectures depend on the size, shape and surface chemistry of AuNP as well as the hosting liposomes and the employed chemistries/methods to prepare the nanocomposites. The starting materials for AuNP in the AuNP-liposome nanocomposites can be either pre-prepared AuNP or gold ions that need to be reduced in-situ. Hydrophilic pre-prepared AuNP can be assembled on the outermost layer of the liposomes (
Figure 1A) if enough attractions (electrostatic or covalent) are provided
[1]. If a suspension of the Hydrophilic pre-prepared AuNP was used as the hydration media to prepare the liposomes, AuNP can be encapsulated into the aqueous core as demonstrated in
Figure 1B
[2]. Alternatively to using pre-prepared hydrophilic AuNP, ionic or molecular precursors of gold can be used as a starting material followed by the successful encapsulation of these precursor into the aqueous cores of liposomes and ultimately the reduction into AuNP
[3]. Hydrophobic pre-prepared AuNP can be incorporated into the bilayer membrane as shown in
Figure 1C
[4] for ultrasmall AuNP with a diameter approaching the lipid membrane thickness (about 3–5 nm) and capped with hydrophobic capping agent (i.e., alkanethiols)
[4][5].
Figure 1. Illustration of different architectures of AuNP-liposome nanocomposites. Pre-prepared hydrophilic AuNP on the outermost shell (A) or in the aqueous reservoir (B) of a liposome. In (C), pre-prepared ultrasmall hydrophobic AuNP are embedded in the bilayer of a liposome. Figure created in BioRender.com.
2. Chemistries to Prepare AuNP-Liposome Nanocomposite
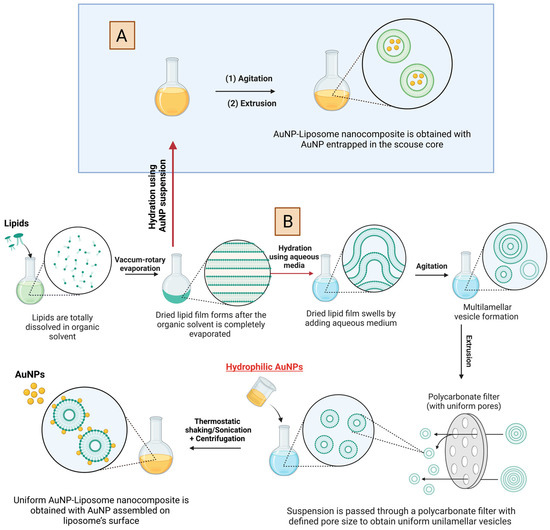
Typical formation of liposomes via thin film hydration method is shown in
Figure 2 [6]. Briefly, the thin film hydration method implies the dissolution of lipids in a volatile organic solvent, followed by rotary evaporation of the solvent to form dried lipid film. Further, the lipid film swells by hydration in an aqueous medium and multilamellar vesicle starts to form. To obtain, uniform unilamellar vesicles, the suspension is passed through a polycarbonate filter with defined pore size
[7]. AuNP can be introduced at different stages.
Figure 2A, shows the introduction of pre-prepared hydrophilic AuNP as an aqueous suspension to hydrate the dried lipid film, which results in the encapsulation of AuNP into the aqueous core of the formed liposomes.
Figure 2B, shows the introduction of AuNP at much later stage, where the liposome is already formed and thus the resulting architecture will be mostly assembled AuNP on the liposome outer shell (i.e., covalent or electrostatic binding). Chemistries described in
Figure 2 requires hydrophilic AuNP with size typically smaller than the size of the liposome and typically results in poor encapsulation yield
[3]. After the formation of AuNP-liposome nanocomposite, unencapsulated AuNPs could be removed using several purification methods including repeated slow-speed centrifugation or density-based fractionation
[8][9][10].
Figure 2. Schematic illustration of the preparation of AuNP-liposome nanocomposite using hydrophilic AuNP. Note that the preparations of two different architectures are sketched: AuNP in the aqueous core of the liposomes (A) and AuNP on the outermost shell of the liposome (B). For the later, attractive forces (electrostatic or covalent interaction) should exist for stable assembly. Figure created in BioRender.com.
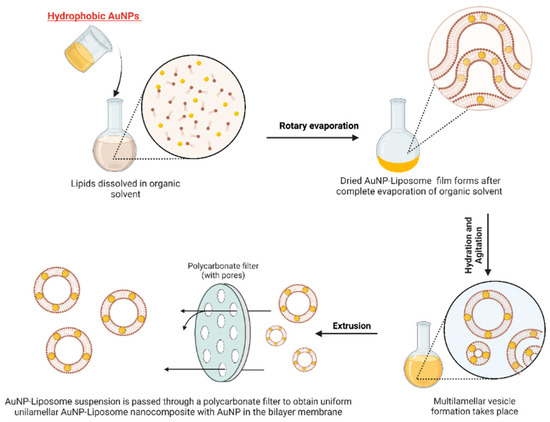
For hydrophobic AuNP with small size (i.e., alkanethiol-capped AuNP with diameter less than 5 nm), AuNP can be introduced in the first step and suspended in the organic solvent with the dissolved phospholipid as shown in
Figure 3. Upon drying, phospholipid and hydrophobic AuNP will form a dried film, which upon hydration will result in liposome that encapsulate the small AuNP in the bilayer membrane
[4]. It is worth to mention that this protocol can only be applied for small AuNP that individually do not exhibit optical properties in the Vis-NIR
[11][12]. Moreover, the incorporation of AuNP in the bilayer may result in significant impact on the stability of the hosting liposome and fragmentation into micelles
[3]. Furthermore, the fundamental drawback of this technique is that it possesses low encapsulation efficiency, which necessitates an extra step for removing the free AuNP
[13]. In addition, drug incorporation after liposomal preparation was correlated with higher encapsulation efficiency compared to drug incorporation during the liposomal formation, which guarantees improved drug bioavailability in the targeted site of action
[14]. It is noteworthy to mention that the choice of the material incorporation (i.e., either after or during liposomal formation) depends on several standards, including the properties of the incorporated material (i.e., hydrophilic or lipophilic), the targeted profile of the drug release, and therapeutic applications of the prepared liposome-based nanocomposite
[15][16][17][18][19]. Although production methods of liposome-based nanocomposites received significant efforts for scaling-up these fabrication techniques, some concerns include the poor colloidal stability profile, low encapsulation efficiencies, toxicity coming from the organic solvents used during synthesis process, and high costs of large-scale fabrication are associated with the production of liposome-based nanocomposites
[14]. However, these limitations are variable based on the production techniques followed during the liposomal formation process. In other words, some liposome production methods (i.e., Freeze Drying, Reverse-Phase Evaporation, and Membrane Contactor) are among the useful techniques that are efficient for large-scale fabrication of liposome-based nanocomposites.
Figure 3. Schematic illustration of the preparation of AuNP-liposome nanocomposite using ultra-small hydrophobic AuNP. Note that the resulting architectures implies the incorporation of AuNP into the lipid membrane bilayer. Figure created in BioRender.com.
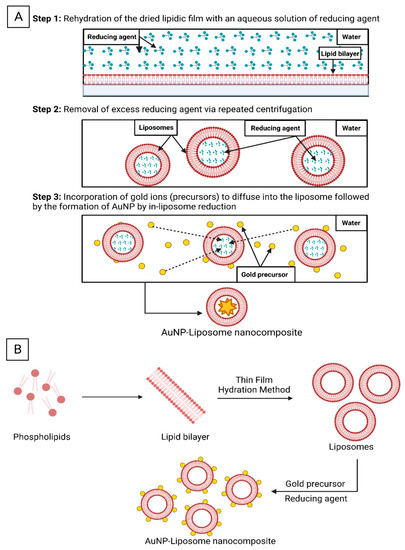
Alternatively, liposomal dried film can be rehydrated with a reducing agent and then gold ions can be introduced and selectively reduced inside the aqueous core of the liposomes as shown in
Figure 4A, which could be called in-liposome reduction approach
[3]. Lee et. al. utilized this approach with sodium citrate or ascorbic acid as reducing agents and tetrachloroaurate ions as the gold precursors, while the reduction was carried out at room temperature overnight. Employing the in-liposome reduction approach, the research group prepared seven types of AuNP-liposome nanocomposites with tunable size, metal compositions and optical properties
[3]. On-liposome reduction approach is another pathway where gold undergoes reduction on the surface of pre-synthesized liposomes (
Figure 4B)
[20].
Table 1 summarizes the reported chemistries used to prepare AuNP-liposome nanocomposites and the physiochemical properties of each component and the resulting products.
Figure 4. Schematic illustration of the AuNP-liposome nanocomposites formation using the “in-liposome” (A) and “on-liposome” (B) reduction approaches. Figure created in BioRender.com.
In the preparation of AuNR, shape-directing agents (i.e., CTAB) are used to promote the anisotropic growth of AuNR. Unfortunately CTAB has cytotoxic profile which limits the biomedical applications of CTAB-capped AuNR
[21][22][23]. In this regard, Gudlur et al. reported the CTAB-free preparation of AuNR using cationic liposomes as a substituent for the CTAB in order to improve the biocompatibility of AuNR
[24]. The authors used cationic pre-prepared liposomes that were mixed with gold precursor (HAuCl
4) in the presence of silver salt, reducing agent (ascorbic acid), and followed by heating at 40 °C to prepare anisotropic AuNR-liposome nanocomposite. Interestingly, AuNR-liposome nanocomposite potentially induced a significant photothermal ability by hyperthermia-induced cell death in different cancer cell lines, and thus resulting in enhanced cellular uptake compared to CTAB-mediated AuNR. However, the actual role of liposomes in the synthesis process of AuNR is still unknown, and much research needs to be conducted to validate this emerging method.
Table 1. Examples of reported AuNP-liposome nanocomposites.
3. Analytical Characterization of AuNP-Liposome Nanocomposites
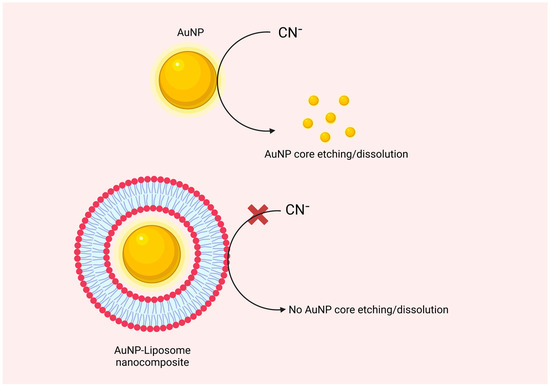
Transmission electron microscopy (TEM) is a typical tool to visualize the architecture of AuNP-liposome nanocomposites and confirm the spatial distribution of AuNP whether on the outermost shell of the liposome, inside the aqueous core, or within the liposome bilayer. However, negative staining is usually required, and thus staining-related artifacts are possible challenge. Another challenge is related to the two-dimensional nature of the TEM imaging, which makes it difficult to confirm if the nanoparticles are “on” or “in” the liposome. A more complex tool is the Cryogenic transmission electron microscopy (Cryo-TEM), where thin film of liposome suspension is frozen under liquid nitrogen temperature
[32]. The resulting vitrified ice film is extremely thin and can be imaged directly by EM. Alternative indirect method is the use of the sodium cyanide test, in which the successful coating of the AuNP with lipids can be tested using the oxidation capacity of cyanide to the encapsulated AuNP and the protection role of the lipid bilayer
[33]. In this assay, the lipidic shell that coats the AuNP surface acts as a non-ion-permeable barrier that protects the golden core from exposure to cyanide ions as shown in
Figure 5. For encapsulated AuNP, addition of cyanide will prevent or delay the oxidation of the protected AuNP, and thus the original color of the suspension will be maintained compared to control naked AuNP
[33]. If AuNP is “on” the liposome or simply suspended in the same media without effective encapsulation, then added cyanide can oxidize the AuNP and the suspension color will disappear. Moreover, brilliant color of nanogold and its unique plasmonic optical extinction in the Visible-NIR region of the spectrum can be followed to confirm the formation of AuNP-liposome nanocomposites
[34]. Coloration of liposome is a visual evidence supporting the formation of AuNP-liposome nanocomposites. Elemental analysis using energy dispersive spectroscopy (EDS) can be employed to further support the formation of AuNP-liposome nanocomposites, looking for the gold fingerprints
[35]. Quantitively, gold content can be measured using inductively coupled mass spectrometry (ICP-MS) and reported as weight percent of the nanocomposite
[36]. The various analytical modalities that can be used to confirm the presence, quantify and visualize the encapsulated AuNP are justifications to use AuNP as labels for liposomes, and thus to understand the biological interactions and fate of labeled liposomes. It is noteworthy to highlight the need to compare information gathered by complementary techniques to confirm the properties of the prepared AuNP-liposome nanocomposites. For instance, morphological information obtained from SEM and TEM could be further correlated with the optical absorption spectra of the nanocomposite where coloration of liposomes and AuNP and its unique plasmonic optical extinction in the Visible-NIR region could confirm the formation of the AuNP-liposome nanocomposites and its size that correlates with unique wavelengths
[20][34][37]. Moreover, correlating the data obtained by FTIR, XPS, and ICP-MS could provide insights on the overall structure, composition, and surface properties of the AuNP-liposome nanocomposites
[35][38].
Figure 6. Graphical illustration of the protective properties of the AuNP encapsulated into the liposomal core against chemical etching with cyanide ions. Figure created in BioRender.com.