Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cho-Ming Chao | -- | 3581 | 2023-07-21 02:51:28 | | | |
| 2 | Catherine Yang | Meta information modification | 3581 | 2023-07-21 03:25:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marega, M.; El-Merhie, N.; Gökyildirim, M.Y.; Orth, V.; Bellusci, S.; Chao, C. Stem/Progenitor Cells and Therapy in BPD. Encyclopedia. Available online: https://encyclopedia.pub/entry/47095 (accessed on 07 February 2026).
Marega M, El-Merhie N, Gökyildirim MY, Orth V, Bellusci S, Chao C. Stem/Progenitor Cells and Therapy in BPD. Encyclopedia. Available at: https://encyclopedia.pub/entry/47095. Accessed February 07, 2026.
Marega, Manuela, Natalia El-Merhie, Mira Y. Gökyildirim, Valerie Orth, Saverio Bellusci, Cho-Ming Chao. "Stem/Progenitor Cells and Therapy in BPD" Encyclopedia, https://encyclopedia.pub/entry/47095 (accessed February 07, 2026).
Marega, M., El-Merhie, N., Gökyildirim, M.Y., Orth, V., Bellusci, S., & Chao, C. (2023, July 21). Stem/Progenitor Cells and Therapy in BPD. In Encyclopedia. https://encyclopedia.pub/entry/47095
Marega, Manuela, et al. "Stem/Progenitor Cells and Therapy in BPD." Encyclopedia. Web. 21 July, 2023.
Copy Citation
Bronchopulmonary dysplasia (BPD) is a chronic lung disease commonly seen in preterm infants, and is triggered by infection, mechanical ventilation, and oxygen toxicity. Among other problems, lifelong limitations in lung function and impaired psychomotor development may result. Despite major advances in understanding the disease pathologies, successful interventions are still limited to only a few drug therapies with a restricted therapeutic benefit, and which sometimes have significant side effects. As a more promising therapeutic option, mesenchymal stem cells (MSCs) have been in focus for several years due to their anti-inflammatory effects and their secretion of growth and development promoting factors.
bronchopulmonary dysplasia
premature infants
lung injury
chronic lung disease
1. Introduction
Preterm birth is the leading cause of infant mortality and long-term morbidity and represents a significant healthcare burden [1][2][3]. Premature newborns may suffer from respiratory distress requiring mechanical ventilation and oxygen support. The prolonged use of oxygen increases its concentration in the lungs (hyperoxia), which can eventually lead to inflammation resulting in abnormal lung development [4][5]. The affected infants are at high risk to develop bronchopulmonary dysplasia (BPD), a multifactorial chronic lung disease characterized by immature lung vasculature, inflammation, restricted lung development, and disordered lung repair [6]. The lower the gestational age and birth weight, the higher the risk to suffer from clinical syndromes which are considered as BPD. To date, the pathologic description of BPD includes disrupted alveolarization and microvasculature development, fibrosis, and cystic emphysema, and is likely to emerge due to disturbed signaling pathways [7].
Alongside a number of postnatal challenges that contribute to the development of BPD, the results of several studies have implicated that adverse conditions during pregnancy like placental vascular disease, intrauterine growth restriction, or chorioamnionitis may already be sufficient to induce or at least promote BPD and its related consequences [8].
Several advances in perinatal and neonatal medicine have improved the survival rates of preterm babies. However, these infants are susceptible to short- or long-term respiratory complications and are at an increased risk of BPD [6]. The current therapy is limited to a few drugs, such as caffeine and vitamin A, or corticosteroids like dexamethasone. However, this pharmacological approach has been controversially discussed, since on the one hand it has been shown to be associated with neurodevelopmental complications [9][10], whereas on the other hand, a low-dose treatment of high-risk infants turned out to have a positive benefit vs danger relation [11][12]. Nevertheless, these drugs failed to address all aspects of the disease, including the impaired alveolarization and the inflammation. Thus, no successful handling is available to prevent the long-lasting consequences of BPD, like pulmonary hypertension, exercise intolerance with sometimes the need for life long oxygen supply, and echocardiographic abnormalities [13]. In this scenario, the need of alternative safe therapies has strongly emerged.
In BPD, inflammation is considered as an initiative characteristic, and infections are deemed as high-risk factors. The levels of proinflammatory cytokines and chemokines, like interleukin (IL)-1b, IL-6, IL-8, tumor necrosis factor (TNF)-α, MCP-1, -2, -3, and transforming growth factor (TGF)-β1 are all increased, while anti-inflammatory molecules, like IL-4, IL-13, and IL-10, growth factors (fibroblast growth factor 10 (FGF-10), and vascular endothelial growth factor A (VEGFA), and platelet-derived growth factor subunit A (PDGF-A) are all decreased, whereas they are required for the physiological development of the lung and its repair during injury [14]. Regarding inflammation, the nuclear factor-kappa B (NF-κB) pathway has been implicated in the pathogenesis of BPD. NF-κB is a transcription factor that plays a critical role in the regulation of the inflammation and cellular stress responses. In BPD, activation of the NF-κB pathway has been observed and linked to the persistent inflammation and oxidative stress that are characteristics of this disease [14].
Stem-cell based therapy has been proven to be promising. Stem/progenitor cells are important in preserving the structure of the lung, and their presence is fundamental for the repair of the damaged tissue in lung diseases.
Therapeutic approaches should therefore address the restoration of the function of the different stem/progenitor cells (as well as their presence) in their compartments, including growth factors modulating the inflammation derived from the damage. Interestingly, mesenchymal stem cells (MSCs) have recently emerged as a good candidate, as they support cells during their development [15][16]. Additionally, MSCs can modulate the immune response with a reduction in inflammation and the levels of pro-inflammatory cytokines, such as IL-6 and TNF-α. MSCs were identified in the tracheal aspirate of preterm newborns, showing phenotypical alterations compared to the MSCs from the control group of healthy babies [17][18]. The expression of proinflammatory cytokines in these cells was found to be higher compared to the control, strongly suggesting a role of MSCs in the pathology of BPD. Replacing these cells with exogenous MSCs could therefore represent a feasible treatment of BPD.
2. Stem/Progenitor Cells in the Lung
In BPD, inflammation, oxidative stress, and mechanical ventilation can lead to abnormal lung growth and a reduced lung function. Studies have demonstrated that the number and function of stem and progenitor cells are altered in the lungs of infants with BPD [19]. Lung stem/progenitor cells belong to the endothelial, mesenchymal, and epithelial lineages [20]. The regeneration and repair of damaged lung tissue occurs by either the proliferation of already differentiated cells, or by the differentiation of stem/progenitor cells. Basal cells and variant club cells (VCCs) are present in the bronchi and in proximity of the neuroepithelial bodies, and they can give rise to club cells (CCs), ciliated cells, and goblet cells (GCs) [21]. In the alveoli, it is possible to identify bronchioalveolar stem cells (BASCs) that can differentiate into CCs, ciliated cells, GCs, and alveolar type 1 and 2 (AT1 and 2) cells, with AT2 cells having the ability to self-renew and give rise to the AT1 cells.
The lung contains several different stem and progenitor cells, including basal cells (BCs), variant cells, GCs, BASCs, distal airway stem cells (DASCs), and AT2 cells (Figure 1 and Table 1 summarize the different localization of the cells and their specific markers). The activation of the stem/progenitor cells is associated with the level of lung injury. A severe injury of the alveolar epithelium leads to the activation of the non-alveolar epithelial type II cells to repair the damage; a mild injury induces the activation of the AT2 cells for the resolution of the damage. All these different types of cells could cover a potential therapeutic role in the treatment of BPD, including the MSCs and their extracellular vesicles (EVs) that have emerged as a promising therapeutic option as will be discussed further below.
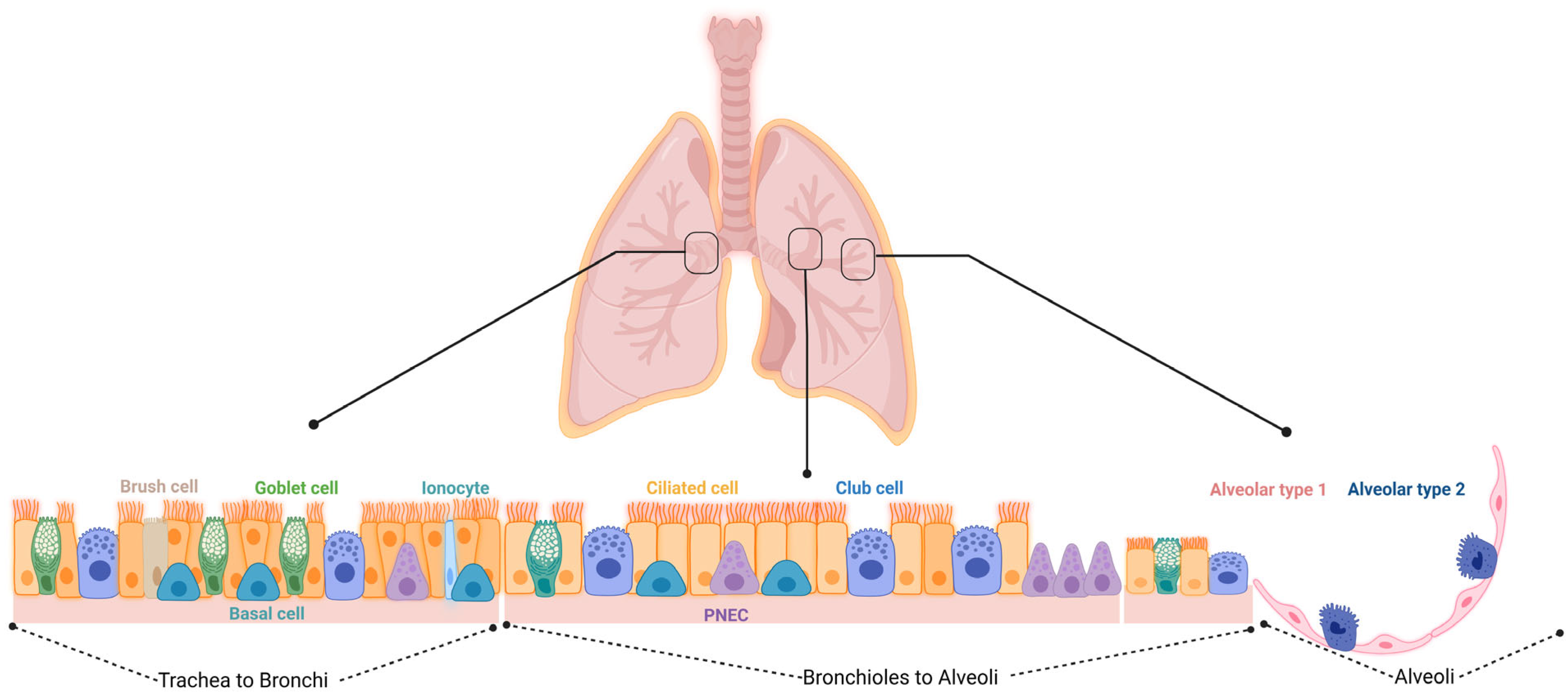
Figure 1. Schematic overview of the different cell types within the respiratory epithelium. Trachea to bronchi: the epithelial surfaces of the trachea and proximal airways are lined by a pseudostratified columnar epithelium consisting of basal, brush, ciliated, goblet, and club cells, along with the less frequent pulmonary neuroendocrine cells (PNECs) and ionocytes. Bronchioles to alveoli: small airways are lined by a simple columnar or cuboidal epithelium that consists of ciliated, club, and a few goblet cells. Alveoli: the alveoli are lined by either squamous alveolar type 1 (AT1) or cuboidal alveolar type 2 (AT2) cell.
2.1. Epithelial Progenitors
2.1.1. Basal Cells (BCs)
BCs are the most primitive cell type in the airways and are capable of self-renewal and differentiation into other cell types, including ciliated, goblet, pulmonary neuroendocrine cells (PNECs), ionocytes, and club cells in steady-state and after acute lung injury to restore lung homeostasis [22][23][24][25]. They are present throughout the respiratory tree and account for about 30% of the total airway epithelial cells in the upper airways, while their number gradually decreases to 6% in the smaller airways, respectively [26][27]. Tumor protein (TP)63 and cytokeratin (KRT)5 are considered as the common markers to identify these BCs, along with podoplanin (PDPN) [28][29]. KRT8 is upregulated in the BCs just before they differentiate into luminal cells, generating an intermediate KRT8+ basal luminal precursor cell under homeostatic conditions [24][25][26]. Intermediate [29][30][31] luminal precursor cells can differentiate into secretory and ciliated cells and PNECs [25][32][33][34][35]. In addition to the KRT5+ progenitors, new KRT17+ basal progenitor cells were identified in the mouse airway epithelium using time-series single-cell transcriptomic analysis combined with in vivo lineage tracing [36]. It has been shown in murine models that basal cells are formed during prenatal development and contribute to the pool of luminal cells [37].
It seems that BCs play a role in regeneration and repair as well as they have been implicated in chronic lung disease. However, little is known about these cells in preterm neonates due to limitations in basal cell isolation techniques. A study has shown that these cells, isolated from the nasopharyngeal aspirates of preterm infants, could differentiate into the airway epithelium, thereby providing the possibility for future therapy in neonatal lung diseases like BPD [38].
2.1.2. Secretory Cells (SCs): Variant Club Cells (VCCs), Club Cells, and Goblet Cells
CCs express secretory proteins (e.g., CC10) in addition to bronchiolar surfactants and proteases important for the regulation of epithelial integrity, immunity, and host-defense interactions [38][39][40]. A destruction and airway ciliated cell abnormality leading to a defect in mucociliary clearance has been found in premature infants developing BPD [40]. Moreover, ciliated cells of preterm infants showed a decline in ciliary beating, thus making the epithelium more vulnerable to external stimuli and injuries [38][41]. They are replenished by the basal cells, and their differentiation is maintained by Notch signaling [29]. It is possible to recognize two different types of CCs based on their sensitivity to naphthalene. The toxin induces the death of most of the CCs. However, a subpopulation is resistant to the injury, and is characterized by the lack of expression of the cytochrome P450 family member CYP2F2 [41]. These cells are the VCCs. They can reconstitute the injured epithelium through self-renewal and differentiation into the ciliated cells and CCs. VCCs can be considered as epithelial progenitors in small airways after injury.
GCs are secretory cells possessing numerous vesicle-bound mucin granules for mucus production, thus creating a mucous gel layer for the efficient mucociliary clearance and pathogen entrapment. Under healthy conditions, there is an equilibrium present between mucus production and clearance, however, in chronic airway diseases, hyperplasia and metaplasia of GCs can occur [41][42][43]. Moreover, they were found to metabolize xenobiotics via the production of P450 mono-oxygenase [44].
In infants with BPD, a decrease in the number of CCs as well as in the expression of the CC-derived protein secretoglobin (SCGB) 1A1 in the bronchiolar epithelium was reported [45][46][47]. This accelerated CC death might be a result of supplemental oxygen therapy in these infants. CCs are termed as the second stem cells in the airways since they serve as progenitors for ciliated and mucus-secreting cells.
Taken together, this could indicate a decrease in the levels of goblet cells in BPD because of CC hypoplasia, and hence this deficiency in the stem cell pool could lead to a lower host defense and interfere with the normal epithelial turnover and regeneration [48]. It has also been shown that GCs are plastic cells, and they may be able of self-renewal and trans-differentiation into a hybrid ciliated epithelial cell, thereby suggesting the presence of a transitional state [49].
2.1.3. Pulmonary Neuroendocrine Cells (PNECs)
The first cells to be formed within the epithelium are the PNECs. They are present throughout the bronchial tree either as single cells in humans, or in clusters as neuroepithelial bodies in rodents, and their number increases from the bronchi to the terminal bronchioles [50][51][52]. A great number of PNECs is present in the fetal lung, suggesting that they play a role during fetal lung development [53]. Moreover, they are involved in the communication between the nervous and immune systems [53][54][55]. It has been shown that the number of PNECs as well as their peptide contents change in babies with BPD [56][57][58], which was also observed in a rodent model of hyperoxia. Additionally, increased levels of gastrin-releasing peptide (GRP)-, and calcitonin- and serotonin-immunoreactive PNECs have been reported in infants who died from BPD. In contrast, post mortem analysis of pre-term infants with respiratory distress syndrome revealed a decrease in the GRP+ PNECs that was assumingly caused by degranulation [56]. The overactivation and hyperplasia of PNECs have been reported in BPD, indicating a changed environment in BPD induced by chronic hyperoxia [56][59]. Hence, the dysfunction of pulmonary progenitor cells in BPD leads to an impairment in pulmonary growth and to the inability of the immature lung to repair itself [60].
2.1.4. Alveolar Progenitor Cells
The distal alveolar region is lined with squamous type 1 and cuboidal 2 alveolar epithelial cells, also termed alveolar type 1 (AT1) and alveolar type 2 (AT2). AT2s are considered as alveolar progenitors with self-renewal abilities. Their turnover within the normal lung is slow, however, upon lung injury, they were shown to rapidly proliferate and differentiate into AT1 cells for maintaining the alveolar homeostasis [61][62][63][64][65][66]. The differentiation of AT2 into AT1 cells was observed in a study on neonatal rats under hyperoxia, and increased AT2 cell proliferation was observed in human severe type 2 BPD as well as in premature baboons following oxygen support and mechanical ventilation [67][68][69][70]. It is known that infants affected with BPD show a reduced formation of alveoli which continues to persist into adulthood [71][72]. Moreover, a decline in AT1 cells has been observed in infants with BPD, which is accompanied with alveolar simplification and the suspension of alveolarization [63][73].
Distal lung progenitor cells represent a subset of AT2 cells. They are responsible for the physiological turnover and for the repair through their ability to self-renew and differentiate into AT1 cells both in vivo and in vitro. In addition, they have regenerative potential: following pneumonectomy, an increase in AT2 cell numbers has been observed in mice, with a compensatory growth of the remaining lung parenchyma [74][75][76][77]. In the in vivo BPD model, hyperoxia exposure has been shown to deplete the AT2 cells [78]. This damage to the lung may contribute to an impaired or incomplete lung development and a resultant lifelong lung disease. The regenerative potential of AT2 cells therefore makes them interesting as a possible therapeutic intervention. In the in vivo model, primary murine AT2 cells were shown to prevent O2-induced functional and structural lung injury. Furthermore, it was demonstrated that induced human pluripotent stem cells also protected against lung injury in mice [79]. It has shown in past research how the intratracheal injection of progenitors can improve the structure and functions of the lung. Isolated AT2 cells were injected to prevent lung injury in an established oxygen-induced mouse model mimicking BPD. The results confirmed the safety of the administration and the efficacy of this procedure. However, the difficulty of isolating a sufficient number of these cells needs further work. Recently, different subpopulations of AT2 cells in terms of their activation status have been identified, thus increasing the complexity of a possible therapeutic approach with AT2 cells [80][81]
2.1.5. Non-Alveolar Progenitors
The proximal airway progenitor cells include the already mentioned basal and secretory cells, while the distal progenitors comprise the VCCs and the non-alveolar epithelial type II derived progenitors, such as DASCs, BASCs, and lineage-negative epithelial progenitors (LNEPs).
2.1.6. BASCs and DASCs
BASCs expressing the cell markers of both AT2 (surfactant protein C (SFTPC)) and secretory cells (secretoglobin 1A1 (SCGB1A1)) differentiate into AT1 and AT2 cells following bleomycin or influenza virus-induced injury, but not after hyperoxia-induced alveolar injury [41][82][83]. AT2 cells are capable of self-renewal and differentiation into AT1 (HOPX+) cells during homeostasis and upon injury [65][67]. In addition, AT2 cells differentiate into SFTPC+ and stem cell antigen (SCA)l+ alveolar progenitor cells, which are capable in differentiating into AT1 cells. In addition to the progenitor AT2 cells, another type of alveolar progenitor cells expressing alpha-6-beta4-integrin but not SFTPC and SCGB1A1 have been identified [84]. These cells can self-renew and differentiate into CCs and AT2 cells. Interestingly, it was found that a small population of AT1 cells expressing HOPX can give rise to AT2 cells following partial pneumonectomy [85]. A new population of stem cells distinct from AT2 and club progenitor cells has been identified, termed DASCs [86][87]. They express TP63/KRT5, can migrate, and give rise to SCGB1A1+/CYP2F2+ secretory club cells, which can self-renew and differentiate into ciliated cells and BCs (SCGB1A1+ and SFTPC+) upon lung injury.
2.1.7. Lineage-Negative Epithelial Stem/Progenitors (LNEPs)
A lineage tracing study identified a very small subpopulation of quiescent club-like cells, characterized by a high expression of H2-K1 and a lack of TP63 that have the potential to differentiate into alveolar epithelial cells. These LNEPs are localized in the small distal airways and can contribute to the repair of the distal lung area [87].
Table 1. Summary of the specific markers that enable the precise identification of the epithelial stem/progenitor cell subpopulations present within the lung.
| Cell Type | Location | Markers | Differentiation Potential | Refs. |
|---|---|---|---|---|
| Basal cells (BCs) | Epithelium in the trachea and bronchioles | TP63, KRT5, KRT14, NGFR, PDPN, KRT8, N2ICD, and c-Myb/TP73 |
Self-renewal; AT1, AT2 basal luminal precursor cell, neuroendocrine cells, ionocytes CCs, GCs, and ciliated cells |
[23][87][88][89][90][91][92][93][94] |
| Variant club cells/ Clara cells |
Bronchi and bronchioles (epithelium) | SCGB1A1 and CYP2F2− | Self-renewal: GCs and ciliated cells | [82][95][96] |
| Neuroendocrine cells (PNECs) | Epithelium, at branching points in the bronchi and bronchioles | GRP, ASCL1, CXCR4, NCAD and ROBO | Multiciliated cells and secretory cells | [49][55][97][98][99][100] |
| Broncho-alveolar stem cells (BASCs) | Epithelium and distal airway alveolar sacs | SCGB1A1 and SFTPC | Self-renewal: AT2 Ciliated cells: GCs and AT1 |
[101][102][103][104] |
| AT2 | Distal airway alveolar sacs | SFTPC and LysM | Self-Renewal: AT1, SCA1+ mesenchymal progenitors, SFTPC+ AT1 progenitors, |
[66][83][105][106][107][108] |
| Distal airway stem cells (DASCs), Alveolar progenitor |
Distal airways, bronchioles, and alveolar regions | TP63 and KRT5 | Self-Renewal: AT2 | [84] |
| Self-Renewal: | [86] | |||
| AT2, SCGB1A1+, and CYP2F2+ CCs | [86][96] | |||
| AT1 | HOPX, | AT2 | [84] | |
| Club cells, ciliated cells | Terminal bronchioles and less in the respiratory bronchioles, trachea, and bronchioles |
SCGB1A1, and CYP2F2 | BCs | [86] |
| Ciliated cells | [96] | |||
| TubIVa and FOXJ1 | GCs | [109] | ||
| Integrin α6β4+ alveolar progenitor | Terminal bronchioles, broncho-alveolar junctions, and less in the respiratory bronchioles | SCGB1A1−, SPC−, ITGα6, and ITGβ4 | Self-Renewal: AT1 Ciliated cells: AT2 and CCs |
[96] |
Abbreviations: ASCL-1, achaete-scute homolog 1; AT1/2, alveolar type 1/2 cells; BCs, basal cells; CCs, club cells; c-Myb, transcription factor; CYP2F2, cytochrome P450 family 2 subfamily f member 2; CXCR4, chemokine receptor; FOXJ1, transcription factor of fox family; GCs, goblet cells; GRP, bombesin or gene-related peptide; HOPX, homeodomain-only protein; ITG, integrin; KRT, cytokeratin; LysM, lysozyme M; N2ICD, Notch intracellular domain 2; NCAD, N-cadherin; NGFR, nerve growth factor receptor; PDPN, podoplanin; ROBO, roundabout receptor; SCA-1, stem cell antigen-1; SCGB1A1, secretoglobin 1A1; SFTPC, surfactant protein C; TP, tumor protein; and TubIVa, human tubulin beta class Iva.
3. Use of Stem Cells for the Treatment of BPD
Protocols for stem cell isolation and its subsequent in vivo administration are just barely developed far enough to be transferred from animal models to initial human clinical trials. A number of such studies have already addressed the safety and dose-responses with promising results, meaning that the next steps can focus more and more on therapeutic applications.
However, successful interventions still require a better understanding of these molecular processes, especially during differentiation of the cell types involved. This includes unique markers to improve cell-specific isolation, as well as a deeper knowledge of stem/progenitor cell physiology.
The mesenchymal compartment plays a fundamental role in supporting the proliferation and maintenance of the stem/progenitor niches in the lung (Figure 2A). Since MSCs give rise to different mesenchymal populations, like LIFs and myofibroblasts (MYFs), depending on the status of the tissues, the disturbance of their physiological function can cause an impaired lung development (Figure 2B). Thus, MSCs appear to be good candidates for stem cell therapy, and also because they are immune-privileged cells that do not induce host responses or cell rejection. Moreover, they can produce key molecules, such as FGF10 and PDGF [110][111], which influence the proliferation and differentiation of the stem/progenitor cells. As another advantage, they can be easily isolated from a wide range of tissues, like the bone marrow, peripheral blood, placenta, umbilical cord, and Wharton’s jelly [16][112]. The umbilical cord and Wharton’s jelly-isolated MSCs display more potent anti-inflammatory and immunomodulatory properties. Additional studies have focused more on the MSCs from the bone marrow and adipose tissues [15]. The safety profile of MSCs has been well established, and it is promising in that not only the MSC administration shows an improvement in the inflammation of the lung and its repair (e.g., alveolarization), but also of their EVs, suggesting a paracrine effect [113][114][115].
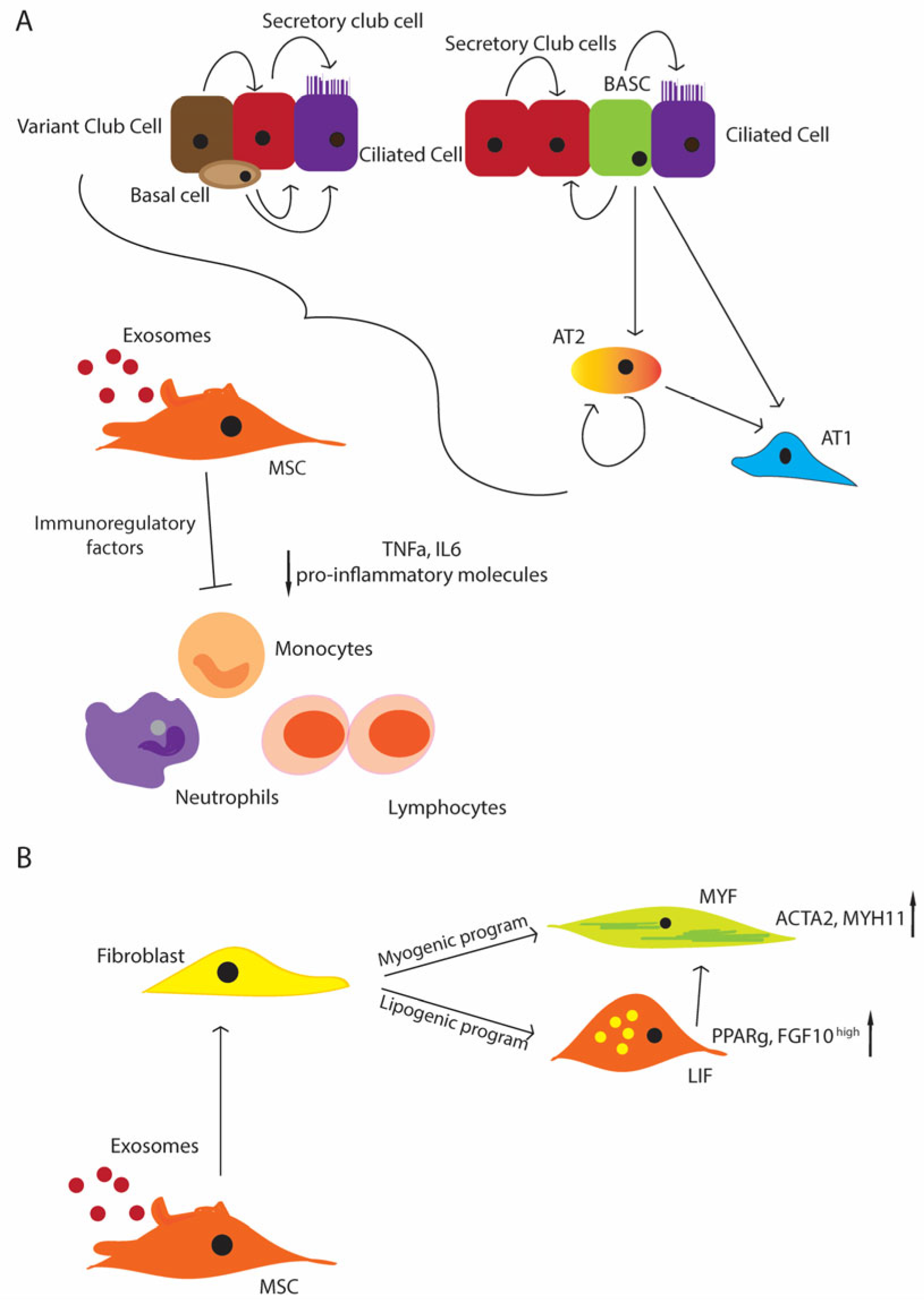
Figure 2. Schematic representation of the regenerative potential of the lung stem/progenitor cells (A) MSCs support the stem cell niche under physiological conditions and upon injury; basal cells differentiate in the secretory and ciliated cells. Variant cells can contribute to the secretory cell pool, and the secretory cells can come from the BASCs and secretory cells themselves. BASCs can differentiate in ciliated cells as well. AT2 cells can differentiate from the BASCs, and they represent the primary source of AT1 cells, and additionally they can maintain themselves; MSCs contribute to suppressing inflammation, inhibiting immune cells, such as monocytes, neutrophils, and lymphocytes (decreased level of pro-inflammatory molecules, TNF-α, IL-6 e.g.,) (B) MSCs give rise to the entire mesenchymal compartment, including lipofibroblasts (LIFs) and myofibroblasts (MYFs), with the activation of the lipogenic or myogenic program that can contribute to the progression or the resolution of injuries.
A recent study showed that a population of repair-supportive mesenchymal cells (RSMCs) rising from the GLI1+ cells is able to activate the club cell progenitors [116]. These RSMCs express high levels of FGF10 and enhanced WNT signaling. GLI1+ cells are usually located in the parabronchial, perivascular, and alveolar regions, and provide the FGF10-producing RSMCs in the lung. The comparison between the RSMCs and the resident MSCs (rMSCs) by scRNA-seq suggested the possibility that rMSCs could originate from the adjacent alveolar region surrounding the bronchi. Further investigations are needed to clarify whether this hypothesis is correct and transferable to human medicine [116].
References
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, Regional, and National Causes of Child Mortality in 2000–13, with Projections to Inform Post-2015 Priorities: An Updated Systematic Analysis. Lancet 2015, 385, 430–440.
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.E.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, Regional, and National Causes of Child Mortality: An Updated Systematic Analysis for 2010 with Time Trends since 2000. Lancet 2012, 379, 2151–2161.
- Botet, F.; Figueras-Aloy, J.; Miracle-Echegoyen, X.; Rodriguez-Miguelez, J.M.; Salvia-Roiges, M.D.; Carbonell-Estrany, X. Trends in Survival among Extremely-Low-Birth-Weight Infants (Less than 1000 g) without Significant Bronchopulmonary Dysplasia. BMC Pediatr. 2012, 12, 63.
- Bhandari, V.; Elias, J.A. Cytokines in Tolerance to Hyperoxia-Induced Injury in the Developing and Adult Lung. Free Radic. Biol. Med. 2006, 41, 4–18.
- Bhandari, V.; Choo-Wing, R.; Lee, C.G.; Zhu, Z.; Nedrelow, J.H.; Chupp, G.L.; Zhang, X.; Matthay, M.A.; Ware, L.B.; Homer, R.J.; et al. Hyperoxia Causes Angiopoietin 2–Mediated Acute Lung Injury and Necrotic Cell Death. Nat. Med. 2006, 12, 1286–1293.
- Smith, L.K.; Hindori-Mohangoo, A.D.; Delnord, M.; Durox, M.; Szamotulska, K.; Macfarlane, A.; Alexander, S.; Barros, H.; Gissler, M.; Blondel, B.; et al. Quantifying the Burden of Stillbirths before 28 Weeks of Completed Gestational Age in High-Income Countries: A Population-Based Study of 19 European Countries. Lancet 2018, 392, 1639–1646.
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary Dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78.
- Mestan, K.K.; Leibel, S.L.; Sajti, E.; Pham, B.; Hietalati, S.; Laurent, L.; Parast, M. Leveraging the Placenta to Advance Neonatal Care. Front. Pediatr. 2023, 11, 1174174.
- Oluwole, I.; Tan, J.B.C.; DeSouza, S.; Hutchinson, M.; Leigh, R.M.; Cha, M.; Rodriguez, A.; Hou, G.; Rao, S.S.; Narang, A.; et al. The Association between Bronchopulmonary Dysplasia Grade and Risks of Adverse Neurodevelopmental Outcomes among Preterm Infants Born at Less than 30 Weeks of Gestation. J. Matern. Fetal Neonatal Med. 2023, 36, 2167074.
- Yeh, T.F.; Lin, Y.J.; Lin, H.C.; Huang, C.C.; Hsieh, W.S.; Lin, C.H.; Tsai, C.H. Outcomes at School Age after Postnatal Dexamethasone Therapy for Lung Disease of Prematurity. N. Engl. J. Med. 2004, 350, 1304–1313.
- Jensen, E.A.; Wiener, L.E.; Rysavy, M.A.; Dysart, K.C.; Gantz, M.G.; Eichenwald, E.C.; Greenberg, R.G.; Harmon, H.M.; Laughon, M.M.; Watterberg, K.L.; et al. Assessment of Corticosteroid Therapy and Death or Disability According to Pretreatment Risk of Death or Bronchopulmonary Dysplasia in Extremely Preterm Infants. JAMA Netw. Open 2023, 6, e2312277.
- Hillman, N.H.; Jobe, A.H. Preterm Lung and Brain Responses to Mechanical Ventilation and Corticosteroids. J. Perinatol. 2023.
- Homan, T.D.; Nayak, R.P. Short-and Long-Term Complications of Bronchopulmonary Dysplasia. Respir. Care 2021, 66, 1618–1629.
- Shahzad, T.; Radajewski, S.; Chao, C.M.; Bellusci, S.; Ehrhardt, H. Pathogenesis of Bronchopulmonary Dysplasia: When Inflammation Meets Organ Development. Mol. Cell. Pediatr. 2016, 3, 23.
- Behnke, J.; Kremer, S.; Shahzad, T.; Chao, C.M.; Bottcher-Friebertshauser, E.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies-New Perspectives for the Injured Lung. J. Clin. Med. 2020, 9, 682.
- Goetz, M.J.; Kremer, S.; Behnke, J.; Staude, B.; Shahzad, T.; Holzfurtner, L.; Chao, C.M.; Morty, R.E.; Bellusci, S.; Ehrhardt, H. MSC Based Therapies to Prevent or Treat BPD-A Narrative Review on Advances and Ongoing Challenges. Int. J. Mol. Sci. 2021, 22, 1138.
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712.
- Popova, A.P.; Bozyk, P.D.; Bentley, J.K.; Linn, M.J.; Goldsmith, A.M.; Schumacher, R.E.; Weiner, G.M.; Filbrun, A.G.; Hershenson, M.B. Isolation of Tracheal Aspirate Mesenchymal Stromal Cells Predicts Bronchopulmonary Dysplasia. Pediatrics 2010, 126, e1127–e1133.
- Lignelli, E.; Palumbo, F.; Myti, D.; Morty, R.E. Recent Advances in Our Understanding of the Mechanisms of Lung Alveolarization and Bronchopulmonary Dysplasia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 317, L832–L887.
- Alysandratos, K.-D.; Herriges, M.J.; Kotton, D.N. Epithelial Stem and Progenitor Cells in Lung Repair and Regeneration. Annu. Rev. Physiol. 2021, 83, 529–550.
- Rock, J.R.; Randell, S.H.; Hogan, B.L.M. Airway Basal Stem Cells: A Perspective on Their Roles in Epithelial Homeostasis and Remodeling. Dis. Models Mech. 2010, 3, 545–556.
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. Basal Cells Are a Multipotent Progenitor Capable of Renewing the Bronchial Epithelium. Am. J. Pathol. 2004, 164, 577–588.
- Hong, K.U.; Reynolds, S.D.; Watkins, S.; Fuchs, E.; Stripp, B.R. In Vivo Differentiation Potential of Tracheal Basal Cells: Evidence for Multipotent and Unipotent Subpopulations. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L643–L649.
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal Cells as Stem Cells of the Mouse Trachea and Human Airway Epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775.
- Montoro, D.T.; Haber, A.L.; Biton, M.; Vinarsky, V.; Lin, B.; Birket, S.E.; Yuan, F.; Chen, S.; Leung, H.M.; Villoria, J.; et al. A Revised Airway Epithelial Hierarchy Includes CFTR-Expressing Ionocytes. Nature 2018, 560, 319–324.
- Nakajima, M.; Kawanami, O.; Jin, E.; Ghazizadeh, M.; Honda, M.; Asano, G.; Horiba, K.; Ferrans, V.J. Immunohistochemical and Ultrastructural Studies of Basal Cells, Clara Cells and Bronchiolar Cuboidal Cells in Normal Human Airways. Pathol. Int. 1998, 48, 944–953.
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B. Number and Proliferation of Clara Cells in Normal Human Airway Epithelium. Am. J. Respir. Crit. Care Med. 1999, 159, 1585–1591.
- Tata, P.R.; Mou, H.; Pardo-Saganta, A.; Zhao, R.; Prabhu, M.; Law, B.M.; Vinarsky, V.; Cho, J.L.; Breton, S.; Sahay, A.; et al. Dedifferentiation of Committed Epithelial Cells into Stem Cells in Vivo. Nature 2013, 503, 218–223.
- Rock, J.R.; Gao, X.; Xue, Y.; Randell, S.H.; Kong, Y.Y.; Hogan, B.L. Notch-Dependent Differentiation of Adult Airway Basal Stem Cells. Cell Stem Cell 2011, 8, 639–648.
- Watson, J.K.; Rulands, S.; Wilkinson, A.C.; Wuidart, A.; Ousset, M.; Van Keymeulen, A.; Göttgens, B.; Blanpain, C.; Simons, B.D.; Rawlins, E.L. Clonal Dynamics Reveal Two Distinct Populations of Basal Cells in Slow-Turnover Airway Epithelium. Cell Rep. 2015, 12, 90–101.
- Teixeira, V.H.; Nadarajan, P.; Graham, T.A.; Pipinikas, C.P.; Brown, J.M.; Falzon, M.; Nye, E.; Poulsom, R.; Lawrence, D.; Wright, N.A.; et al. Stochastic Homeostasis in Human Airway Epithelium Is Achieved by Neutral Competition of Basal Cell Progenitors. eLife 2013, 2, e00966.
- Plasschaert, L.W.; Zilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A Single-Cell Atlas of the Airway Epithelium Reveals the CFTR-Rich Pulmonary Ionocyte. Nature 2018, 560, 377–381.
- Carraro, G.; Mulay, A.; Yao, C.; Mizuno, T.; Konda, B.; Petrov, M.; Lafkas, D.; Arron, J.R.; Hogaboam, C.M.; Chen, P.; et al. Single-Cell Reconstruction of Human Basal Cell Diversity in Normal and Idiopathic Pulmonary Fibrosis Lungs. Am. J. Respir. Crit. Care Med. 2020, 202, 1540–1550.
- Zaragosi, L.E.; Deprez, M.; Barbry, P. Using Single-Cell RNA Sequencing to Unravel Cell Lineage Relationships in the Respiratory Tract. Biochem. Soc. Trans. 2020, 48, 327–336.
- Ruiz Garcia, S.; Deprez, M.; Lebrigand, K.; Cavard, A.; Paquet, A.; Arguel, M.J.; Magnone, V.; Truchi, M.; Caballero, I.; Leroy, S.; et al. Novel Dynamics of Human Mucociliary Differentiation Revealed by Single-Cell RNA Sequencing of Nasal Epithelial Cultures. Development 2019, 146, 177428.
- Jaeger, B.; Schupp, J.C.; Plappert, L.; Terwolbeck, O.; Artysh, N.; Kayser, G.; Engelhard, P.; Adams, T.S.; Zweigerdt, R.; Kempf, H.; et al. Airway Basal Cells Show a Dedifferentiated KRT17highPhenotype and Promote Fibrosis in Idiopathic Pulmonary Fibrosis. Nat. Commun. 2022, 13, 5637.
- Yang, Y.; Riccio, P.; Schotsaert, M.; Mori, M.; Lu, J.; Lee, D.K.; Garcia-Sastre, A.; Xu, J.; Cardoso, W. V Spatial-Temporal Lineage Restrictions of Embryonic P63(+) Progenitors Establish Distinct Stem Cell Pools in Adult Airways. Dev. Cell 2018, 44, 752–761.e4.
- Shui, J.E.; Wang, W.; Liu, H.; Stepanova, A.; Liao, G.; Qian, J.; Ai, X.; Ten, V.; Lu, J.; Cardoso, W.V. Prematurity Alters the Progenitor Cell Program of the Upper Respiratory Tract of Neonates. Sci. Rep. 2021, 11, 10799.
- Wang, H.; Liu, Y.; Liu, Z. Clara Cell 10-KD Protein in Inflammatory Upper Airway Diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 25–30.
- Lee, R.M.; O’Brodovich, H. Airway Epithelial Damage in Premature Infants with Respiratory Failure. Am. Rev. Respir. Dis. 1988, 137, 450–457.
- Zheng, D.; Limmon, G.V.; Yin, L.; Leung, N.H.; Yu, H.; Chow, V.T.; Chen, J. Regeneration of Alveolar Type I and II Cells from Scgb1a1-Expressing Cells Following Severe Pulmonary Damage Induced by Bleomycin and Influenza. PLoS ONE 2012, 7, e48451.
- Whitsett, J.A. Airway Epithelial Differentiation and Mucociliary Clearance. Ann. Am. Thorac. Soc. 2018, 15, S143–S148.
- Lumsden, A.B.; McLean, A.; Lamb, D. Goblet and Clara Cells of Human Distal Airways: Evidence for Smoking Induced Changes in Their Numbers. Thorax 1984, 39, 844–849.
- Vieira Braga, F.A.; Kar, G.; Berg, M.; Carpaij, O.A.; Polanski, K.; Simon, L.M.; Brouwer, S.; Gomes, T.; Hesse, L.; Jiang, J.; et al. A Cellular Census of Human Lungs Identifies Novel Cell States in Health and in Asthma. Nat. Med. 2019, 25, 1153–1163.
- De Water, R.; Willems, L.N.; Van Muijen, G.N.; Franken, C.; Fransen, J.A.; Dijkman, J.H.; Kramps, J.A. Ultrastructural Localization of Bronchial Antileukoprotease in Central and Peripheral Human Airways by a Gold-Labeling Technique Using Monoclonal Antibodies. Am. Rev. Respir. Dis. 1986, 133, 882–890.
- Barth, P.J.; Wolf, M.; Ramaswamy, A. Distribution and Number of Clara Cells in the Normal and Disturbed Development of the Human Fetal Lung. Pediatr. Pathol. 1994, 14, 637–651.
- Thomas, W.; Seidenspinner, S.; Kawczyńska-Leda, N.; Chmielnicka-Kopaczyk, M.; Marx, A.; Wirbelauer, J.; Szymankiewicz, M.; Speer, C.P. Clara Cell Secretory Protein in Tracheobronchial Aspirates and Umbilical Cord Serum of Extremely Premature Infants with Systemic Inflammation. Neonatology 2010, 97, 228–234.
- Ramsay, P.L.; DeMayo, F.J.; Hegemier, S.E.; Wearden, M.E.; Smith, C.V.; Welty, S.E. Clara Cell Secretory Protein Oxidation and Expression in Premature Infants Who Develop Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2001, 164, 155–161.
- Schilders, K.A.; Eenjes, E.; van Riet, S.; Poot, A.A.; Stamatialis, D.; Truckenmuller, R.; Hiemstra, P.S.; Rottier, R.J. Regeneration of the Lung: Lung Stem Cells and the Development of Lung Mimicking Devices. Respir. Res. 2016, 17, 44.
- Evans, M.J.; Plopper, C.G. The Role of Basal Cells in Adhesion of Columnar Epithelium to Airway Basement Membrane. Am. Rev. Respir. Dis. 1988, 138, 481–483.
- Hoyt, R.F., Jr.; Feldman, H.; Sorokin, S.P. Neuroepithelial Bodies (NEB) and Solitary Endocrine Cells in the Hamster Lung. Exp. Lung Res. 1982, 3, 299–311.
- Kuo, C.S.; Krasnow, M.A. Formation of a Neurosensory Organ by Epithelial Cell Slithering. Cell 2015, 163, 394–405.
- Weichselbaum, M.; Sparrow, M.P.; Hamilton, E.J.; Thompson, P.J.; Knight, D.A. A Confocal Microscopic Study of Solitary Pulmonary Neuroendocrine Cells in Human Airway Epithelium. Respir. Res. 2005, 6, 115.
- Cutz, E.; Pan, J.; Yeger, H.; Domnik, N.J.; Fisher, J.T. Recent Advances and Contraversies on the Role of Pulmonary Neuroepithelial Bodies as Airway Sensors. Semin. Cell Dev. Biol. 2013, 24, 40–50.
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and Sweet Taste Receptors Regulate Human Upper Respiratory Innate Immunity. J. Clin. Investig. 2014, 124, 1393–1405.
- Branchfield, K.; Nantie, L.; Verheyden, J.M.; Sui, P.; Wienhold, M.D.; Sun, X. Pulmonary Neuroendocrine Cells Function as Airway Sensors to Control Lung Immune Response. Science 2016, 351, 707–710.
- Johnson, D.E.; Lock, J.E.; Elde, R.P.; Thompson, T.R. Pulmonary Neuroendocrine Cells in Hyaline Membrane Disease and Bronchopulmonary Dysplasia. Pediatr. Res. 1982, 16, 446–454.
- Johnson, D.E.; Kulik, T.J.; Lock, J.E.; Elde, R.P.; Thompson, T.R. Bombesin-, Calcitonin-, and Serotonin-Immunoreactive Pulmonary Neuroendocrine Cells in Acute and Chronic Neonatal Lung Disease. Pediatr. Pulmonol. 1985, 1, S13–S20.
- Cullen, A.; Van Marter, L.J.; Allred, E.N.; Moore, M.; Parad, R.B.; Sunday, M.E. Urine Bombesin-like Peptide Elevation Precedes Clinical Evidence of Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2002, 165, 1093–1097.
- Cutz, E.; Yeger, H.; Pan, J. Pulmonary Neuroendocrine Cell System in Pediatric Lung Disease-Recent Advances. Pediatr. Dev. Pathol. 2007, 10, 419–435.
- Fujinaga, H.; Baker, C.D.; Ryan, S.L.; Markham, N.E.; Seedorf, G.J.; Balasubramaniam, V.; Abman, S.H. Hyperoxia Disrupts Vascular Endothelial Growth Factor-Nitric Oxide Signaling and Decreases Growth of Endothelial Colony-Forming Cells from Preterm Infants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1160–L1169.
- Evans, M.J.; Cabral, L.J.; Stephens, R.J.; Freeman, G. Transformation of Alveolar Type 2 Cells to Type 1 Cells Following Exposure to NO2. Exp. Mol. Pathol. 1975, 22, 142–150.
- Fehrenbach, H. Alveolar Epithelial Type II Cell: Defender of the Alveolus Revisited. Respir. Res. 2001, 2, 33–46.
- Beers, M.F.; Moodley, Y. When Is an Alveolar Type 2 Cell an Alveolar Type 2 Cell? A Conundrum for Lung Stem Cell Biology and Regenerative Medicine. Am. J. Respir. Cell Mol. Biol. 2017, 57, 18–27.
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L. Type 2 Alveolar Cells Are Stem Cells in Adult Lung. J. Clin. Investig. 2013, 123, 3025–3036.
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-Cell Wnt Signaling Niches Maintain Stemness of Alveolar Type 2 Cells. Science 2018, 359, 1118–1123.
- Desai, T.J.; Brownfield, D.G.; Krasnow, M.A. Alveolar Progenitor and Stem Cells in Lung Development, Renewal and Cancer. Nature 2014, 507, 190–194.
- Uhal, B.D. Cell Cycle Kinetics in the Alveolar Epithelium. Am. J. Physiol. 1997, 272, L1031–L1045.
- Raman, T.; O’Connor, T.P.; Hackett, N.R.; Wang, W.; Harvey, B.G.; Attiyeh, M.A.; Dang, D.T.; Teater, M.; Crystal, R.G. Quality Control in Microarray Assessment of Gene Expression in Human Airway Epithelium. BMC Genom. 2009, 10, 493.
- Maniscalco, W.M.; Watkins, R.H.; O’Reilly, M.A.; Shea, C.P. Increased Epithelial Cell Proliferation in Very Premature Baboons with Chronic Lung Disease. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2002, 283, L991–L1001.
- Abman, S.H.; Collaco, J.M.; Shepherd, E.G.; Keszler, M.; Cuevas-Guaman, M.; Welty, S.E.; Truog, W.E.; McGrath-Morrow, S.A.; Moore, P.E.; Rhein, L.M.; et al. Interdisciplinary Care of Children with Severe Bronchopulmonary Dysplasia. J. Pediatr. 2017, 181, 12–28.e1.
- Caskey, S.; Gough, A.; Rowan, S.; Gillespie, S.; Clarke, J.; Riley, M.; Megarry, J.; Nicholls, P.; Patterson, C.; Halliday, H.L.; et al. Structural and Functional Lung Impairment in Adult Survivors of Bronchopulmonary Dysplasia. Ann. Am. Thorac. Soc. 2016, 13, 1262–1270.
- Thebaud, B.; Abman, S.H. Bronchopulmonary Dysplasia: Where Have All the Vessels Gone? Roles of Angiogenic Growth Factors in Chronic Lung Disease. Am. J. Respir. Crit. Care Med. 2007, 175, 978–985.
- Angusamy, S.; Mansour, T.; Abdulmageed, M.; Han, R.; Schutte, B.C.; LaPres, J.; Harkema, J.R.; Omar, S.A. Altered Thymocyte and T Cell Development in Neonatal Mice with Hyperoxia-Induced Lung Injury. J. Perinat. Med. 2018, 46, 441–449.
- Liu, S. Mouse Pneumonectomy Model of Compensatory Lung Growth. J. Vis. Exp. 2014, 94, e52294.
- Voswinckel, R.; Motejl, V.; Fehrenbach, A.; Wegmann, M.; Mehling, T.; Fehrenbach, H.; Seeger, W. Characterisation of Post-Pneumonectomy Lung Growth in Adult Mice. Eur. Respir. J. 2004, 24, 524–532.
- Lechner, A.J.; Driver, I.H.; Lee, J.; Conroy, C.M.; Nagle, A.; Locksley, R.M.; Rock, J.R. Recruited Monocytes and Type 2 Immunity Promote Lung Regeneration Following Pneumonectomy. Cell Stem Cell 2017, 21, 120–134.e7.
- Ahmadvand, N.; Khosravi, F.; Lingampally, A.; Wasnick, R.; Vazquez-Armendariz, I.; Carraro, G.; Heiner, M.; Rivetti, S.; Lv, Y.; Wilhelm, J.; et al. Identification of a Novel Subset of Alveolar Type 2 Cells Enriched in PD-L1 and Expanded Following Pneumonectomy. Eur. Respir. J. 2021, 58, 2004168.
- Kalymbetova, T.V.; Selvakumar, B.; Rodríguez-Castillo, J.A.; Gunjak, M.; Malainou, C.; Heindl, M.R.; Moiseenko, A.; Chao, C.M.; Vadász, I.; Mayer, K.; et al. Resident Alveolar Macrophages Are Master Regulators of Arrested Alveolarization in Experimental Bronchopulmonary Dysplasia. J. Pathol. 2018, 245, 153–159.
- Shafa, M.; Ionescu, L.I.; Vadivel, A.; Collins, J.J.P.; Xu, L.; Zhong, S.; Kang, M.; de Caen, G.; Daneshmand, M.; Shi, J.; et al. Human Induced Pluripotent Stem Cell–Derived Lung Progenitor and Alveolar Epithelial Cells Attenuate Hyperoxia-Induced Lung Injury. Cytotherapy 2018, 20, 108–125.
- Choi, J.; Park, J.-E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.-K.; Han, N.; Lee, J.-H. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors That Mediate Alveolar Regeneration. Cell Stem Cell 2020, 27, 366–382.e7.
- Lee, J.H.; Bhang, D.H.; Beede, A.; Huang, T.L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.H.; Ryeom, S.; Kim, C.F. Lung Stem Cell Differentiation in Mice Directed by Endothelial Cells via a BMP4-NFATc1-Thrombospondin-1 Axis. Cell 2014, 156, 440–455.
- Rawlins, E.L.; Okubo, T.; Xue, Y.; Brass, D.M.; Auten, R.L.; Hasegawa, H.; Wang, F.; Hogan, B.L. The Role of Scgb1a1+ Clara Cells in the Long-Term Maintenance and Repair of Lung Airway, but Not Alveolar, Epithelium. Cell Stem Cell 2009, 4, 525–534.
- Chapman, H.A.; Li, X.; Alexander, J.P.; Brumwell, A.; Lorizio, W.; Tan, K.; Sonnenberg, A.; Wei, Y.; Vu, T.H. Integrin Alpha6beta4 Identifies an Adult Distal Lung Epithelial Population with Regenerative Potential in Mice. J. Clin. Investig. 2011, 121, 2855–2862.
- Jain, R.; Barkauskas, C.E.; Takeda, N.; Bowie, E.J.; Aghajanian, H.; Wang, Q.; Padmanabhan, A.; Manderfield, L.J.; Gupta, M.; Li, D.; et al. Plasticity of Hopx(+) Type I Alveolar Cells to Regenerate Type II Cells in the Lung. Nat. Commun. 2015, 6, 6727.
- Zuo, W.; Zhang, T.; Wu, D.Z.; Guan, S.P.; Liew, A.A.; Yamamoto, Y.; Wang, X.; Lim, S.J.; Vincent, M.; Lessard, M.; et al. P63(+)Krt5(+) Distal Airway Stem Cells Are Essential for Lung Regeneration. Nature 2015, 517, 616–620.
- Vaughan, A.E.; Brumwell, A.N.; Xi, Y.; Gotts, J.E.; Brownfield, D.G.; Treutlein, B.; Tan, K.; Tan, V.; Liu, F.C.; Looney, M.R.; et al. Lineage-Negative Progenitors Mobilize to Regenerate Lung Epithelium after Major Injury. Nature 2015, 517, 621–625.
- Paul, M.K.; Bisht, B.; Darmawan, D.O.; Chiou, R.; Ha, V.L.; Wallace, W.D.; Chon, A.T.; Hegab, A.E.; Grogan, T.; Elashoff, D.A.; et al. Dynamic Changes in Intracellular ROS Levels Regulate Airway Basal Stem Cell Homeostasis through Nrf2-Dependent Notch Signaling. Cell Stem Cell 2014, 15, 199–214.
- Zhao, R.; Fallon, T.R.; Saladi, S.V.; Pardo-Saganta, A.; Villoria, J.; Mou, H.; Vinarsky, V.; Gonzalez-Celeiro, M.; Nunna, N.; Hariri, L.P.; et al. Yap Tunes Airway Epithelial Size and Architecture by Regulating the Identity, Maintenance, and Self-Renewal of Stem Cells. Dev. Cell 2014, 30, 151–165.
- Mori, M.; Mahoney, J.E.; Stupnikov, M.R.; Paez-Cortez, J.R.; Szymaniak, A.D.; Varelas, X.; Herrick, D.B.; Schwob, J.; Zhang, H.; Cardoso, W. V Notch3-Jagged Signaling Controls the Pool of Undifferentiated Airway Progenitors. Development 2015, 142, 258–267.
- Gao, X.; Bali, A.S.; Randell, S.H.; Hogan, B.L. GRHL2 Coordinates Regeneration of a Polarized Mucociliary Epithelium from Basal Stem Cells. J. Cell Biol. 2015, 211, 669–682.
- Morimoto, M.; Nishinakamura, R.; Saga, Y.; Kopan, R. Different Assemblies of Notch Receptors Coordinate the Distribution of the Major Bronchial Clara, Ciliated and Neuroendocrine Cells. Development 2012, 139, 4365–4373.
- Shan, L.; Aster, J.C.; Sklar, J.; Sunday, M.E. Notch-1 Regulates Pulmonary Neuroendocrine Cell Differentiation in Cell Lines and in Transgenic Mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L500–L509.
- Ito, T.; Udaka, N.; Yazawa, T.; Okudela, K.; Hayashi, H.; Sudo, T.; Guillemot, F.; Kageyama, R.; Kitamura, H. Basic Helix-Loop-Helix Transcription Factors Regulate the Neuroendocrine Differentiation of Fetal Mouse Pulmonary Epithelium. Development 2000, 127, 3913–3921.
- Kumar, P.A.; Hu, Y.; Yamamoto, Y.; Hoe, N.B.; Wei, T.S.; Mu, D.; Sun, Y.; Joo, L.S.; Dagher, R.; Zielonka, E.M.; et al. Distal Airway Stem Cells Yield Alveoli In Vitro and during Lung Regeneration Following H1N1 Influenza Infection. Cell 2011, 147, 525–538.
- Guha, A.; Vasconcelos, M.; Cai, Y.; Yoneda, M.; Hinds, A.; Qian, J.; Li, G.; Dickel, L.; Johnson, J.E.; Kimura, S.; et al. Neuroepithelial Body Microenvironment Is a Niche for a Distinct Subset of Clara-like Precursors in the Developing Airways. Proc. Natl. Acad. Sci. USA 2012, 109, 12592–12597.
- Linnoila, R.I. Functional Facets of the Pulmonary Neuroendocrine System. Lab. Investig. 2006, 86, 425–444.
- Minoo, P.; Su, G.; Drum, H.; Bringas, P.; Kimura, S. Defects in Tracheoesophageal and Lung Morphogenesis in Nkx2.1(−/−) Mouse Embryos. Dev. Biol. 1999, 209, 60–71.
- Noguchi, M.; Sumiyama, K.; Morimoto, M. Directed Migration of Pulmonary Neuroendocrine Cells toward Airway Branches Organizes the Stereotypic Location of Neuroepithelial Bodies. Cell Rep. 2015, 13, 2679–2686.
- Yao, E.; Lin, C.; Wu, Q.; Zhang, K.; Song, H.; Chuang, P.T. Notch Signaling Controls Transdifferentiation of Pulmonary Neuroendocrine Cells in Response to Lung Injury. Stem Cells 2018, 36, 377–391.
- Borges, M.; Linnoila, R.I.; Van De Velde, H.J.K.; Chen, H.; Nelkin, B.D.; Mabry, M.; Baylin, S.B.; Ball, D.W. An Achaete-Scute Homologue Essential for Neuroendocrine Differentiation in the Lung. Nature 1997, 386, 852–855.
- Bender Kim, C.F.; Jackson, E.L.; Woolfenden, A.E.; Lawrence, S.; Babar, I.; Vogel, S.; Crowley, D.; Bronson, R.T.; Jacks, T. Identification of Bronchioalveolar Stem Cells in Normal Lung and Lung Cancer. Cell 2005, 121, 823–835.
- Zhang, Y.; Goss, A.M.; Cohen, E.D.; Kadzik, R.; Lepore, J.J.; Muthukumaraswamy, K.; Yang, J.; DeMayo, F.J.; Whitsett, J.A.; Parmacek, M.S.; et al. A Gata6-Wnt Pathway Required for Epithelial Stem Cell Development and Airway Regeneration. Nat. Genet. 2008, 40, 862–870.
- Parekh, K.R.; Nawroth, J.; Pai, A.; Busch, S.M.; Senger, C.N.; Ryan, A.L. Stem Cells and Lung Regeneration. Am. J. Physiol. Cell Physiol. 2020, 319, C675–C693.
- Lynch, T.J.; Liu, X.; Wei, J.; Engelhardt, J.F. Stem Cell Niches in the Lung. In Lung Stem Cells in the Epithelium and Vasculature; Firth, A., Yuan, J.X.J., Eds.; Springer International Publishing: Cham, Switzeland, 2015; pp. 35–58. ISBN 978-3-319-16232-4.
- Kapere Ochieng, J.; Schilders, K.; Kool, H.; Buscop-van Kempen, M.; Boerema-De Munck, A.; Grosveld, F.; Wijnen, R.; Tibboel, D.; Rottier, R.J. Differentiated Type II Pneumocytes Can Be Reprogrammed by Ectopic Sox2 Expression. PLoS ONE 2014, 9, e107248.
- Liu, Y.; Kumar, V.S.; Zhang, W.; Rehman, J.; Malik, A.B. Activation of Type II Cells into Regenerative Stem Cell Antigen-1(+) Cells during Alveolar Repair. Am. J. Respir. Cell Mol. Biol. 2015, 53, 113–124.
- Summer, R.; Fitzsimmons, K.; Dwyer, D.; Murphy, J.; Fine, A. Isolation of an Adult Mouse Lung Mesenchymal Progenitor Cell Population. Am. J. Respir. Cell Mol. Biol. 2007, 37, 152–159.
- Raiser, D.M.; Kim, C.F. Commentary: Sca-1 and Cells of the Lung: A Matter of Different Sorts. Stem Cells 2009, 27, 606–611.
- Prince, L.S. FGF10 and Human Lung Disease Across the Life Spectrum. Front. Genet. 2018, 9, 517.
- Popova, A.P.; Bentley, J.K.; Cui, T.X.; Richardson, M.N.; Linn, M.J.; Lei, J.; Chen, Q.; Goldsmith, A.M.; Pryhuber, G.S.; Hershenson, M.B. Reduced Platelet-Derived Growth Factor Receptor Expression Is a Primary Feature of Human Bronchopulmonary Dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L231–L239.
- Pierro, M.; Ionescu, L.; Montemurro, T.; Vadivel, A.; Weissmann, G.; Oudit, G.; Emery, D.; Bodiga, S.; Eaton, F.; Peault, B.; et al. Short-Term, Long-Term and Paracrine Effect of Human Umbilical Cord-Derived Stem Cells in Lung Injury Prevention and Repair in Experimental Bronchopulmonary Dysplasia. Thorax 2013, 68, 475–484.
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823.
- Worthington, E.N.; Hagood, J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020, 21, 2318.
- Gowen, A.; Shahjin, F.; Chand, S.; Odegaard, K.E.; Yelamanchili, S. V Mesenchymal Stem Cell-Derived Extracellular Vesicles: Challenges in Clinical Applications. Front. Cell Dev. Biol. 2020, 8, 149.
- Chu, X.; Lingampally, A.; Moiseenko, A.; Kheirollahi, V.; Vazquez-Armendariz, A.I.; Koepke, J.; Khadim, A.; Kiliaris, G.; Shahriari Felordi, M.; Zabihi, M.; et al. GLI1+ Cells Are a Source of Repair-Supportive Mesenchymal Cells (RSMCs) during Airway Epithelial Regeneration. Cell. Mol. Life Sci. 2022, 79, 581.
More
Information
Subjects:
Respiratory System
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
941
Revisions:
2 times
(View History)
Update Date:
21 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




