Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hosam M. Saleh | -- | 2223 | 2023-07-19 12:03:56 | | | |
| 2 | Sirius Huang | -1 word(s) | 2222 | 2023-07-20 11:59:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Saleh, H.M.; Hassan, A.I. Applications of Nanomaterials in Electrochemical Devices. Encyclopedia. Available online: https://encyclopedia.pub/entry/46971 (accessed on 13 January 2026).
Saleh HM, Hassan AI. Applications of Nanomaterials in Electrochemical Devices. Encyclopedia. Available at: https://encyclopedia.pub/entry/46971. Accessed January 13, 2026.
Saleh, Hosam M., Amal I. Hassan. "Applications of Nanomaterials in Electrochemical Devices" Encyclopedia, https://encyclopedia.pub/entry/46971 (accessed January 13, 2026).
Saleh, H.M., & Hassan, A.I. (2023, July 19). Applications of Nanomaterials in Electrochemical Devices. In Encyclopedia. https://encyclopedia.pub/entry/46971
Saleh, Hosam M. and Amal I. Hassan. "Applications of Nanomaterials in Electrochemical Devices." Encyclopedia. Web. 19 July, 2023.
Copy Citation
Nanomaterials have gained significant attention as a remarkable class of materials due to their unique properties and the fact that they encompass a wide range of samples with at least one dimension ranging from 1 to 100 nm. The deliberate design of nanoparticles enables the achievement of extremely large surface areas. In the field of cost-effective electrochemical devices for energy storage and conversion applications, nanomaterials have emerged as a key area of research.
nanomaterials
green nanotechnology
electrochemical devices
cost-effective
biosensors
energy storage
1. Introduction
The development of cost-effective electrochemical devices is crucial for efficient energy storage and conversion, which is essential for sustainable development. Nanomaterials have demonstrated great potential in enhancing the performance of electrochemical devices, but the synthesis of these materials for large-scale applications remains a challenge [1]. Several synthesis methods have been developed to address this challenge, including solution-based, template-based, and microwave-assisted synthesis. These methods have shown promise in producing high-quality nanomaterials in large quantities, and they have the potential to reduce production costs. Integrating nanomaterials into electrochemical devices is also a significant challenge, as the properties of nanomaterials differ significantly from those of their bulk counterparts [2]. The interface between the nanomaterials and other components of the electrochemical device is critical, and it can significantly influence device performance and efficiency. Therefore, optimizing the integration of nanomaterials into electrochemical devices is crucial for realizing their full potential.
The synthesis of nanomaterials on a large scale for practical applications remains a challenge. However, significant progress has been made in developing various synthesis methods, such as solution-based methods, template-based methods, and microwave-assisted synthesis [3][4]. These approaches show promise in producing high-quality nanomaterials in large quantities while also potentially reducing production costs.
Integrating nanomaterials into electrochemical devices presents another significant challenge. The properties of nanomaterials differ significantly from their bulk counterparts, necessitating careful consideration of their integration with other components of the device. The interface between nanomaterials and the device’s other constituents critically influences device performance and efficiency [5]. Therefore, optimizing the integration of nanomaterials is crucial to fully exploit their potential in electrochemical devices [5].
2. Classification of Nanomaterials
Nanomaterials can be classified based on their dimensions, such as 0D, 1D, and 2D structures. Zero-dimensional (0D) nanomaterials are spherical nanoparticles and are the simplest type of nanomaterials. Examples of 0D nanomaterials include metal nanoparticles like gold and silver, semiconductor nanoparticles like quantum dots, and oxide nanoparticles like titanium dioxide [6]. These nanomaterials have unique physical and chemical properties compared to their bulk counterparts, such as a high surface-to-volume ratio and quantum confinement effects.
Moving on to one-dimensional (1D) nanomaterials, they have one dimension in the nanoscale range. Examples of 1D nanomaterials include nanowires, nanotubes, and nanorods. Carbon nanotubes, silicon nanowires, and zinc oxide nanorods are some common examples of 1D nanomaterials. These nanomaterials are appealing for use in a wide range of electrical, photonic, and energy storage applications because of their excellent mechanical, electronic, and optical capabilities that result from their one-dimensional structure. Finally, two-dimensional (2D) nanomaterials have two dimensions in the nanoscale range. Examples of 2D nanomaterials include graphene, transition metal dichalcogenides (TMDs) like MoS2 and WS2, and black phosphorus [7]. These nanomaterials have unique electronic, mechanical, and optical properties that make them promising candidates for various applications, including sensors, energy storage, and electronic devices.
Nanomaterials can also be classified based on their electric properties, such as insulating, semiconducting, and metal-like properties. Insulating nanomaterials do not conduct electricity and have a wide band gap. Examples of insulating nanomaterials include metal oxides like titanium dioxide, zinc oxide, and aluminum oxide. These nanomaterials are widely used in various applications, including catalysis, energy storage, and environmental remediation.
Scheme 1 and Table 1 summarize the use of nanomaterials in various types of electrochemical devices.
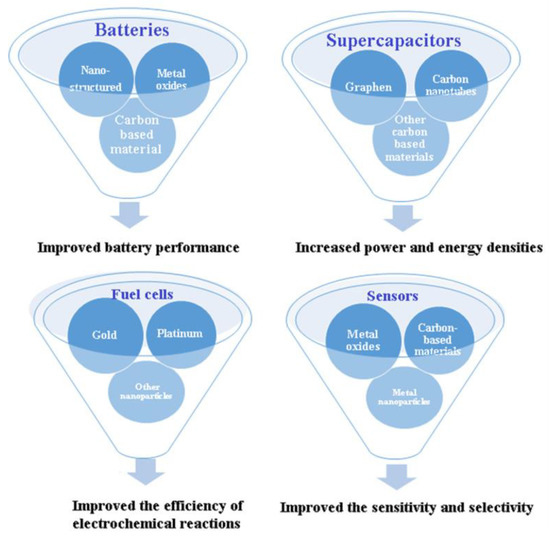
Scheme 1. Nanomaterials in various types of electrochemical devices.
Table 1. Nanomaterials used in various electrochemical devices.
| Electrochemical Device | Nanomaterials Used | Applications | References |
|---|---|---|---|
| Batteries | Nanostructured metals, metal oxides, and carbon-based materials, MOFs, COFs, MXenes. | Electrode materials to improve performance, increase surface area, improve conductivity, and provide higher energy and power densities | [6][8] |
| Supercapacitors | Carbon nanotubes, graphene, and other carbon-based materials, MOFs, COFs, and MXenes. | Electrode materials to increase surface area, improve conductivity, and provide high power and energy densities | [7][9][10][11][12] |
| Fuel Cells | Platinum, gold, and other metal nanoparticles, MOFs, COFs, MXenes. | Catalysts to improve the efficiency of electrochemical reactions that generate electricity | [13] |
| Sensors | Metal nanoparticles, metal oxides, and carbon-based materials, MOFs, COFs, MXenes | Sensing elements to improve sensitivity and selectivity due to their high surface area, high catalytic activity, and unique optical and electrical properties | [14] |
Semiconducting nanomaterials have intermediate electrical conductivity, and their conductivity can be tuned by doping or changing their size. Examples of semiconducting nanomaterials include quantum dots, carbon nanotubes, and TMDs [15]. Various electrochemical devices can benefit from the use of certain nanomaterials because of their distinctive electrical and optical capabilities in the fields of electronics, photonics, and energy conversion. For example, metal nanoparticles like gold and silver have been used in electrochemical sensing and catalysis. Carbon nanotubes have shown potential as electrodes in batteries and fuel cells, while graphene has shown promise as a material for supercapacitors. Some studies focused on the use of graphene as a means of stabilizing the interface between silicon-based electrodes and electrolytes to achieve a stable cycle life. While coating graphene on the surface of nanostructured silicon electrodes is an effective approach, most of the graphene-supported anode reports involve silicon nanoparticle structures. The study by Yang et al. [16] suggested that coating graphene layers on silicon nanowires could be a viable option for stabilizing the interface between silicon-based electrodes and electrolytes. After 500 charge–discharge cycles, the resultant silicon nanowires showed excellent Coulombic efficiencies of over 99% and a huge reversible capacity of 1650 mA h g1. The nanowires were composed of silicon carbide nanocrystals and surrounded by a homogenous graphene shell. Furthermore, Si nanowires were formed in situ by a gold-catalyzed procedure. These nanowires, when backed by graphene, showed an initial reversible lithium extraction capacity of 2009 mA h g1, and after 30 cycles at 420 mA g1, a reversible capacity of 1400 mA h g1 was achieved. These findings demonstrate the potential of graphene-supported silicon nanowires and suggest a promising avenue for further research in this area. Furthermore, insulating nanomaterials like titanium dioxide have been used as anodes in lithium-ion batteries, while semiconducting nanomaterials like quantum dots have been used in solar cells [2].
To better understand and categorize the different types of nanomaterials that can be used in electrochemical applications, a classification based on different classes of materials has been proposed. These classes include carbon-based nanomaterials, metal oxides, and hydroxides, conducting polymers, and hybrid materials. Carbon-based nanomaterials such as graphene, carbon nanotubes, and fullerenes have been extensively studied and show great potential for improving the performance of electrochemical devices, particularly in energy storage applications such as batteries and supercapacitors [17].
3. Applications of Nanomaterials in Electrochemical Devices
Metal oxides and hydroxides, including titanium dioxide, zinc oxide, and iron oxide, have also shown promise in electrochemical devices due to their high surface area and redox properties [18]. Conducting polymers such as polyaniline and polypyrrole offer unique properties such as high conductivity and tunable redox potential, making them suitable for a range of electrochemical applications. Hybrid materials, which combine two or more classes of nanomaterials, have also been investigated and offer the potential for improved performance and functionality. When electroactive materials are nanostructured, significant alterations are made to the devices’ electro-chemical characteristics. Assembled from quasi-zero-dimensional structures or building blocks (nanoparticles), two- and three-dimensional nanometer-sized structures like dye-sensitized solar cells (DSSC) are one example. Moreover, 0D nanoparticles and nanodots have attracted significant attention for their potential use in batteries due to their small size and high surface area. In particular, 0D nanoparticles and nanodots exhibit unique physical and chemical properties that make them promising candidates for electrode materials in batteries. These materials can significantly improve the performance of batteries by providing high capacity, fast charging and discharging rates, and a long cycle life [19].
One advantage of 0D nanoparticles and nanodots is their high surface area, which provides numerous active sites for electrochemical reactions. This property can enhance the capacity of batteries by increasing the amount of active material available for electrochemical reactions [20]. Additionally, the small size of 0D nanoparticles and nanodots enables fast diffusion of lithium ions, leading to faster charging and discharging rates. More efficient ion transfer and enhanced electrochemical performance are two benefits of using 0D nanoparticles and nanodots in batteries, which benefit from their high surface area-to-volume ratio [20].
Research has been conducted on various types of 0D nanoparticles and nanodots, including metal oxide nanoparticles, carbon nanodots, and quantum dots. For instance, studies have shown that tin oxide nanoparticles can improve the cycling stability and rate capability of lithium-ion batteries, while carbon nanodots have been proven to enhance the capacity and rate performance of sodium-ion batteries [21]. Quantum dots have also been investigated for their potential use in next-generation batteries because of their unique electronic and optical properties [21]. Moreover, 0D nanoparticles and nanodots have unique properties that make them attractive for use in various electrochemical devices beyond just batteries. For instance, their high surface area-to-volume ratio and tunable size and shape make them ideal for use in sensors, catalysts, and supercapacitors. Additionally, their small size can facilitate electron transfer and enhance ion diffusion, improving overall device performance. Recent research has focused on developing novel synthetic methods for producing highly uniform and monodisperse 0D nanoparticles and nanodots, as well as exploring their electrochemical properties and potential applications in various electrochemical devices [22].
Extensive research has been carried out on 1D nanowires and 2D nanosheets for their applications in various electrochemical devices, such as supercapacitors and fuel cells [23]. These materials exhibit high specific capacitance and fast charge/discharge rates in supercapacitors due to their large surface area and excellent conductivity. In fuel cells, 1D nanowires and 2D nanosheets have been used as catalysts to improve reaction kinetics and reduce the cost of electrochemical reactions. The unique properties of 1D and 2D nanomaterials, such as their mechanical strength, flexibility, and transparency, make them attractive for various electrochemical device applications [24].
Three-dimensional (3D) nanometer-sized structures, also known as nanoarchitectures or nanostructures, have also been investigated for their potential use in electrochemical devices. These structures can offer a high surface area and unique porosity, which can enhance electrochemical reactions and improve the performance of electrochemical devices [25]. Examples of 3D nanostructures include nanocubes, nanorods, and mesoporous silica. Researchers have explored their use in batteries, supercapacitors, and fuel cells with promising results. However, challenges remain in synthesizing and integrating 3D nanostructures into practical electrochemical devices, and more research is needed to overcome these challenges and fully exploit the potential of these materials.
Definite analytical techniques are used for nanoparticle detection and characterization. Advanced technologies include electron microscopes for scanning or transmission (SEM, TEM) [23][24][26][27]. Different optical procedures can provide various types of physicochemical information for nanoparticles, but their usefulness depends on the technology and particular material properties, such as composition and scale [28]. Electrochemical technologies can address some problems, such as low-cost, easy-to-use portable controllers and self-powered devices [29]. As many nanoparticles can generate electrical signals, have high biocompatibility and stability, and are simple to operate, nanotechnology may be a promising solution for many electrochemical technologies [30].
Quantitative techniques are required to quantify exposure to foreign organisms to support the creation and implementation of biomonitoring programs. The electrochemical phenomenon has many technological applications, such as chemical synthesis, electroextraction, metal refining, batteries, fuel cells, sensors, and surface modification by electrostatic precipitation, separation, and corrosion. Recent studies have emphasized the importance of nanotechnology in constructing promising electrode materials for high-performance supercapacitors [28][29][30][31][32]. The synthesis of nitrogen-rich activated nanosized carbon with hierarchical micro/mesoporous and ultra-high specific surface area was greatly simplified and chemical waste was reduced when Zheng et al. [33] revealed a template-free and one-step carbonization–activation technology. Activated carbon with a high nitrogen content and strong electrochemical characteristics was obtained by synthesizing chitin nanoparticles in a NaOH/urea solvent using a mechanically driven sol–gel transition process.
Table 2 compares different types of nanomaterials used in various electrochemical devices. Metal oxides and hydroxides, such as titanium dioxide, zinc oxide, and iron oxide, offer high surface area and redox properties, making them suitable for electrochemical applications. Conducting polymers like polyaniline and polypyrrole exhibit high conductivity and tunable redox potential, providing versatility in electrochemical devices. Hybrid materials, which combine different nanomaterial classes, offer improved performance and functionality due to synergistic effects. Furthermore, nanomaterials designed for electrode materials in electrochemical devices demonstrate tailored properties for high-performance devices. By understanding the distinctive characteristics and synthesis methods of these nano-materials, researchers can develop cost-effective electrochemical devices with enhanced performance.
Table 2. Comparative Analysis of Morphological Features and Properties of Nanomaterials Synthesized via Different Methods.
| Nanomaterials | Synthesis Method | Morphological Features | Properties | References |
|---|---|---|---|---|
| Metal oxides and hydroxides | Various | High surface area, redox properties | Suitable for electrochemical devices | [31][32][34][35] |
| Conducting polymers (e.g., polyaniline, polypyrrole) | Various | High conductivity, tunable redox potential | Versatile for electrochemical applications | [36] |
| Hybrid materials | Combination of nanomaterial classes | Improved performance and functionality | Enhanced properties through synergy | [37] |
| 0D nanoparticles and nanodots | Various synthesis methods | Small size, high surface area | Promising for batteries, sensors, catalysts, supercapacitors | [38] |
| 1D nanowires and 2D nanosheets | Various synthesis methods | Large surface area, excellent conductivity | Suitable for supercapacitors, fuel cells, and other electrochemical devices | [39] |
| 3D nanostructures (nanocubes, nanorods, mesoporous silica) | Various synthesis methods | High surface area, unique porosity | Potential for batteries, supercapacitors, fuel cells | [40] |
| Nanomaterials for electrode materials | Various synthesis methods | Tailored properties for high-performance devices | Potential for cost-effective electrochemical devices | [41] |
References
- Xu, H.; Ci, S.; Ding, Y.; Wang, G.; Wen, Z. Recent Advances in Precious Metal-Free Bifunctional Catalysts for Electrochemical Conversion Systems. J. Mater. Chem. A 2019, 7, 8006–8029.
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871.
- Pottathara, Y.B.; Grohens, Y.; Kokol, V.; Kalarikkal, N.; Thomas, S. Synthesis and Processing of Emerging Two-Dimensional Nanomaterials. In Nanomaterials Synthesis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–25.
- Ouyang, D.; Huang, Z.; Choy, W.C.H. Solution-processed Metal Oxide Nanocrystals as Carrier Transport Layers in Organic and Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1804660.
- Devasahayam, S.; Hussain, C.M. Thin-Film Nanocomposite Devices for Renewable Energy Current Status and Challenges. Sustain. Mater. Technol. 2020, 26, e00233.
- Chen, Y.; Zhao, X.; Li, Y.; Jin, Z.-Y.; Yang, Y.; Yang, M.-B.; Yin, B. Light-and Magnetic-Responsive Synergy Controlled Reconfiguration of Polymer Nanocomposites with Shape Memory Assisted Self-Healing Performance for Soft Robotics. J. Mater. Chem. C 2021, 9, 5515–5527.
- Zhang, L.; Li, M.; Lyu, Q.; Zhu, J. Bioinspired Structural Color Nanocomposites with Healable Capability. Polym. Chem. 2020, 11, 6413–6422.
- Ehab, M.; Salama, E.; Ashour, A.; Attallah, M.; Saleh, H.M. Optical Properties and Gamma Radiation Shielding Capability of Transparent Barium Borosilicate Glass Composite. Sustainability 2022, 14, 13298.
- Lu, C.; Fang, R.; Chen, X. Single-atom Catalytic Materials for Advanced Battery Systems. Adv. Mater. 2020, 32, 1906548.
- Zhao, J.; Burke, A.F. Electrochemical Capacitors: Materials, Technologies and Performance. Energy Storage Mater. 2021, 36, 31–55.
- Verma, K.D.; Sinha, P.; Banerjee, S.; Kar, K.K. Characteristics of Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials I: Characteristics; Springer: Berlin/Heidelberg, Germany, 2020; pp. 269–285.
- Forouzandeh, P.; Pillai, S.C. Two-Dimensional (2D) Electrode Materials for Supercapacitors. Mater. Today Proc. 2021, 41, 498–505.
- Kim, S.; Lee, Y.M. Two-Dimensional Nanosheets and Membranes for Their Emerging Technologies. Curr. Opin. Chem. Eng. 2023, 39, 100893.
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent Advances in Two-Dimensional-Material-Based Sensing Technology toward Health and Environmental Monitoring Applications. Nanoscale 2020, 12, 3535–3559.
- Kim, H.; Beack, S.; Han, S.; Shin, M.; Lee, T.; Park, Y.; Kim, K.S.; Yetisen, A.K.; Yun, S.H.; Kwon, W. Multifunctional Photonic Nanomaterials for Diagnostic, Therapeutic, and Theranostic Applications. Adv. Mater. 2018, 30, 1701460.
- Yang, Y.; Liu, S.; Bian, X.; Feng, J.; An, Y.; Yuan, C. Morphology-and Porosity-Tunable Synthesis of 3D Nanoporous SiGe Alloy as a High-Performance Lithium-Ion Battery Anode. ACS Nano 2018, 12, 2900–2908.
- Salahdin, O.D.; Sayadi, H.; Solanki, R.; Parra, R.M.R.; Al-Thamir, M.; Jalil, A.T.; Izzat, S.E.; Hammid, A.T.; Arenas, L.A.B.; Kianfar, E. Graphene and Carbon Structures and Nanomaterials for Energy Storage. Appl. Phys. A 2022, 128, 703.
- Dong, Q.; Ryu, H.; Lei, Y. Metal Oxide Based Non-Enzymatic Electrochemical Sensors for Glucose Detection. Electrochim. Acta 2021, 370, 137744.
- Li, C.; Wu, H.; Hong, S.; Wang, Y.; Song, N.; Han, Z.; Dong, H. 0D/2D Heterojunction Constructed by High-Dispersity Mo-Doped Ni2P Nanodots Supported on g-C3N4 Nanosheets towards Enhanced Photocatalytic H2 Evolution Activity. Int. J. Hydrogen Energy 2020, 45, 22556–22566.
- Jin, X.; Gu, T.-H.; Lee, K.-G.; Kim, M.J.; Islam, M.S.; Hwang, S.-J. Unique Advantages of 2D Inorganic Nanosheets in Exploring High-Performance Electrocatalysts: Synthesis, Application, and Perspective. Coord. Chem. Rev. 2020, 415, 213280.
- Zhang, C.; Chen, M.; Pan, Y.; Li, Y.; Wang, K.; Yuan, J.; Sun, Y.; Zhang, Q. Carbon Nanodots Memristor: An Emerging Candidate toward Artificial Biosynapse and Human Sensory Perception System. Adv. Sci. 2023, 10, 2207229.
- Rasal, A.S.; Yadav, S.; Yadav, A.; Kashale, A.A.; Manjunatha, S.T.; Altaee, A.; Chang, J.-Y. Carbon Quantum Dots for Energy Applications: A Review. ACS Appl. Nano Mater. 2021, 4, 6515–6541.
- Baruah, B.; Kumar, A. Platinum-Free Anode Electrocatalysts for Methanol Oxidation in Direct Methanol Fuel Cells. In Ceramic and Specialty Electrolytes for Energy Storage Devices; CRC Press: Boca Raton, FL, USA, 2021; pp. 261–283. ISBN 1003144810.
- Niu, H.; Xia, C.; Huang, L.; Zaman, S.; Maiyalagan, T.; Guo, W.; You, B.; Xia, B.Y. Rational Design and Synthesis of One-Dimensional Platinum-Based Nanostructures for Oxygen-Reduction Electrocatalysis. Chin. J. Catal. 2022, 43, 1459–1472.
- Mei, J.; Liao, T.; Ayoko, G.A.; Bell, J.; Sun, Z. Cobalt Oxide-Based Nanoarchitectures for Electrochemical Energy Applications. Prog. Mater. Sci. 2019, 103, 596–677.
- Inkson, B.J. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) for Materials Characterization. In Materials Characterization Using Nondestructive Evaluation (NDE) Methods; Elsevier: Amsterdam, The Netherlands, 2016; pp. 17–43.
- Cao, D.; Gong, S.; Shu, X.; Zhu, D.; Liang, S. Preparation of ZnO Nanoparticles with High Dispersibility Based on Oriented Attachment (OA) Process. Nanoscale Res. Lett. 2019, 14, 210.
- Wartel, F.; Kosmidis, L.; Gogonel, A.; Baldovino, A.; Stephenson, Z.; Triquet, B.; Quinones, E.; Lo, C.; Mezzetta, E.; Broster, I. Timing Analysis of an Avionics Case Study on Complex Hardware/Software Platforms. In Proceedings of the 2015 Design, Automation & Test in Europe Conference & Exhibition (DATE), Grenoble, France, 9–13 March 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 397–402.
- Shrivastava, S.; Trung, T.Q.; Lee, N.-E. Recent Progress, Challenges, and Prospects of Fully Integrated Mobile and Wearable Point-of-Care Testing Systems for Self-Testing. Chem. Soc. Rev. 2020, 49, 1812–1866.
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Rodrigo, M.A. Renewable Energies Driven Electrochemical Wastewater/Soil Decontamination Technologies: A Critical Review of Fundamental Concepts and Applications. Appl. Catal. B Environ. 2020, 270, 118857.
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and Its Derived Composite Electrodes toward Supercapacitors: Fabrication, Properties, and Challenges. J. Bioresour. Bioprod. 2022, 7, 245–269.
- Parveen, N.; Ansari, M.O.; Ansari, S.A.; Kumar, P. Nanostructured Titanium Nitride and Its Composites as High-Performance Supercapacitor Electrode Material. Nanomaterials 2022, 13, 105.
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin Derived Nitrogen-Doped Porous Carbons with Ultrahigh Specific Surface Area and Tailored Hierarchical Porosity for High Performance Supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151.
- Yu, X.-Y.; Liu, Z.-G.; Huang, X.-J. Nanostructured Metal Oxides/Hydroxides-Based Electrochemical Sensor for Monitoring Environmental Micropollutants. Trends Environ. Anal. Chem. 2014, 3, 28–35.
- Obodo, R.M.; Shinde, N.M.; Chime, U.K.; Ezugwu, S.; Nwanya, A.C.; Ahmad, I.; Maaza, M.; Ejikeme, P.M.; Ezema, F.I. Recent Advances in Metal Oxide/Hydroxide on Three-Dimensional Nickel Foam Substrate for High Performance Pseudocapacitive Electrodes. Curr. Opin. Electrochem. 2020, 21, 242–249.
- Yang, T.; Xu, C.; Liu, C.; Ye, Y.; Sun, Z.; Wang, B.; Luo, Z. Conductive Polymer Hydrogels Crosslinked by Electrostatic Interaction with PEDOT: PSS Dopant for Bioelectronics Application. Chem. Eng. J. 2022, 429, 132430.
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, Synthesis, and Characterization of Graphene–Nanoparticle Hybrid Materials for Bioapplications. Chem. Rev. 2015, 115, 2483–2531.
- Li, B.L.; Setyawati, M.I.; Zou, H.L.; Dong, J.X.; Luo, H.Q.; Li, N.B.; Leong, D.T. Emerging 0D Transition-metal Dichalcogenides for Sensors, Biomedicine, and Clean Energy. Small 2017, 13, 1700527.
- Rajesh, D.; Francis, M.K.; Bhargav, P.B.; Nafis, A.; Balaji, C. 2D Layered Nickel-Cobalt Double Hydroxide Nano 1D Silver Nanowire-Graphitic Carbon Nitrides for High Performance Super Capacitors. J. Alloys Compd. 2022, 898, 162803.
- Qian, C.; Guo, X.; Zhang, W.; Yang, H.; Qian, Y.; Xu, F.; Qian, S.; Lin, S.; Fan, T. Co3O4 Nanoparticles on Porous Bio-Carbon Substrate as Catalyst for Oxygen Reduction Reaction. Microporous Mesoporous Mater. 2019, 277, 45–51.
- Mary, B.; Vijaya, J.J.; Nair, R.R.; Mustafa, A.; Selvamani, P.S.; Saravanakumar, B.; Bououdina, M.; Kennedy, L.J. Reduced Graphene Oxide-Tailored CuFe2O4 Nanoparticles as an Electrode Material for High-Performance Supercapacitors. J. Nanomater. 2022, 2022, 9861440.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
838
Revisions:
2 times
(View History)
Update Date:
20 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




