Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeffery Tyler McGarr | -- | 3266 | 2023-07-17 19:36:30 | | | |
| 2 | Sirius Huang | Meta information modification | 3266 | 2023-07-18 03:02:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mcgarr, J.T.; Mbonimpa, E.G.; Mcavoy, D.C.; Soltanian, M.R. Per- and Polyfluoroalkyl Substances (PFAS) Sorption. Encyclopedia. Available online: https://encyclopedia.pub/entry/46892 (accessed on 07 February 2026).
Mcgarr JT, Mbonimpa EG, Mcavoy DC, Soltanian MR. Per- and Polyfluoroalkyl Substances (PFAS) Sorption. Encyclopedia. Available at: https://encyclopedia.pub/entry/46892. Accessed February 07, 2026.
Mcgarr, Jeffery Tyler, Eric Gentil Mbonimpa, Drew Clifton Mcavoy, Mohamad Reza Soltanian. "Per- and Polyfluoroalkyl Substances (PFAS) Sorption" Encyclopedia, https://encyclopedia.pub/entry/46892 (accessed February 07, 2026).
Mcgarr, J.T., Mbonimpa, E.G., Mcavoy, D.C., & Soltanian, M.R. (2023, July 17). Per- and Polyfluoroalkyl Substances (PFAS) Sorption. In Encyclopedia. https://encyclopedia.pub/entry/46892
Mcgarr, Jeffery Tyler, et al. "Per- and Polyfluoroalkyl Substances (PFAS) Sorption." Encyclopedia. Web. 17 July, 2023.
Copy Citation
Per- and polyfluorinated alkyl substances (PFAS) are an environmentally persistent group of chemicals that can pose an imminent threat to human health through groundwater and surface water contamination. The primary adsorption mechanisms identified in the literature are electrostatic interactions and hydrophobic interactions.
PFAS
aqueous film forming foam
1. Introduction
Poly- and perfluoroalkyl substances (PFAS) are an environmentally persistent group of chemicals consisting of over 5000 chemical species [1][2]. The United States Environmental Protection Agency (USEPA) has selected 29 PFAS compounds to be included in their fifth Unregulated Contaminant Monitoring Rule, which identifies contaminants that should be monitored in drinking water but are not included in the Safe Drinking Water Act. PFAS are especially problematic as they can lead to detrimental health effects, including increased cancer risk, reproductive health issues, and birth defects [3][4][5]. Furthermore, setting PFAS-related health standards is problematic as sufficient toxicity data are unavailable [6].
PFAS have been used in a wide variety of industrial processes and consumer products [7][8][9][10]. Their widespread usage and utility can be attributed to a variety of properties, including but not limited to high thermal stability and amphiphilic properties (i.e., being simultaneously hydrophobic and hydrophilic) [11]. Properties that make PFAS attractive to manufacturers and inherently (perhaps unknowingly) attractive to consumers also make them a threat to human and environmental health [3][12][13]. PFAS-containing products have had the opportunity to spread around the world for nearly a century. As a result, PFAS as an environmental contaminant on regional and global scales also simultaneously grew unchecked and unnoticed until the early 2000s. It was then that studies found long-chain PFAS in wildlife from remote habitats [14] and in human blood serum samples [15]. Today, PFAS contamination is a global issue, with the compounds being detected in water, sediments, animal and plant tissues, and humans on every continent [16][17].
2. PFAS Sorption
2.1. PFAS Adsorption Mechanisms
The primary adsorption mechanisms identified in the literature are electrostatic interactions and hydrophobic interactions. Hydrogen bonding has been discussed in previous reviews; however, this mode of adsorption was found to be insignificant in typical environmental conditions [18][19]. The following subsections examine both electrostatic and hydrophobic interactions. Note that sorption and adsorption are technically different, with sorption being used when the mechanism for sorptive behavior is not specified and adsorption used when the mechanism is adhesion to a surface.
2.1.1. Electrostatic Interaction
PFAS exist in numerous ionic states (i.e., anionic, cationic, or zwitterionic). Thus, electrostatic interactions play a significant role in PFAS sorption [20][21][22]. In typical environmental conditions, many PFAS are found to be anionic (negatively charged), resulting in attraction to positively charged soil and sediment surfaces [23][24] (Figure 1b,c). With many sediment surfaces being negatively charged, cation bridging could also be an important mechanism [25]. Electrostatic interactions can be affected by a variety of factors, including pH, ionic concentrations, presence of metals such as iron and aluminum, organic carbon (OC), and PFAS chain length, all discussed further in Section 2.3.
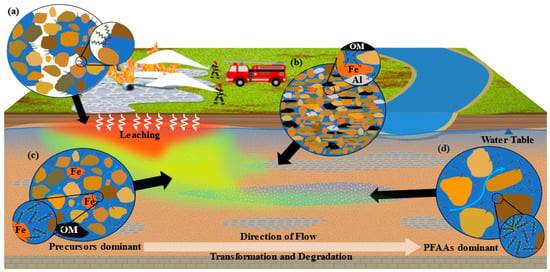
Figure 1. Conceptual model of PFAS fate and transport in a fire training area (FTA). Depicted are the impacts of physical and chemical heterogeneity in the subsurface, air–water interface (AWI), and transformation and degradation. A PFOS molecule is used to represent PFAS throughout the model. Black arrows indicate where popouts (a–d) are representative of. (a) PFAS sorption to AWI due to hydrophobic interactions in the unsaturated zone. (b) PFAS sorption in silts and clays. (c) PFAS sorption in higher-permeability materials, such as sand. (d) PFAS sorption in gravel-dominated sediments. Depicted as well is the formation of a hemi-micelle.
Cationic (positively charged) and zwitterionic (variably charged) PFAS have not received as much attention as their anionic counterparts. Barzen-Hansen et al. found that cationic PFAA precursor compounds, such as 6:2 fluorotelomer sulfonamidoamine (6:2 FtSaAm), and zwitterionic PFAA precursor compounds, such as fluorotelomer sulfonamido betaines (FtSaB), sorb more via electrostatic interactions when compared to anionic fluorotelomer sulfonates (FtS) [20]. Xiao et al. generated similar findings using cationic perfluorooctaneamido ammonium salt (PFOAAmS) and zwitterionic perfluorooctaneamido betaine (PFOAB) when compared to PFOA. Zhi and Liu performed unique batch sorption experiments as they included a variety of pyrogenic carbonaceous materials (i.e., biochar and chimney soot) to simulate potential soil constituents after a fire [22]. They also found that cationic PFOAAmS and zwitterionic PFOAB and 6:2 fluorotelomer sulfonamido betaine (6:2 FTAB) sorbed more readily than their anionic counterparts PFOA and PFOS due to electrostatic interactions. Although not discussed here, these differences may also be influenced by differences in dissociation constants (pKa) of the select PFAS compounds. They concluded that the presence of pyrogenic carbonaceous material enhanced sorption of cationic and zwitterionic compounds, which likely has implications for AFFF discharge sites.
2.1.2. Hydrophobic Interaction
Hydrophobic interactions are commonly identified as a primary contributor of PFAS sorption in subsurface sediments and soils [20][26][27][28][29][30][31]. Hydrophobic interactions are driven by the hydrophobic PFAS C–F tail and its attraction to hydrophobic surfaces in the subsurface environment [11][23]. This process is seemingly counterintuitive as many of the attractions would seem to conflict with anticipated electrostatic repulsion. For example, anionic PFAS can sorb to negatively charged OC surfaces exhibiting electrostatic repulsion. However, the hydrophobicity of both the C–F tail and the hydrophobic portions of the OC surface will overcome the electrostatic interactions, resulting in hydrophobic-induced sorption (Figure 1b,c) [32][33][34]. Long-chained PFAS will exhibit increased potential for hydrophobic interaction when compared to shorter-chained PFAS [28][35][36][37]. A linear PFAS will exhibit higher hydrophobicity than a branched PFAS of the same chemical formula [38]. In the unsaturated zone, PFAS’s amphiphilic properties result in adsorption of PFAS at air–water interfaces (AWI) (Figure 1a) [39][40][41][42] and NAPL–water interfaces [23][24][43]. Additionally, the hydrophobic C–F tails have been shown to coalesce, resulting in the creation of micelles and hemi-micelles at relatively high concentration (Figure 1d) [44][45][46].
Hydrophobic interaction is not the primary sorption mechanism for cationic and zwitterionic PFAS [20][21]. Barzen-Hansen et al. found that hydrophobic interactions of cationic FtSaAm and zwitterionic FtSaB were negligible due to the electrostatic interaction between positively charged PFAS and negatively charged surfaces [20]. They note that hydrophobic interactions play some role in zwitterionic sorption as C–F chain length in zwitterionic PFAS was found to affect sorption. Similar results were observed in batch experiments using cationic PFOAAmS, the zwitterionic PFOAB, and 6:2 FTAB, where hydrophobic interaction was negligible [21][22].
2.2. PFAS Sorption Isotherms and Kinetics
Sorption isotherms are used to quantify PFAS sorption by determining a sorption distribution (or partition) coefficient, Kd. Sorption isotherms can be calculated with laboratory data; however, field data typically show higher retardation than can be attributed to laboratory-derived Kd values [25]. This is a common issue for solutes undergoing sorption. Studies on other compounds (e.g., volatile organic compounds and trace metals) have attributed this discrepancy to scale effects [47][48][49]. The most frequently used isotherms to describe PFAS sorption are the linear, Freundlich, and Langmuir isotherms. Other isotherms found in the literature include the Virial isotherm [26] and the Donnan model [29]. Commonly used models of PFAS sorption kinetics include the first-order, pseudo-first-order, pseudo-second-order, and intraparticle diffusion models [19][21][26][30].
2.2.1. Linear Isotherm
The linear isotherm has been used to describe PFAS sorption either exclusively or in conjunction with a non-linear model, such as Freundlich or Langmuir [30][31][37][50][51]. It has been shown that the linear sorption isotherm is acceptable when low PFAS concentrations (environmentally relevant) are considered [52][53]. The linear isotherm is written as:
where Cs is the solid phase concentration at equilibrium, Kd is the distribution coefficient, and Cw is the aqueous concentration at equilibrium.
2.2.2. Freundlich Isotherm
The Freundlich isotherm is one of the most commonly used non-linear isotherms [21][42][54][55][56]. The Freundlich isotherm is written as:
where KF is the Freundlich distribution coefficient and n is the Freundlich exponent used to adjust linearity. When examining PFAS sorption in sediment and soil, n is typically near 1, indicating a near-linear sorption isotherm [27][31][56]. The non-linearity of the isotherm indicates that the distribution coefficient is dependent on solute concentration.
2.2.3. Langmuir Isotherm
The Langmuir isotherm is another common choice when calculating a non-linear PFAS sorption isotherm. Langmuir includes a term to quantify the maximum sorption capacity, Sm, which is affected by properties of both porous media and the PFAS compound. Additionally, the Langmuir isotherm could be attractive for examining sorption behavior when competitive PFAS sorption occurs [2]. The Langmuir isotherm is written as:
where KL is the Langmuir distribution coefficient [57][58].
2.2.4. Sorption Kinetics
Because PFAS sorption can be rate-limited, kinetic models are used to better understand transport beyond just equilibrium isotherms. Commonly used models of PFAS sorption kinetics include the first-order, pseudo-first-order, pseudo-second-order, and intraparticle diffusion models [2][19][26]. The first-order model is used to describe sorption when multiple kinds of sorption sites are present (can be modified to include more or fewer sorption sites) and is written as:
where Fw is the fraction of PFAS in aqueous solution at time t (h), F0 is the fraction of PFAS in aqueous solution at equilibrium, F1 and F2 are the fraction of PFAS sorbed to two kinds of sorption sites, k1 and k2 are rate coefficients for those sorption sites, and e is Euler’s number [26].
Pseudo-first-order and pseudo-second-order models are written as follows:
where St is PFAS sorbed at time t (µg/g), Se is PFAS sorbed at equilibrium, and µ1 (1/h) and µ2 (g/µg h) are sorption rate coefficients for pseudo-first-order and pseudo-second-order models, respectively [2].
The intraparticle diffusion model is written as:
where µi (µg/g/h0.5) is the sorption rate coefficient and Bi is a constant representing the boundary layer effect on sediment surfaces (µg/g) [2].
2.3. Factors Impacting PFAS Fate and Transport in Both Saturated and Unsaturated Media
There are numerous factors that affect the fate and transport of PFAS in subsurface environments (Figure 1). Researchers split these into three categories: characteristics of the adsorbent (e.g., soil and sediments), characteristics of the adsorbate (e.g., PFAS), and solution characteristics. Additionally, researchers explore phenomena unique to the unsaturated zone as it has important implications for the AFFF discharge sites. Li et al. and Anderson et al. both performed meta-analysis and found that it is impossible to reliably attribute sorption of PFAS to an individual factor but instead must be attributed to a combination of factors (e.g., OC, clay content, and pH) [25][59]. It should be noted that these characteristics along with the sorption mechanism and time can also affect the ability of PFAS to desorb from sediment, potentially resulting in irreversible sorption in environmental conditions [21][26][30][31][60].
2.3.1. Characteristics of the Adsorbent
Field- and laboratory-scale studies have analyzed properties of sediments to better understand PFAS fate and transport. Key factors include OC content, mineralogy, clay content, and ion exchange capacities. The most frequently examined characteristic is OC content [25][26][51][59]. A higher OC content will typically result in increased sorption. Prior work has shown that OC is the primary driver of PFAS sorption [26][51][61][62]. Wang et al. (2021) showed that 19–42% of PFOS sorption is controlled by OC in sand-dominated media with varying geochemical properties (except in pure sand, where 100% of sorption was attributed to OC). However, Li et al. (2018) found that OC alone is not statistically significant when attempting to correlate with changes in Kd values. A significant statistical relationship was observed when OC was combined with clay content and pH in multiple linear regression analysis [25].
Mineralogy, clay content, and cation and anion exchange capacities are not well-studied. Mineralogy and elemental composition are important to the sorption of anionic PFAS, especially in sediments and soils with limited OC [50][57][63][64]. Lyu et al. performed column experiments with different soils and showed an interesting trend in soils with negligible OC fractions [63]. Differential retention of PFOA was explained by differing elemental compositions, where the soil with higher retention had twice the aluminum and iron contents. Lyu et al. found that aluminum oxides had a greater impact than iron oxides on PFOA sorption [64]. Hellsing et al. similarly found that PFOA, PFOS, PFHxA, and PFNA display an affinity for aluminum oxides due to electrostatic interactions with shorter-chained PFAS shown to sorb at higher concentrations [57]. Additionally, Hellsing et al. showed that electrostatic attraction to aluminum was quite weak as PFAS desorbed with a water rinse [57]. This behavior indicates that a rainfall event could remobilize anionic PFAS sorbed to aluminum. Adamson et al. (2020) and Nickerson et al. (2021) both studied an AFFF discharge site and attributed higher concentrations of zwitterionic and cationic PFAS to silt- and clay-rich areas of high cation exchange capacity [28][38]. Similarly, other studies concluded that anion exchange can augment sorption of anionic PFAS, whereas cation exchange can promote anionic PFAS transport [59][65]. Li et al. determined clay content to be one of the significant characteristics dictating PFAS sorption alongside OC content and pH [25].
2.3.2. Characteristics of the Adsorbate
One of the difficulties in understanding PFAS fate and transport is the simple fact that there are thousands of unique PFAS compounds differing in the C–F chain length, functional group, and electrical charge, with each affecting transport behavior. The C–F chain length drives hydrophobic interactions, with longer chains displaying higher degrees of hydrophobicity. This trend has been shown in both field- and laboratory-scale studies [23][35][37][66]. Higgins and Luthy found that, for each additional CF2 in the C–F chain, the log Kd will increase by 0.5 to 0.6 [27]. Anderson et al. used regression analysis to estimate the Koc of 15 unique PFAS compounds. They found that the log Koc of short-chained PFAS falls between 2.5 and 3, whereas long-chained PFAS have estimated log Koc values of 3.5 to 4.25 [59]. While not directly discussed in the PFAS literature, it is also possible that longer chain lengths can render the sorption process more irreversible. This is because one stage of the sorption process is driven by diffusion (mass transfer from dissolved phase to solid phase), which can decrease for larger chain length or particle/molecule size according to Stokes–Einstein equation [67][68]. The second stage of the sorption process is the physio-chemical binding of the sorbate molecules to the sorbent. This binding can be stronger for longer chain lengths [69]. Additionally, PFAS functional groups have also been shown to impact sorption [27][37][70][71]. Sörengård et al. performed batch sorption experiments with 17 PFAS compounds and 44 unique sorbents and found that sorption of PFAS with identical chain length increased from FTSA, PFCA, PFSA to perflouooctanesulfonamide (FOSA) [37]. Higgins and Luthy reported similar findings, with PFSAs sorbing more than PFCAs [27].
2.3.3. Characteristics of the Solution
The characteristics of the solution (beyond PFAS) have also been shown to impact fate and transport of PFAS in the subsurface environment. Two solution characteristics typically investigated are pH and inorganic ion concentration. It has also been found that sorption to particulate in the solution (e.g., particulate OC) can have a significant effect on PFAS transport; however, this effect is less studied [36]. The influence of pH is reported as an inverse relationship with sorption. A decrease in pH will typically result in an increase in sorption of anionic PFAS and vice versa due to alterations in charge at potential sorption sites in soils with variably charged clays [27][35][49][56][70]. However, the majority of prior studies hold pH constant. Studies have also found that pH will control how PFAS compounds interact with sediment and soil (i.e., hydrophobic vs. electrostatic). At lower pH, electrostatic interactions are expected to dominate. At environmentally relevant pH conditions, hydrophobic interactions are favorable [27][60]. Additionally, Campos-Pereira et al. (2018) found that changes in pH affected longer-chained PFAS more than short-chain PFAS. Functional groups also seem to have an effect on how PFAS react to changes in pH, with PFOA found to be less impacted by alterations to pH than PFOS [72]. Although not discussed herein, these differences may also be influenced by differences in dissociation constants (pKa) of the select PFAS compounds. Two meta-analyses identified pH as one of three important controls on PFAS sorption, which also included OC and clay content of soils and sediments [25][59].
Ionic strength of solution has also been identified as a solution characteristic that affects PFAS sorption. The cations Ca2+ (calcium), Na+ (sodium), Mg2+ (magnesium), and K+ (potassium) are the most commonly investigated. Typically, an increase in ionic strength correlates to an increase in sorption [27][40][56][73]. Higgins and Luthy (2006) investigated the impact of altering Ca2+ and Na+ ionic strength on sorption of a variety of anionic PFAS. They found that log Kd increased by 0.36 ± 0.04 with each log unit increase of Ca2+, but there were no significant changes correlated to changes in Na+. Chen et al. (2012) performed similar experiments to investigate the impact of altering the concentration of Ca2+, Na+, Mg2+, and K+ on the sorption of PFOS in a saltwater environment. For PFOS at a concentration of 10 µg/L, they found the log Kd increased 0.48 ± 0.03 per log unit of salinity. For the monovalent cations, they found that an increase in K+ resulted in no changes, whereas an increase in Na+ doubled the sorbed PFOS. For divalent cations, it was found that, per log unit increase of Ca2+ and Mg2+, the log Kd increased by 0.50 and 0.52, respectively. Meta-analysis by Li et al. (2018) found no significant relationships between the change in Ca2+ and Na+ and the sorption of PFOS and PFOA. However, they did find that Ca2+ displayed significant relations with EtFOSAA and PFDS. It has been noted that it is difficult to isolate the impact of changes in ionic strength as these changes are likely to alter pH [25][27].
2.3.4. Transport in Unsaturated Zone
In the unsaturated zone, surfactant behavior of PFAS (e.g., hydrophobicity of the C–F tail) results in adsorption (or accumulation) to AWIs [39][40][41][42] and fluid–fluid interfaces (FFI), such as NAPL–water [23][24][43]. Due to surficial discharge (e.g., AFFF discharge), PFAS behavior in the unsaturated zone is of critical importance. As such, investigation of interfacial sorption has increased in recent years [74][75]. Both AWI and FFI enhance retardation, with air–water interface adsorption (AWIA) found to be more significant. AWIA can account for over 70% of PFAS mass [42][76]. Both forms of interfacial sorption have been fitted with a variety of sorption isotherms, including the linear [77], Freundlich [58], and Langmuir isotherms [43][76]. Prior studies found that the linear isotherm is sufficient to describe low environmentally relevant PFAS concentrations [77]. Others have concluded that the Langmuir isotherm can only be reliably used at higher concentrations as it significantly underestimates interfacial sorption at low concentrations [58]. This underestimation is because the surface activity of PFAS increases with lower concentrations, resulting in elevated interfacial sorption [58].
AWIA is influenced by characteristics of the solution and adsorbate. Silva et al. (2019) found that AWIA of PFCAs is influenced by chain length, with longer chains (higher hydrophobicity) resulting in increased AWIA. Additionally, pH and ionic strength of solution have also been found to affect AWIA, with increases in both resulting in enhanced sorption [40][41][43]. Lyu and Brusseau (2020) found that ionic strength was more important than pH [40]. Li et al. (2019) found that increases in both Ca2+ and Na+ result in enhanced AWIA, with Ca2+ having a larger impact [26]. Saturation is also a critical factor determining the amount of PFAS sorbed. Guo et al. (2020) modeled the fate and transport of PFOS in the unsaturated zone [42]. They found that sediments of higher permeability (e.g., sand) had higher retardation factors due to enhanced AWIA when compared to materials of lower permeability (e.g., clay). They attributed this to increased AWI area at lower saturations (fine-grained media retains more water compared to larger-grained media). Similar findings of both the influence of saturation and grain size on AWIA have been generated in several recent studies [41][78]. Some studies have proposed estimating AWI area by using soil water content and the van Genuchten model equations [78][79][80][81]. Additionally, AWIA can account for PFAS residence times on decadal time scales and has been observed in field studies, which has implications for AFFF discharge sites [28][38][62].
References
- Bolan, N.; Sarkar, B.; Yan, Y.; Li, Q.; Wijesekara, H.; Kannan, K.; Tsang, D.C.W.; Schauerte, M.; Bosch, J.; Noll, H.; et al. Remediation of poly- and perfluoroalkyl substances (PFAS) contaminated soils–To mobilize or to immobilize or to degrade? J. Hazard. Mater. 2021, 401, 123892.
- Sima, M.W.; Jaffé, P.R. A critical review of modeling Poly- and Perfluoroalkyl Substances (PFAS) in the soil-water environment. Sci. Total Environ. 2021, 757, 143793.
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648.
- Fenton, S.E.; Ducatman, A.; Boobis, A.; Dewitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per-and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40, 606–630.
- Savitz, D.A.; Stein, C.R.; Elston, B.; Wellenius, G.A.; Bartell, S.M.; Shin, H.M.; Vieira, V.M.; Fletcher, T. Relationship of perfluorooctanoic acid exposure to pregnancy outcome based on birth records in the mid-Ohio valley. Environ. Health Perspect. 2012, 120, 1201–1207.
- Simon, J.A.; Abrams, S.; Bradburne, T.; Bryant, D.; Burns, M.; Newell, C.J.; Parker, B.L.; Singh, T.; Tomiczek, P.; Wice, R. PFAS Experts Symposium: Statements on regulatory policy, chemistry and analytics, toxicology, transport/fate, and remediation for per-and polyfluoroalkyl substances (PFAS) contamination issues. Remediation 2019, 29, 31–48.
- Ahrens, L.; Bundschuh, M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929.
- Phong Vo, H.N.; Ngo, H.H.; Guo, W.; Hong Nguyen, T.M.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Hang Nguyen, T.A. Poly-and perfluoroalkyl substances in water and wastewater: A comprehensive review from sources to remediation. J. Water Process Eng. 2020, 36, 101393.
- Sepulvado, J.G.; Blaine, A.C.; Hundal, L.S.; Higgins, C.P. Occurrence and Fate of Perfluorochemicals in Soil Following the Land Application of Municipal Biosolids. Environ. Sci. Technol. 2011, 45, 8106–8112.
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732.
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.; Kannan, K.; Mabury, S.A.; Pj Van Leeuwenkk, S. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541.
- Ghisi, R.; Vamerali, T.; Manzetti, S. Accumulation of perfluorinated alkyl substances (PFAS) in agricultural plants: A review. Environ. Res. 2019, 169, 326–341.
- Jiao, X.; Shi, Q.; Gan, J. Uptake, accumulation, and metabolism of PFASs in plants and health perspectives: A critical review. Crit. Rev. Environ. Sci. Technol. 2020, 51, 2745–2776.
- Giesy, J.P.; Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342.
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.S.; Loganathan, B.G.; Mohd, M.A.; Olivero, J.; Van Wouwe, N.; Yang, J.H.; et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489–4495.
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Weifeng, J.; Chen, B.; Zhang, T.; Qiu, W. PFAS and their substitutes in groundwater: Occurrence, transformation, and remediation. J. Hazard. Mater. 2021, 412, 125159.
- Kurwadkar, S.; Dane, J.; Kanel, S.R.; Nadagouda, M.N.; Cawdrey, R.W.; Ambade, B.; Struckhoff, G.C.; Wilkin, R. Per-and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 2022, 809, 151003.
- Du, Z.; Deng, S.; Bei, Y.; Huang, Q.; Wang, B.; Huang, J.; Yu, G. Adsorption behavior and mechanism of perfluorinated compounds on various adsorbents-A review. J. Hazard. Mater. 2014, 274, 443–454.
- Zhang, D.Q.; Zhang, W.L.; Liang, Y.N. Adsorption of perfluoroalkyl and polyfluoroalkyl substances (PFASs) from aqueous solution–A review. Sci. Total Environ. 2019, 694, 133606.
- Barzen-Hanson, K.A.; Davis, S.E.; Kleber, M.; Field, J.A. Sorption of Fluorotelomer Sulfonates, Fluorotelomer Sulfonamido Betaines, and a Fluorotelomer Sulfonamido Amine in National Foam Aqueous Film-Forming Foam to Soil. Environ. Sci. Technol. 2017, 51, 12394–12404.
- Xiao, F.; Jin, B.; Golovko, S.A.; Golovko, M.Y.; Xing, B. Sorption and Desorption Mechanisms of Cationic and Zwitterionic Per- and Polyfluoroalkyl Substances in Natural Soils: Thermodynamics and Hysteresis. Environ. Sci. Technol. 2019, 53, 11818–11827.
- Zhi, Y.; Liu, J. Sorption and desorption of anionic, cationic and zwitterionic polyfluoroalkyl substances by soil organic matter and pyrogenic carbonaceous materials. Chem. Eng. J. 2018, 346, 682–691.
- Guelfo, J.L.; Higgins, C.P. Subsurface transport potential of perfluoroalkyl acids at aqueous film-forming foam (AFFF)-impacted sites. Environ. Sci. Technol. 2013, 47, 4164–4171.
- McKenzie, E.R.; Siegrist, R.L.; McCray, J.E.; Higgins, C.P. The influence of a non-aqueous phase liquid (NAPL) and chemical oxidant application on perfluoroalkyl acid (PFAA) fate and transport. Water Res. 2016, 92, 199–207.
- Li, Y.; Oliver, D.P.; Kookana, R.S. A critical analysis of published data to discern the role of soil and sediment properties in determining sorption of per and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 2018, 628–629, 110–120.
- Li, F.; Fang, X.; Zhou, Z.; Liao, X.; Zou, J.; Yuan, B.; Sun, W. Adsorption of perfluorinated acids onto soils: Kinetics, isotherms, and influences of soil properties. Sci. Total Environ. 2019, 649, 504–514.
- Higgins, C.P.; Luthy, R.G. Sorption of perfluorinated surfactants on sediments. Environ. Sci. Technol. 2006, 40, 7251–7256.
- Adamson, D.T.; Nickerson, A.; Kulkarni, P.R.; Higgins, C.P.; Popovic, J.; Field, J.; Rodowa, A.; Newell, C.; Deblanc, P.; Kornuc, J.J. Mass-Based, Field-Scale Demonstration of PFAS Retention within AFFF-Associated Source Areas. Environ. Sci. Technol 2020, 54, 15768–15777.
- Higgins, C.P.; Luthy, R.G. Modeling sorption of anionic surfactants onto sediment materials: An a priori approach for perfluoroalkyl surfactants and linear alkylbenzene sulfonates. Environ. Sci. Technol. 2007, 41, 3254–3261.
- Miao, Y.; Guo, X.; Dan Peng Fan, T.; Yang, C. Rates and equilibria of perfluorooctanoate (PFOA) sorption on soils from different regions of China. Ecotoxicol. Environ. Saf. 2017, 139, 102–108.
- Milinovic, J.; Lacorte, S.; Vidal, M.; Rigol, A. Sorption behaviour of perfluoroalkyl substances in soils. Sci. Total Environ. 2015, 511, 63–71.
- Chen, H.; Zhang, C.; Yu, Y.; Han, J. Sorption of perfluorooctane sulfonate (PFOS) on marine sediments. Mar. Pollut. Bull. 2012, 64, 902–906.
- Wang, F.; Shih, K. Adsorption of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) on alumina: Influence of solution pH and cations. Water Res. 2011, 45, 2925–2930.
- Zhao, L.; Bian, J.; Zhang, Y.; Zhu, L.; Liu, Z. Comparison of the sorption behaviors and mechanisms of perfluorosulfonates and perfluorocarboxylic acids on three kinds of clay minerals. Chemosphere 2014, 114, 51–58.
- Campos Pereira, H.; Ullberg, M.; Kleja, D.B.; Gustafsson, J.P.; Ahrens, L. Sorption of perfluoroalkyl substances (PFASs) to an organic soil horizon–Effect of cation composition and pH. Chemosphere 2018, 207, 183–191.
- Chen, H.; Reinhard, M.; Yin, T.; Nguyen, T.V.; Tran, N.H.; Yew-Hoong Gin, K. Multi-compartment distribution of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in an urban catchment system. Water Res. 2019, 154, 227–237.
- Sörengård, M.; Östblom, E.; Köhler, S.; Ahrens, L. Adsorption behavior of per- And polyfluoralkyl substances (PFASs) to 44 inorganic and organic sorbents and use of dyes as proxies for PFAS sorption. J. Environ. Chem. Eng. 2020, 8, 103744.
- Nickerson, A.; Rodowa, A.E.; Adamson, D.T.; Field, J.A.; Kulkarni, P.R.; Kornuc, J.J.; Higgins, C.P. Spatial Trends of Anionic, Zwitterionic, and Cationic PFASs at an AFFF-Impacted Site. Environ. Sci. Technol 2021, 55, 313–323.
- Lyu, Y.; Brusseau, M.L.; Chen, W.; Yan, N.; Fu, X.; Lin, X. Adsorption of PFOA at the Air-Water Interface during Transport in Unsaturated Porous Media. Environ. Sci. Technol. 2018, 52, 7745–7753.
- Lyu, Y.; Brusseau, M.L. The influence of solution chemistry on air-water interfacial adsorption and transport of PFOA in unsaturated porous media. Sci. Total Environ. 2020, 713, 136744.
- Brusseau, M.L. Estimating the relative magnitudes of adsorption to solid-water and air/oil-water interfaces for per- and poly-fluoroalkyl substances. Environ. Pollut. 2019, 254, 113102.
- Guo, B.; Zeng, J.; Brusseau, M.L. A Mathematical Model for the Release, Transport, and Retention of Per- and Polyfluoroalkyl Substances (PFAS) in the Vadose Zone. Water Resour. Res. 2020, 56, e2019WR026667.
- Silva JA, K.; Martin, W.A.; Johnson, J.L.; McCray, J.E. Evaluating air-water and NAPL-water interfacial adsorption and retention of Perfluorocarboxylic acids within the Vadose zone. J. Contam. Hydrol. 2019, 223, 103472.
- Bhhatarai, B.; Gramatica, P. Prediction of aqueous solubility, vapor pressure and critical micelle concentration for aquatic partitioning of perfluorinated chemicals. Environ. Sci. Technol. 2011, 45, 8120–8128.
- Brusseau, M.L. The influence of molecular structure on the adsorption of PFAS to fluid-fluid interfaces: Using QSPR to predict interfacial adsorption coefficients. Water Res. 2019, 152, 148–158.
- Brusseau, M.L.; van Glubt, S. The influence of surfactant and solution composition on PFAS adsorption at fluid-fluid interfaces. Water Res. 2019, 161, 17–26.
- Dai, Z.; Wolfsberg, A.; Reimus, P.; Deng, H.; Kwicklis, E.; Ding, M.; Ware, D.; Ye, M. Identification of sorption processes and parameters for radionuclide transport in fractured rock. J. Hydrol. 2012, 414, 220–230.
- Deng, H.; Dai, Z.; Wolfsberg, A.; Lu, Z.; Ye, M.; Reimus, P. Upscaling of reactive mass transport in fractured rocks with multimodal reactive mineral facies. Water Resour. Res. 2010, 46.
- Soltanian, M.R.; Sun, A.; Dai, Z. Reactive transport in the complex heterogeneous alluvial aquifer of Fortymile Wash, Nevada. Chemosphere 2017, 179, 379–386.
- Johnson, R.L.; Anschutz, A.J.; Smolen, J.M.; Simcik, M.F.; Penn, R.L. The adsorption of perfluorooctane sulfonate onto sand, clay, and iron oxide surfaces. J. Chem. Eng. Data 2007, 52, 1165–1170.
- Ahrens, L.; Yeung, L.W.Y.; Taniyasu, S.; Lam, P.K.S.; Yamashita, N. Partitioning of perfluorooctanoate (PFOA), perfluorooctane sulfonate (PFOS) and perfluorooctane sulfonamide (PFOSA) between water and sediment. Chemosphere 2011, 85, 731–737.
- You, C.; Jia, C.; Pan, G. Effect of salinity and sediment characteristics on the sorption and desorption of perfluorooctane sulfonate at sediment-water interface. Environ. Pollut. 2010, 158, 1343–1347.
- Silva, J.A.K.; Martin, W.A.; McCray, J.E. Air-water interfacial adsorption coefficients for PFAS when present as a multi-component mixture. J. Contam. Hydrol. 2021, 236, 103731.
- Wang, Y.; Khan, N.; Huang, D.; Carroll, K.C.; Brusseau, M.L. Transport of PFOS in aquifer sediment: Transport behavior and a distributed-sorption model. Sci. Total Environ. 2021, 779, 146444.
- van Glubt, S.; Brusseau, M.L.; Yan, N.; Huang, D.; Khan, N.; Carroll, K.C. Column versus batch methods for measuring PFOS and PFOA sorption to geomedia. Environ. Pollut. 2021, 268, 115917.
- Zhi, Y.; Liu, J. Column chromatography approach to determine mobility of fluorotelomer sulfonates and polyfluoroalkyl betaines. Sci. Total Environ. 2019, 683, 480–488.
- Hellsing, M.S.; Josefsson, S.; Hughes, A.V.; Ahrens, L. Sorption of perfluoroalkyl substances to two types of minerals. Chemosphere 2016, 159, 385–391.
- Schaefer, C.E.; Culina, V.; Nguyen, D.; Field, J. Uptake of Poly-and Perfluoroalkyl Substances at the Air−Water Interface. Environ. Sci. Technol 2019, 53, 46.
- Anderson, R.; Adamson, D.T.; Stroo, H.F. Partitioning of poly- and perfluoroalkyl substances from soil to groundwater within aqueous film-forming foam source zones. J. Contam. Hydrol. 2019, 220, 59–65.
- Zhao, L.; Zhang, Y.; Fang, S.; Zhu, L.; Liu, Z. Comparative sorption and desorption behaviors of PFHxS and PFOS on sequentially extracted humic substances. J. Environ. Sci. 2014, 26, 2517–2525.
- Brusseau, M.L.; Khan, N.; Wang, Y.; Yan, N.; van Glubt, S.; Carroll, K.C. Nonideal Transport and Extended Elution Tailing of PFOS in Soil. Environ. Sci. Technol. 2019, 53, 10654–10664.
- McGuire, M.E.; Schaefer, C.; Richards, T.; Backe, W.J.; Field, J.A.; Houtz, E.; Sedlak, D.L.; Guelfo, J.L.; Wunsch, A.; Higgins, C.P. Evidence of Remediation-Induced Alteration of Subsurface Poly-and Perfluoroalkyl Substance Distribution at a Former Firefighter Training Area. Environ. Sci. Technol. 2014, 48, 6644–6652.
- Lyu, X.; Liu, X.; Sun, Y.; Ji, R.; Gao, B.; Wu, J. Transport and retention of perfluorooctanoic acid (PFOA) in natural soils: Importance of soil organic matter and mineral contents, and solution ionic strength. J. Contam. Hydrol. 2019, 225, 103477.
- Lyu, X.; Liu, X.; Wu, X.; Sun, Y.; Gao, B.; Wu, J. Importance of Al/Fe oxyhydroxide coating and ionic strength in perfluorooctanoic acid (PFOA) transport in saturated porous media. Water Res. 2020, 175, 115685.
- Wang, F.; Shih, K.; Leckie, J.O. Effect of humic acid on the sorption of perfluorooctane sulfonate (PFOS) and perfluorobutane sulfonate (PFBS) on boehmite. Chemosphere 2015, 118, 213–218.
- Anderson, R.H.; Long, G.C.; Porter, R.C.; Anderson, J.K. Occurrence of select perfluoroalkyl substances at U.S. Air Force aqueous film-forming foam release sites other than fire-training areas: Field-validation of critical fate and transport properties. Chemosphere 2016, 150, 678–685.
- Alperin, M.J.; Albert, D.B.; Martens, C.S. Seasonal variations in production and consumption rates of dissolved organic carbon in an organic-rich coastal sediment. Geochim. Et Cosmochim. Acta 1994, 58, 4909–4930.
- Babakhani, P.; Bridge, J.; Doong, R.A.; Phenrat, T. Continuum-based models and concepts for the transport of nanoparticles in saturated porous media: A state-of-the-science review. Adv. Colloid Interface Sci. 2017, 246, 75–104.
- Curti, L.; Moore, O.W.; Babakhani, P.; Xiao, K.-Q.; Woulds, C.; Bray, A.W.; Fisher, B.J.; Kazemian, M.; Kaulich, B.; Peacock, C.L. Carboxyl-richness controls organic carbon preservation during coprecipitation with iron (oxyhydr) oxides in the natural environment. Commun. Earth Environ. 2021, 2, 229.
- Ahrens, L.; Yamashita, N.; Yeung, L.W.Y.; Taniyasu, S.; Horii, Y.; Lam, P.K.S.; Ebinghaus, R. Partitioning behavior of per- and polyfluoroalkyl compounds between pore water and sediment in two sediment cores from Tokyo Bay, Japan. Environ. Sci. Technol. 2009, 43, 6969–6975.
- Le, S.T.; Kibbey TC, G.; Weber, K.P.; Glamore, W.C.; O’Carroll, D.M. A group-contribution model for predicting the physicochemical behavior of PFAS components for understanding environmental fate. Sci. Total Environ. 2021, 764, 142882.
- Xing, Y.; Li, Q.; Chen, X.; Fu, X.; Ji, L.; Wang, J.; Li, T.; Zhang, Q. Different transport behaviors and mechanisms of perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) in saturated porous media. J. Hazard. Mater. 2021, 402, 123435.
- Chen, Y.C.; Lo, S.L.; Li, N.H.; Lee, Y.C.; Kuo, J. Sorption of perfluoroalkyl substances (PFASs) onto wetland soils. Desalination Water Treat. 2013, 51, 7469–7475.
- Brusseau, M.L. Simulating PFAS transport influenced by rate-limited multi-process retention. Water Res. 2020, 168, 115179.
- Brusseau, M.L.; Lyu, Y.; Yan, N.; Guo, B. Low-concentration tracer tests to measure air-water interfacial area in porous media. Chemosphere 2020, 250, 126305.
- Costanza, J.; Arshadi, M.; Abriola, L.M.; Pennell, K.D. Accumulation of PFOA and PFOS at the Air-Water Interface. Environ. Sci. Technol. Lett. 2019, 6, 487–491.
- Brusseau, M.L.; Guo, B.; Huang, D.; Yan, N.; Lyu, Y. Ideal versus Nonideal Transport of PFAS in Unsaturated Porous Media. Water Res. 2021, 202, 117405.
- Zeng, J.; Guo, B. Multidimensional simulation of PFAS transport and leaching in the vadose zone: Impact of surfactant-induced flow and subsurface heterogeneities. Adv. Water Resour. 2021, 155, 104015.
- Leverett, M. Capillary behavior in porous solids. Trans. AIME 1941, 142, 152–169.
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898.
- Silva, J.A.; Šimůnek, J.; McCray, J.E. Comparison of methods to estimate air-water interfacial areas for evaluating PFAS transport in the vadose zone. J. Contam. Hydrol. 2022, 247, 103984.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
816
Revisions:
2 times
(View History)
Update Date:
18 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




