
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | -- | 4420 | 2023-07-17 18:19:29 |
Video Upload Options
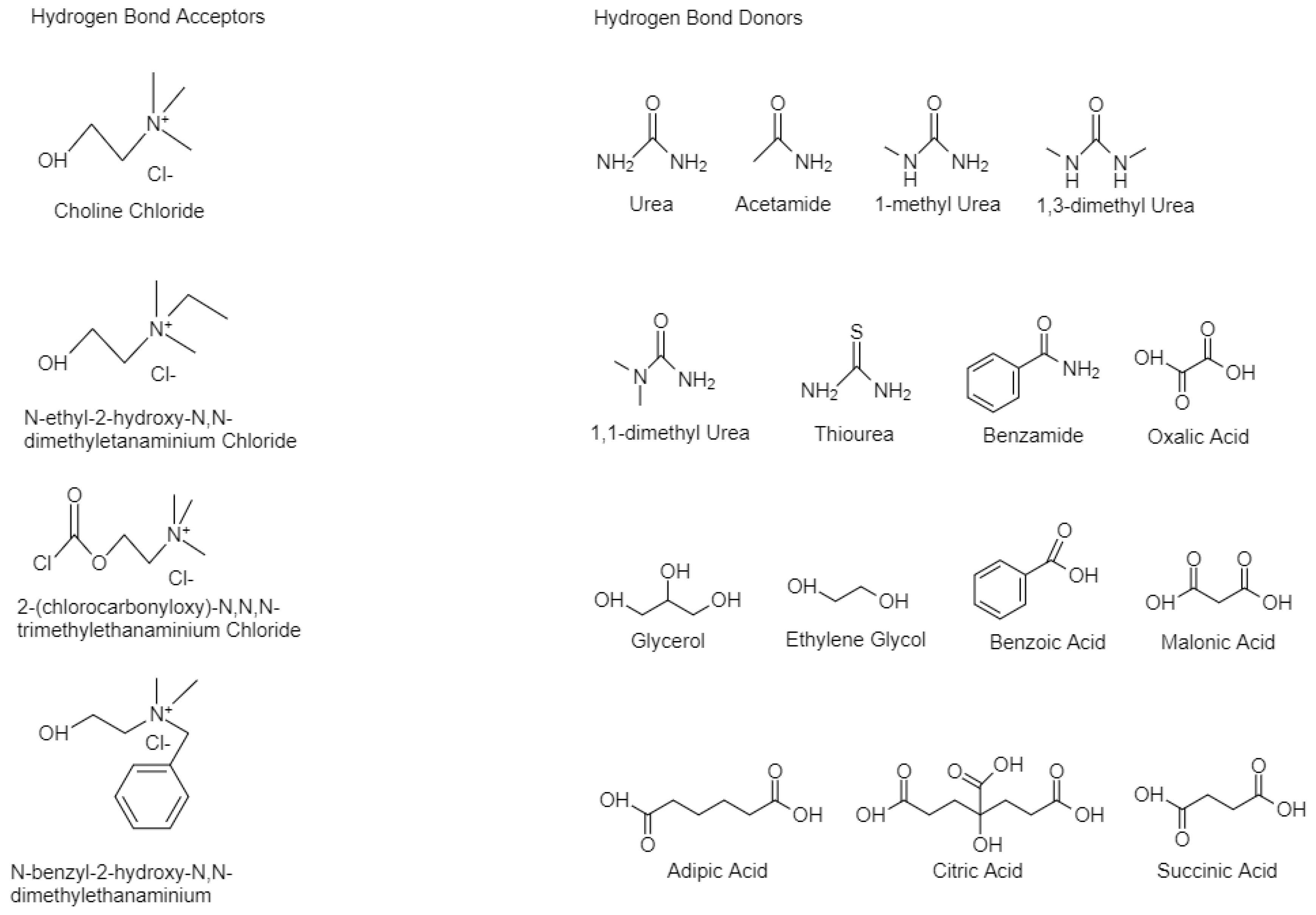
The development and application of sustainable solvents has been a hot topic in different scientific and technological areas. Deep eutectic solvents (DESs), were introduced in 2001 as an alternative to ionic liquids (ILs). These showed a stronger ecofriendly profile, with easier and cheaper production, while having similar properties. DESs contain large, asymmetrical ions that have low lattice energy and, thus, low melting points. They are often acquired by the complexation of a quaternary ammonium salt with a metal salt or hydrogen bond donor (HBD).
1. Introduction
2. Synthesis and Properties of Deep Eutectic Solvents
2.1. Synthesis of Deep Eutectic Solvents
|
Type |
Formula |
Terms |
|---|---|---|
|
I |
Cat+X− + zMClx |
M = Zn, Sn, Al, Ga, Fe, In |
|
II |
Cat+X− + zMClx · yH2O |
M = Co, Cu, Ni, Fe, Cr |
|
III |
Cat+X− + zRZ |
Z = OH, COOH, CONH2 |
|
IV |
MClx + RZ = MClx−1+ · RZ + MClx+1 − |
M = Zn, Al and Z = OH, CONH2 |
Cat+ = any phosphonium, ammonium or sulfonium cation, X− = a Lewis base, generally a halide anion, MClx = metal chloride, RZ = organic compound.
2.2. Properties of Deep Eutectic Solvents
3. Microextraction Techniques Using DES
3.1. Solid Phase Microextraction
3.1.1. Solid Phase Extraction
3.1.2. Solid Phase Microextraction
3.1.3. Stir Bar Sorptive Extraction
3.2. Liquid Phase Microextraction
References
- Moldoveanu, S.; David, V. The Role of Sample Preparation. Modern Sample Preparation for Chromatography; Elsevier: Amsterdam, The Netherlands, 2015; pp. 33–49.
- Wen, Y. Recent advances in solid-phase extraction techniques with nanomaterials. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–73.
- Moldoveanu, C.S.; David, V. Preparatory Information. J. Chromatogr. Libr. 2020, 65, 3–111.
- Armenta, S.; Garrigues, S.; Esteve-Turrillas, F.A.; De La Guardia, M. Green extraction techniques in green analytical chemistry. TrAC Trends Anal. Chem. 2019, 116, 248–253.
- Armenta, S.; Garrigues, S.; De La Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal. Chem. 2015, 71, 2–8.
- Armenta, S.; Garrigues, S.; De La Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511.
- Lord, H.; Pawliszyn, J. Microextraction of drugs. J. Chromatogr. A 2000, 902, 17–63.
- Sanderson, K. Chemistry: It’s not easy being green. Nature 2011, 469, 18–20.
- Ávalos, M.; Babiano, R.; Cintas, P.; Jiménez, J.L.; Palacios, J.C. Greener Media in Chemical Synthesis and Processing. Angew. Chem. Int. Ed. 2006, 45, 3904–3908.
- Clark, J.H. Chemistry goes green. Nat. Chem. 2009, 1, 12.
- Ranke, J.; Stolte, S.; Störmann, R.; Aming, A.J.; Jastorff, B. Design of Sustainable Chemical Products the Example of Ionic Liquids. Chem. Rev. 2007, 107, 2183–2206.
- Kunz, W.; Maurer, E.; Klein, R.; Touraud, D.; Rengstl, D.; Harrar, A.; Dengler, S.; Zech, O. Low Toxic Ionic Liquids, Liquid Catanionics, and Ionic Liquid Microemulsions. J. Dispers. Sci. Technol. 2011, 32, 1694–1699.
- Rogers, R.D.; Seddon, K.R. CHEMISTRY: Ionic Liquids—Solvents of the Future? Science 2003, 302, 792–793.
- Zhao, H.; Xia, S.; Ma, P. Use of ionic liquids as ‘green’ solvents for extractions. J. Chem. Technol. Biotechnol. 2005, 80, 1089–1096.
- Li, Z.; Pei, Y.; Wang, H.; Fan, J.; Wang, J. Ionic liquid-based aqueous two-phase systems and their applications in green separation processes. TrAC Trends Anal. Chem. 2010, 29, 1336–1346.
- Pena-Pereira, F.; Namieśnik, J. Ionic Liquids and Deep Eutectic Mixtures: Sustainable Solvents for Extraction Processes. ChemSusChem 2014, 7, 1784–1800.
- Romero, A.; Santos, A.; Tojo, J.; Rodríguez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273.
- Kissoudi, M.; Samanidou, V. Recent Advances in Applications of Ionic Liquids in Miniaturized Microextraction Techniques. Molecules 2018, 23, 1437.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem. Commun. 2001, 1, 2010–2011.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Shishov, A.; Pochivalov, A.; Nugbienyo, L.; Andruch, V.; Bulatov, A. Deep eutectic solvents are not only effective extractants. TrAC Trends Anal. Chem. 2020, 129, 115956.
- Kabeer, M.; Hakami, Y.; Asif, M.; Alrefaei, T.; Sajid, M. Modern solutions in magnetic analytical extractions of metals: A review. TrAC Trends Anal. Chem. 2020, 130, 115987.
- Xu, K.; Wang, Y.; Huang, Y.; Li, N.; Wen, Q. A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal. Chim. Acta 2014, 864, 9–20.
- Yao, X.-H.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based-deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123.
- Wang, M.; Wang, J.; Zhang, Y.; Xia, Q.; Bi, W.; Yang, X.; Chen, D.D.Y. Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J. Chromatogr. A 2016, 1443, 262–266.
- Werner, J.; Grześkowiak, T.; Zgoła-Grześkowiak, A.; Stanisz, E. Recent trends in microextraction techniques used in determination of arsenic species. TrAC Trends Anal. Chem. 2018, 105, 121–136.
- Moghadam, A.G.; Rajabi, M.; Asghari, A. Efficient and relatively safe emulsification microextraction using a deep eutectic solvent for influential enrichment of trace main anti-depressant drugs from complicated samples. J. Chromatogr. B 2018, 1072, 50–59.
- Kanberoglu, G.S.; Yilmaz, E.; Soylak, M. Application of deep eutectic solvent in ultrasound-assisted emulsification microextraction of quercetin from some fruits and vegetables. J. Mol. Liq. 2019, 279, 571–577.
- Li, L.; Liu, Y.; Wang, Z.; Yang, L.; Liu, H. Development and applications of deep eutectic solvent derived functional materials in chromatographic separation. J. Sep. Sci. 2020.
- Doğan, B.; Elik, A.; Altunay, N. Determination of paracetamol in synthetic urea and pharmaceutical samples by shaker-assisted deep eutectic solvent microextraction and spectrophotometry. Microchem. J. 2020, 154, 104645.
- Othman, Z.A.A.L.; Habila, M.A.; Yilmaz, E.; Alabdullkarem, E.A.; Soylak, M. A novel deep eutectic solvent microextraction procedure for enrichment, separation and atomic absorption spectrometric determination of palladium at ultra-trace levels in environmental samples. Measurement 2020, 153, 107394.
- Abbott, A.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- Sereshti, H.; Jamshidi, F.; Nouri, N.; Nodeh, H.R. Hyphenated dispersive solid- and liquid-phase microextraction technique based on a hydrophobic deep eutectic solvent: Application for trace analysis of pesticides in fruit juices. J. Sci. Food Agric. 2020, 100, 2534–2543.
- Abbott, A.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71.
- Chen, J.; Liu, M.; Wang, Q.; Du, H.; Zhang, L. Deep Eutectic Solvent-Based Microwave-Assisted Method for Extraction of Hydrophilic and Hydrophobic Components from Radix Salviae miltiorrhizae. Molecules 2016, 21, 1383.
- Doi, H.; Song, X.; Minofar, B.; Kanzaki, R.; Takamuku, T.; Umebayashi, Y. A New Proton Conductive Liquid with No Ions: Pseudo-Protic Ionic Liquids. Chem. A Eur. J. 2013, 19, 11522–11526.
- Aryafard, M.; Karimi, A.; Harifi-Mood, A.R.; Minofar, B. Molecular Dynamics Simulations, Solvatochromic Parameters, and Preferential Solvation in Aqueous Solutions of Ethaline, Ethylene Glycol, and Choline Chloride. J. Chem. Eng. Data 2020, 65, 4556–4566.
- Aryafard, M.; Abbasi, M.; Řeha, D.; Harifi-Mood, A.R.; Minofar, B. Experimental and theoretical investigation of solvatochromic properties and ion solvation structure in DESs of reline, glyceline, ethaline and their mixtures with PEG 400. J. Mol. Liq. 2019, 284, 59–67.
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425.
- Liska, I. Fifty years of solid-phase extraction in water analysis—Historical development and overview. J. Chromatogr. A 2000, 885, 3–16.
- Makoś, P.; Słupek, E.; Gębicki, J. Hydrophobic deep eutectic solvents in microextraction techniques—A review. Microchem. J. 2020, 152, 104384.
- Keçili, R.; Büyüktiryaki, S.; Dolak, I.; Hussain, C.M. The use of magnetic nanoparticles in sample preparation devices and tools. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–95.
- Hou, X.; Tang, S.; Wang, J. Recent advances and applications of graphene-based extraction materials in food safety. TrAC Trends Anal. Chem. 2019, 119, 115603.
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends Anal. Chem. 2014, 59, 50–58.
- Mirzaee, M.T.; Seidi, S.; Alizadeh, R. Pipette-tip SPE based on Graphene/ZnCr LDH for Pb(II) analysis in hair samples followed by GFAAS. Anal. Biochem. 2020, 612, 113949.
- Khezeli, T.; Daneshfar, A. Dispersive micro-solid-phase extraction of dopamine, epinephrine and norepinephrine from biological samples based on green deep eutectic solvents and Fe3O4@MIL-100 (Fe) core–shell nanoparticles grafted with pyrocatechol. RSC Adv. 2015, 5, 65264–65273.
- Jeong, K.M.; Lee, M.S.; Nam, M.W.; Zhao, J.; Jin, Y.; Lee, D.-K.; Kwon, S.W.; Jeong, J.H.; Lee, J. Tailoring and recycling of deep eutectic solvents as sustainable and efficient extraction media. J. Chromatogr. A 2015, 1424, 10–17.
- Fu, N.; Lv, R.; Guo, Z.; Guo, Y.; You, X.; Tang, B.; Han, D.; Yan, H.; Row, K.H. Environmentally friendly and non-polluting solvent pretreatment of palm samples for polyphenol analysis using choline chloride deep eutectic solvents. J. Chromatogr. A 2017, 1492, 1–11.
- Li, G.; Wang, W.; Wang, Q.; Zhu, T. Deep Eutectic Solvents Modified Molecular Imprinted Polymers for Optimized Purification of Chlorogenic Acid from Honeysuckle. J. Chromatogr. Sci. 2016, 54, 271–279.
- Wang, X.; Li, G.; Row, K.H. Graphene and Graphene Oxide Modified by Deep Eutectic Solvents and Ionic Liquids Supported on Silica as Adsorbents for Solid-Phase Extraction. Bull. Korean Chem. Soc. 2017, 38, 251–257.
- Li, G.; Wang, X.; Row, K.H. Magnetic Solid-phase Extraction with Fe3O4/Molecularly Imprinted Polymers Modified by Deep Eutectic Solvents and Ionic Liquids for the Rapid Purification of Alkaloid Isomers (Theobromine and Theophylline) from Green Tea. Molecules 2017, 22, 1061.
- Li, X.; Row, K.H. Application of novel ternary deep eutectic solvents as a functional monomer in molecularly imprinted polymers for purification of levofloxacin. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1068–1069, 56–63.
- Chen, J.; Wanga, Y.; Wei, X.; Xu, P.; Xu, W.; Ni, R.; Meng, J. Magnetic solid-phase extraction for the removal of mercury from water with ternary hydrosulphonyl-based deep eutectic solvent modified magnetic graphene oxide. Talanta 2018, 188, 454–462.
- Ma, W.; Dai, Y.; Row, K.H. Molecular imprinted polymers based on magnetic chitosan with different deep eutectic solvent monomers for the selective separation of catechins in black tea. Electrophoresis 2018, 39, 2039–2046.
- Ma, W.; Row, K.H. Solid-Phase Extraction of Catechins from Green Tea with Deep Eutectic Solvent Immobilized Magnetic Molybdenum Disulfide Molecularly Imprinted Polymer. Molecules 2020, 25, 280.
- Liu, L.; Tang, W.; Tang, B.; Han, D.; Row, K.H.; Zhu, T. Pipette-tip solid-phase extraction based on deep eutectic solvent modified graphene for the determination of sulfamerazine in river water. J. Sep. Sci. 2017, 40, 1887–1895.
- Sun, L.; Deng, Q.; Zhu, T. Optimization of heteroatom doped graphene oxide by deep eutectic solvents and the application for pipette-tip solid-phase extraction of flavonoids. J. Sep. Sci. 2019, 42, 2371–2378.
- Yousefi, S.M.; Shemirani, F.; Ghorbanian, S.A. Deep eutectic solvent magnetic bucky gels in developing dispersive solid phase extraction: Application for ultra trace analysis of organochlorine pesticides by GC-micro ECD using a large-volume injection technique. Talanta 2017, 168, 73–81.
- Lamei, N.; Ezoddin, M.; Ardestani, M.S.; Abdi, K. Dispersion of magnetic graphene oxide nanoparticles coated with a deep eutectic solvent using ultrasound assistance for preconcentration of methadone in biological and water samples followed by GC–FID and GC–MS. Anal. Bioanal. Chem. 2017, 409, 6113–6121.
- Zarei, A.; Nedaei, M.; Ghorbanian, S.A. Application of deep eutectic solvent based magnetic colloidal gel for dispersive solid phase extraction of ultra-trace amounts of some nitroaromatic compounds in water samples. J. Mol. Liq. 2017, 246, 58–65.
- Majidi, S.M.; Hadjmohammadi, M.R. Alcohol-based deep eutectic solvent as a carrier of SiO2 @Fe3O4 for the development of magnetic dispersive micro-solid-phase extraction method: Application for the preconcentration and determination of morin in apple and grape juices, diluted and acidic extract of dried onion and green tea infusion samples. J. Sep. Sci. 2019, 42, 2842–2850.
- Li, G.; Row, K.H. Ternary deep eutectic solvent magnetic molecularly imprinted polymers for the dispersive magnetic solid-phase microextraction of green tea. J. Sep. Sci. 2018, 41, 3424–3431.
- Li, G.; Rwo, K.H. Hydrophilic molecularly imprinted chitosan based on deep eutectic solvents for the enrichment of gallic acid in red ginseng tea. Polymers (Basel) 2019, 11, 1434.
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148.
- Hasan, A.K.; Xin, D.; Manjree, A.; Oh, Y.J.; Lin, R.Y. Application of Direct Immersion Solid-Phase Microextraction (DI-SPME) for Understanding Biological Changes of Mediterranean Fruit Fly (Ceratitis capitata) During Mating Procedures. Molecules 2018, 23, 2951.
- Wang, R.; Li, W.; Chen, Z. Solid phase microextraction with poly(deep eutectic solvent) monolithic column online coupled to HPLC for determination of non-steroidal anti-inflammatory drugs. Anal. Chim. Acta 2018, 1018, 111–118.
- Li, T.; Song, Y.; Xu, J.; Fan, J. A hydrophobic deep eutectic solvent mediated sol-gel coating of solid phase microextraction fiber for determination of toluene, ethylbenzene and o-xylene in water coupled with GC-FID. Talanta 2019, 195, 298–305.
- Mirzajani, R.; Kardani, F.; Ramezani, Z. Fabrication of UMCM-1 based monolithic and hollow fiber—Metal-organic framework deep eutectic solvents/molecularly imprinted polymers and their use in solid phase microextraction of phthalate esters in yogurt, water and edible oil by GC-FID. Food Chem. 2020, 314, 126179.
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), A novel extraction technique for aqueous samples: Theory and principles. J. Microcolumn Sep. 1999, 11, 737–747.
- Benedé, J.L.; Chisvert, A.; Giokas, D.L.; Salvador, A. Development of stir bar sorptive-dispersive microextraction mediated by magnetic nanoparticles and its analytical application to the determination of hydrophobic organic compounds in aqueous media. J. Chromatogr. A 2014, 1362, 25–33.
- Zarei, A.; Nedaei, M.; Ghorbanian, S.A. Deep eutectic solvent based magnetic nanofluid in the development of stir bar sorptive dispersive microextraction: An efficient hyphenated sample preparation for ultra-trace nitroaromatic explosives extraction in wastewater. J. Sep. Sci. 2017, 40, 4757–4764.
- Nemati, M.; Farajzadeh, M.A.; Mohebbi, A.; Khodadadeian, F.; Mogaddam, M.R.A. Development of a stir bar sorptive extraction method coupled to solidification of floating droplets dispersive liquid–liquid microextraction based on deep eutectic solvents for the extraction of acidic pesticides from tomato samples. J. Sep. Sci. 2020, 43, 1119–1127.
- He, Y.; Lee, H.K. Liquid-Phase Microextraction in a Single Drop of Organic Solvent by Using a Conventional Microsyringe. Anal. Chem. 1997, 69, 4634–4640.
- He, Y. Recent advances in application of liquid-based micro-extraction: A review. Chem. Pap. 2014, 68, 995–1007.
- Sarafraz-Yazdi, A.; Amiri, A. Liquid-phase microextraction. TrAC Trends Anal. Chem. 2010, 29, 1–14.
- Anthemidis, A.N.; Mitani, C. Advances in Liquid Phase Micro-Extraction Techniques for Metal, Metalloid and Organometallic Species Determination. Curr. Anal. Chem. 2013, 9, 250–278.
- Karimi, M.; Dadfarnia, S.; Shabani, A.M.H.; Tamaddon, F.; Azadi, D. Deep eutectic liquid organic salt as a new solvent for liquid-phase microextraction and its application in ligandless extraction and preconcentraion of lead and cadmium in edible oils. Talanta 2015, 144, 648–654.
- Arain, M.B.; Yilmaz, E.; Soylak, M. Deep eutectic solvent based ultrasonic assisted liquid phase microextraction for the FAAS determination of cobalt. J. Mol. Liq. 2016, 224, 538–543.
- Yilmaz, E.; Soylak, M. Ultrasound assisted-deep eutectic solvent based on emulsification liquid phase microextraction combined with microsample injection flame atomic absorption spectrometry for valence speciation of chromium(III/VI) in environmental samples. Talanta 2016, 160, 680–685.
- Aydin, F.; Yilmaz, E.; Soylak, M. A simple and novel deep eutectic solvent based ultrasound-assisted emulsification liquid phase microextraction method for malachite green in farmed and ornamental aquarium fish water samples. Microchem. J. 2017, 132, 280–285.
- Zounr, R.A.; Tuzen, M.; Deligonul, N.; Khuhawar, M.Y. A highly selective and sensitive ultrasonic assisted dispersive liquid phase microextraction based on deep eutectic solvent for determination of cadmium in food and water samples prior to electrothermal atomic absorption spectrometry. Food Chem. 2018, 253, 277–283.
- Kanberoglu, G.S.; Yilmaz, E.; Soylak, M. Developing a new and simple ultrasound-assisted emulsification liquid phase microextraction method built upon deep eutectic solvents for Patent Blue V in syrup and water samples. Microchem. J. 2019, 145, 813–818.
- Thongsaw, A.; Udnan, Y.; Ross, G.M.; Chaiyasith, W.C. Speciation of mercury in water and biological samples by eco-friendly ultrasound-assisted deep eutectic solvent based on liquid phase microextraction with electrothermal atomic absorption spectrometry. Talanta 2019, 197, 310–318.
- Li, X.; Wang, M.; Zhao, J.; Guo, H.; Gao, X.; Xiong, Z.; Zhao, L. Ultrasound-assisted emulsification liquid phase microextraction method based on deep eutectic solvent as extraction solvent for determination of five pesticides in traditional Chinese medicine. J. Pharm. Biomed. Anal. 2019, 166, 213–221.




