
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | -- | 2779 | 2023-07-17 18:19:06 |
Video Upload Options
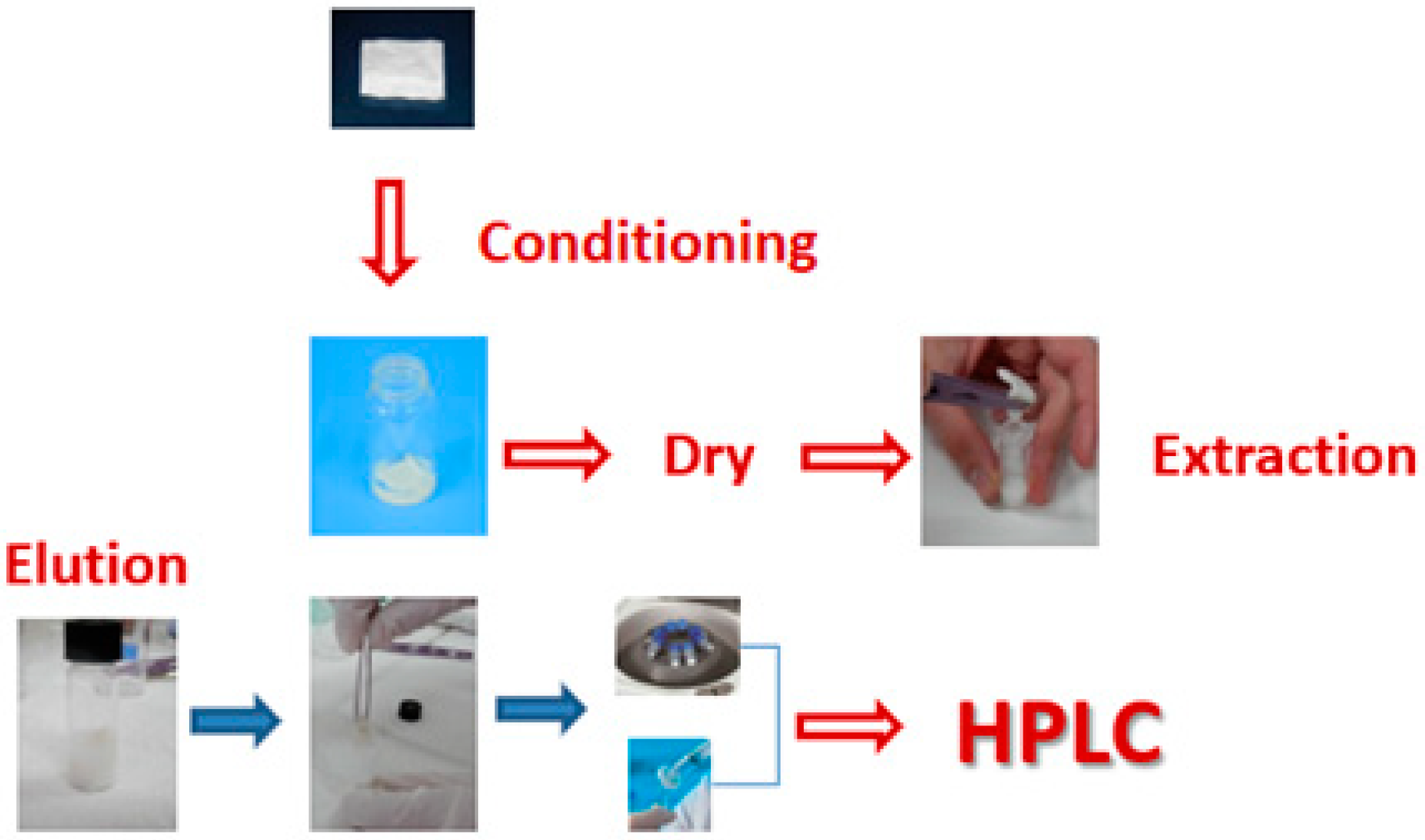
Fabric phase sorptive extraction (FPSE) is a novel and green sample preparation technique introduced in 2014. FPSE utilizes a natural or synthetic permeable and flexible fabric substrate chemically coated with a sol-gel organic-inorganic hybrid sorbent in the form of ultra-thin coating, which leads to a fast and sensitive micro-extraction device. The flexible FPSE requires no modification of samples and allows direct extraction of analytes. Sol-gel sorbent-coated FPSE media possesses high chemical, solvent, and thermal stability due to the strong covalent bonding between the substrate and the sol-gel sorbent.
1. Introduction
|
Analytical Technique |
Fabric Substrate |
Sol-Gel Coating |
E.T. (min) |
Elution System |
Sample |
Type of Analyte |
Analyte |
E.F. |
LOD ng/L |
LOQ ng/L |
R% |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Environmental Samples |
||||||||||||
|
FPSE-HPLC-UV |
Cellulose |
PEG |
40 |
MeOH-ACN |
Tap-Pond-Reclaimed water |
Substituted phenols |
4-CP |
- |
30 |
- |
91.0–109.5 |
[2] |
|
3,5-DMP |
10 |
73.2–90.7 |
||||||||||
|
2,6-DCP |
40 |
19.7–40.7 |
||||||||||
|
2,4,6-TCP |
20 |
26.9–57.0 |
||||||||||
|
2,4-DIPP |
20 |
35.8–90.8 |
||||||||||
|
FPSE-HPLC-UV |
Cellulose |
PTHF |
25 |
MeOH |
Ground-River water, WWTP, Soil, Sludge |
Alkylphenols |
4-TBP |
- |
182 |
601 |
90.1–96.0 |
[13] |
|
4-SBP |
179 |
599 |
90.6–96.3 |
|||||||||
|
4-TAP |
192 |
640 |
89.0–96.5 |
|||||||||
|
4-CP |
161 |
531 |
91.1–96.9 |
|||||||||
|
FPSE-HPLC-FLD |
Cellulose |
PTHF |
20 |
MeOH |
Ground-River-Drinking, WWTP, Hospital wastewater |
Estrogens |
BPA |
13.9 |
42 |
139 |
88.7–96.4 |
[12] |
|
E2 |
14.4 |
20 |
66 |
89.4–97.4 |
||||||||
|
EE2 |
14.7 |
36 |
119 |
89.0–98.0 |
||||||||
|
FPSE-UHPLC-MS/MS |
Cellulose |
PTHF |
20 |
MeOH |
WWTP, Untreated Hospital wastewater, Tap water |
Steroid hormones: Androgens Progestogens |
NORET |
- |
33.5 |
- |
79.3–95.4 |
[14] |
|
NOR |
1.7 |
79.5–103.5 |
||||||||||
|
MGA |
21.4 |
102.2–121.2 |
||||||||||
|
PRO |
6.9 |
79.8–96.9 |
||||||||||
|
BOL |
46.9 |
66.6–92.4 |
||||||||||
|
NAN |
50.7 |
82.2–102.4 |
||||||||||
|
TES |
2.2 |
75.6–91.8 |
||||||||||
|
DHEA |
264 |
77.6–92.7 |
||||||||||
|
AND |
63.6 |
70.0–98.9 |
||||||||||
|
ADTD |
19.4 |
65.9–97.8 |
||||||||||
|
FPSE-HS-GC-MS |
Fiber glass |
PDMDPS |
- |
He |
Environmental air |
Sexual pheromone |
(3E,8Z,11Z)- |
- |
1.6 μg |
5.3 μg |
- |
[15] |
|
tetradecatrien-1-yl |
||||||||||||
|
acetate |
||||||||||||
|
(3E,8Z)- |
0.8 μg |
2.6 μg |
||||||||||
|
tetradecadien-1-yl |
||||||||||||
|
acetate |
||||||||||||
|
FPSE-HPLC-UV |
Cellulose |
PTHF |
20 |
ACN |
Industrial-Ground water, Borcher-Oakay alloy |
Heavy metal ions |
Co(II) |
- |
20 |
66 |
89.6–98.7 |
[16] |
|
Ni(II) |
18 |
59 |
87.0–98.6 |
|||||||||
|
Pd(II) |
10 |
30 |
89.0–99.0 |
|||||||||
|
FPSE-HPLC-UV |
Cellulose |
PTHF |
15 |
ACN |
Industrial-Bore well-Drinking water |
Heavy metal ions |
Cr(III) |
- |
1 |
3 |
89.6–98.7 |
[17] |
|
Cr(IV) |
3 |
9 |
87.0–98.6 |
|||||||||
|
FDSE-FI-FAAS |
Polyester |
PDMDPS |
1.5 |
MIBK |
River-Coastal-Ditch water |
Toxic metals |
Lead |
140 |
1800 |
6000 |
95.0–101.0 |
[18] |
|
Cadmium |
38 |
400 |
1200 |
94.0–98.0 |
||||||||
|
FPSE-UHPLC-MS/MS |
Polyester |
PDMDPS |
60 |
MeOH |
Sewage water |
UV stabilizers in personal care products |
UV P |
10 |
12.8–25.3 |
42.7–84.3 |
83–99 |
[19] |
|
UV 329 |
12.2–19.8 |
40.7–66.0 |
51–65 |
|||||||||
|
UV 326 |
51.6–60.7 |
172–202 |
49–65 |
|||||||||
|
UV 328 |
9.44–18.1 |
31.5–60.3 |
43–64 |
|||||||||
|
UV 327 |
36.2–38.6 |
121–129 |
65–87 |
|||||||||
|
UV 571 |
40.0–44.3 |
133–148 |
49–57 |
|||||||||
|
UV 360 |
6.01–7.34 |
20.0–24.5 |
35–63 |
|||||||||
|
FPSE-UHPLC-MS/MS |
Polyester |
PDMDPS |
60 |
MeOH |
Seawater |
UV stabilizers in personal care products |
UV P |
25 |
5.63 |
18.8 |
- |
[20] |
|
UV 329 |
4.33 |
14.5 |
||||||||||
|
UV 326 |
8.96 |
29.9 |
||||||||||
|
UV 328 |
1.63 |
5.44 |
||||||||||
|
UV 327 |
1.06 |
3.54 |
||||||||||
|
UV 360 |
2.72 |
9.08 |
||||||||||
|
FPSE-LC-MS/MS |
Cellulose |
PEG |
240 |
MeOH |
River water, Effluent/influent wastewater |
Pharmaceuticals Personal care products |
MPB |
- |
10 |
50 |
9–27 a |
[21] |
|
CBZ |
10 |
50 |
20–92 a |
|||||||||
|
PrPB |
2 |
20 |
41–65 a |
|||||||||
|
DHB |
5 |
50 |
44–74 a |
|||||||||
|
BzPB |
1 |
20 |
45–67 a |
|||||||||
|
DHMB |
2 |
20 |
50–74 a |
|||||||||
|
DICLO |
1 |
20 |
44–73 a |
|||||||||
|
BP-3 |
2 |
20 |
59–93 a |
|||||||||
|
TCC |
3 |
10 |
57–59 a |
|||||||||
|
TCS |
50 |
200 |
43–54 a |
|||||||||
|
DFPSE-LC-MS/MS |
Cellulose |
PEG |
10 |
EtOAc |
River water, Effluent/influent wastewater |
Pharmaceuticals Personal care products |
MPB |
- |
4 |
50 |
12–30 a |
[22] |
|
CBZ |
4 |
50 |
18–53 a |
|||||||||
|
PrPB |
2 |
50 |
20–64 a |
|||||||||
|
DHB |
2 |
50 |
21–68 a |
|||||||||
|
BzPB |
2 |
50 |
33–70 a |
|||||||||
|
DHMB |
2 |
20 |
39–76 a |
|||||||||
|
DICLO |
2 |
50 |
23–50 a |
|||||||||
|
BP-3 |
2 |
100 |
45–52 a |
|||||||||
|
TCC |
8 |
50 |
15–49 a |
|||||||||
|
TCS |
20 |
100 |
22–43 a |
|||||||||
|
FPSE-GC-MS |
Cellulose |
PEG |
120 |
EtOAc |
River water, Effluent/influent WWTP |
Non-steroidal Anti-inflammatory drugs |
IBU |
418 |
0.8 |
3 |
82–109 b |
[23] |
|
NAP |
263 |
2 |
5 |
93–111 b |
||||||||
|
KET |
223 |
5 |
15 |
92–108 b |
||||||||
|
DIC |
162 |
2 |
7 |
94–116 b |
||||||||
|
FPSE-UHPLC-MS/MS |
Cellulose |
M-PEG |
60 |
MeOH |
Wastewater from WWTP, Wastewater from hospital effluent |
Cytostatic drugs |
ETO |
- |
7.403 |
24.68 |
44.49–78.57 |
[24] |
|
CP |
3.825 |
12.75 |
40.50–70.20 |
|||||||||
|
VINC |
98.04 |
326.8 |
42.18–82.82 |
|||||||||
|
VINB |
39.93 |
132.8 |
30.18–101.8 |
|||||||||
|
TAM |
0.093 |
0.309 |
81.90–200.5 |
|||||||||
|
Stir-FPSE-UPLC-DAD |
Cellulose |
PEG |
60 |
MeOH |
River-Stream water |
Herbicides |
Simazine |
444 |
140 |
460 |
84–124 |
[25] |
|
Atrazine |
729 |
240 |
790 |
75–126 |
||||||||
|
Secbumeton |
988 |
80 |
260 |
76–103 |
||||||||
|
Terbumeton |
1165 |
80 |
260 |
75–104 |
||||||||
|
Propazine |
996 |
110 |
360 |
75–97 |
||||||||
|
Prometryn |
1286 |
470 |
1500 |
78–111 |
||||||||
|
Terbutryn |
1411 |
80 |
260 |
78–99 |
||||||||
|
Stir-FPSE-HPLC-DAD |
Cellulose |
PTHF |
15 |
ACN |
Wastewater, Reservoir water |
Brominated flame retardants |
TBBPA |
- |
30 |
- |
93 |
[26] |
|
TBBPA-BAE |
20 |
95 |
||||||||||
|
TBBPA-BDBPE |
40 |
92–99 |
||||||||||
|
Stir-bar-FPSE-HPLC-DAD |
Cellulose |
PTHF |
10 |
ACN |
Wastewater, Reservoir water |
Brominated flame retardants |
TBBPA |
- |
10 |
- |
92–95 |
[26] |
|
TBBPA-BAE |
50 |
90–97 |
||||||||||
|
TBBPA-BDBPE |
10 |
91–98 |
||||||||||
|
Food Samples |
||||||||||||
|
FPSE-UPLC-MS |
Cellulose |
PTHF |
20 |
ACN |
Food simulants |
Non-volatile Additives Migrants |
DEP |
3.1 |
5.0 c |
15 d |
67.6 |
[8] |
|
TBC |
6.4 |
1.0 c |
3 d |
104.8 |
||||||||
|
DBM |
6.6 |
3.0 c |
10 d |
112 |
||||||||
|
TBoAC |
7.3 |
1.0 c |
3 d |
83.3 |
||||||||
|
TXIB |
5.1 |
1.0 c |
3 d |
87.4 |
||||||||
|
DBP |
5.8 |
10 c |
30 d |
91.5 |
||||||||
|
2EHAdip |
- |
1.0 c |
3 d |
9.1 |
||||||||
|
2EHseb |
2.9 |
1.0 c |
3 d |
64.7 |
||||||||
|
IRGA38 |
- |
1.0 c |
3 d |
78.1 |
||||||||
|
TOPAC |
- |
5.0 c |
15 d |
33.3 |
||||||||
|
IRGA1076 |
12 |
3.0 c |
10 d |
80.4 |
||||||||
|
IRGA168 |
- |
3.0 c |
10 d |
45.7 |
||||||||
|
IRGA1010 |
- |
3.0 c |
10 d |
67.6 |
||||||||
|
TINU326 |
11 |
10 c |
25 d |
72.1 |
||||||||
|
CHIMA81 |
1.8 |
2.0 c |
10 d |
100.8 |
||||||||
|
TINU327 |
3.2 |
10 c |
30 d |
80.6 |
||||||||
|
CYA1084 |
- |
12 c |
30 d |
86.5 |
||||||||
|
HAAC12 |
- |
7.0 c |
20 d |
53.1 |
||||||||
|
FPSE-GC-MS |
Cellulose |
PEG |
60 |
MeOH |
Oranges |
Freshness markers |
Furfuryl alcohol |
- |
12.5 c |
37.8 d |
98.9 |
[27] |
|
Butyric acid |
150 c |
445 d |
70.1 |
|||||||||
|
Cis-3-hexen-1-ol |
12.5 c |
37.0 d |
93.7 |
|||||||||
|
Ethyl butyrate |
31.1 c |
93.4 d |
70.5 |
|||||||||
|
Vanillin |
31.0 c |
93.0 d |
89.4 |
|||||||||
|
Ethyl isovalerate |
10.0 c |
42.5 d |
80.8 |
|||||||||
|
Linalool |
10.0 c |
40.7 d |
102.1 |
|||||||||
|
1-Octen-3-one |
12.5 c |
37.6 d |
104.1 |
|||||||||
|
Eugenol |
12.5 c |
37.8 d |
86.7 |
|||||||||
|
Octanal |
11.1 c |
33.4 d |
106.5 |
|||||||||
|
Ethyl octanoate |
15.0 c |
43.6 d |
102.6 |
|||||||||
|
Limonene |
10.0 c |
40.8 d |
85.4 |
|||||||||
|
FPSE-HPLC-DAD |
Cellulose |
PEG |
30 |
MeOH |
Raw milk |
Antibiotic drugs Amphenicols |
TAP |
- |
- |
- |
90.5–103.3 |
[9] |
|
FFC |
92.3–103.3 |
|||||||||||
|
CAP |
97.0–106.0 |
|||||||||||
|
FPSE-HPLC-UV |
Cellulose |
PEG |
30 |
MeOH-ACN |
Raw milk |
Antibiotic drugs Sulfonamides |
SMTH |
- |
- |
- |
94.7–107.0 |
[28] |
|
SIX |
93.0–104.6 |
|||||||||||
|
SDMX |
96.1–102.5 |
|||||||||||
|
FPSE-HPLC-DAD |
Cellulose |
PEG |
40 |
ACN |
Intact bovine milk |
Antibiotic drugs Penicillin |
PENG |
- |
3.0 c |
10.0 d |
86.7–115.1 |
[29] |
|
CLO |
6.0 c |
20.0 d |
82.8–107.2 |
|||||||||
|
DICLO |
7.5 c |
25.0 d |
80.8–95.8 |
|||||||||
|
OXA |
9.0 c |
30.0 d |
82.6–92.4 |
|||||||||
|
FPSE-HPLC-UV |
Cellulose |
Graphene |
40 |
MeOH-ACN |
Cow milk, Human breast milk |
Organic monomers |
BPA |
- |
16.7 c |
50 d |
- |
[30] |
|
TEGDMA |
||||||||||||
|
UDMA |
||||||||||||
|
BisGMA |
||||||||||||
|
SAIL-FPSE-HPLC-DAD |
Nonwoven Polypropylene |
[HMIM]NTf2 |
2 |
ACN |
Tea |
Fungicides |
Azoxystrobin |
156–161 |
90–110 |
300–370 |
80.3–90.4 |
[31] |
|
Chlorothalonil |
86–93 |
180–230 |
600–770 |
78.1–84.9 |
||||||||
|
Cyprodinil |
103 |
120–170 |
400–570 |
79.9–86.4 |
||||||||
|
Trifloxystrobin |
217–234 |
100–120 |
330–400 |
81.2–101.2 |
||||||||
|
Biological Samples |
||||||||||||
|
FPSE-HPLC-FLD |
Cellulose |
PHTF |
20 |
MeOH |
Urine |
Estrogens |
BPA |
13.9 |
42 |
139 |
90.0–91.0 |
[12] |
|
E2 |
14.4 |
20 |
66 |
90.7–90.9 |
||||||||
|
EE2 |
14.7 |
36 |
119 |
91.0–91.4 |
||||||||
|
FPSE-UHPLC-MS/MS |
Cellulose |
PTHF |
20 |
MeOH |
Urine |
Steroid Hormones: Androgens Progestogens |
NORET |
- |
35.2 |
- |
- |
[14] |
|
NOR |
132.3 |
|||||||||||
|
MGA |
11.1 |
|||||||||||
|
PRO |
12.8 |
|||||||||||
|
BOL |
37.9 |
|||||||||||
|
NAN |
50.1 |
|||||||||||
|
TES |
8.9 |
|||||||||||
|
DHEA |
110.6 |
|||||||||||
|
AND |
80 |
|||||||||||
|
ADTD |
25.6 |
|||||||||||
|
FPSE-HPLC-PDA |
Cellulose |
PEG |
30 |
MeOH |
Plasma Urine |
Antimicrobial drugs Azoles |
Ketoconazol |
- |
30,000 |
100,000 |
- |
[32] |
|
Terconazole |
||||||||||||
|
Voriconazole |
||||||||||||
|
Bifonazole |
||||||||||||
|
Clotrimazole |
||||||||||||
|
Tioconazole |
||||||||||||
|
Econazole |
||||||||||||
|
Butoconazole |
||||||||||||
|
Miconazole |
||||||||||||
|
Posaconazole |
||||||||||||
|
Ravuconazole |
||||||||||||
|
Anditraconazole |
||||||||||||
|
FPSE-HPLC-DAD |
Cellulose |
PEG |
20 |
MeOH-ACN |
Blood serum |
Drugs Benzodiazepines |
APZ |
- |
10,000 |
30,000 |
91.4–106.0 |
[33] |
|
BRZ |
87.6–97.6 |
|||||||||||
|
DZP |
90.0–104.0 |
|||||||||||
|
LRZ |
86.0–102.4 |
|||||||||||
|
FPSE-HPLC-PDA |
Cellulose |
PEG PCAP-PDMS-PCAP |
30 |
MeOH |
Whole blood Plasma Urine |
Inflammatory |
Ciprofloxacin |
25.8–29.1 |
20,000–10,0000 |
50,000–25,0000 |
- |
[34] |
|
bowel disease |
Sulfasalazine |
56.7–63.9 |
||||||||||
|
drugs |
Cortisone |
26.9–105.4 |
||||||||||
LOD, Limit of detection, calculated as S/N = 3; LOQ, Limit of quantitation, calculated as S/N = 10; E.T., Extraction time; E.F., Enrichment factor; R%, Relative recovery %; Ref., Reference; a Rapp(%)—Apparent recovery that includes the extraction recovery and matrix effect; b Relative recoveries from calibrations in ultrapure water; c,d LOD and LOQ in ng/g.
2. Fabric Phase Sorptive Extraction: Features of Merit—Comparison with Other Sample Extraction Techniques
|
Extraction Technique |
Instrumentation |
Sample Volume (mL) |
E.T. (min) |
LOD ng/L |
LOQ ng/L |
RSD% |
R% |
Ref. |
|---|---|---|---|---|---|---|---|---|
|
FPSE |
GC-MS |
30 |
120 |
0.8–5 |
3–15 |
4–18 |
82–116 |
[23] |
|
SBSE |
GC-MS |
15 |
240 |
13–21 a |
43–70 |
3–20 |
77–107 |
[35] |
|
SPME |
GC-MS |
22 |
40 |
– |
15–40 |
4–9 |
– |
[36] |
|
MEPS |
GC-MS |
5 |
6 |
3–110 |
11–360 |
3–13 |
60–160 |
[37] |
LOD, Limit of detection, calculated as S/N = 3; a LOD calculated as blank ±3 SDblank; LOQ, Limit of quantitation, calculated as S/N = 10.
References
- Samanidou, V.F. Trends in microextraction techniques for sample preparation. Separations 2018, 5, 1.
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K. Fabric phase sorptive extraction explained. Separations 2017, 4, 21.
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC-Trends Anal. Chem. 2015, 71, 2–8.
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC-Trends Anal. Chem. 2015, 73, 19–38.
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A 2013, 1321, 1–13.
- Kazantzi, V.; Anthemidis, A. Fabric sol-gel phase sorptive extraction technique: A review. Separations 2017, 4, 20.
- Silva, F.; Universidade, S.; Fluminense, F.; Semaan, F.S. Sample preparation in food analysis: Practices, problems and future outlook. In Thermal Techniques and Their Applications; Locatelli, M., Celia, C., Eds.; Nova Science Publishers: New York, NY, USA, 2017; pp. 23–53. ISBN 978-1-53612-282-4.
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107.
- Samanidou, V.; Galanopoulos, L.D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50.
- Samanidou, V.F. Fabric phase sorptive extraction in pharmaceutical analysis. Pharm. Anal. Acta 2015, 6, 6–8.
- Kabir, A.; Locatelli, M.; Ulusoy, H. Recent trends in microextraction techniques employed in analytical and bioanalytical sample preparation. Separations 2017, 4, 36.
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25.
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238.
- Guedes-Alonso, R.; Ciofi, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Del Bubba, M.; Kabir, A.; Furton, K.G. Determination of androgens and progestogens in environmental and biological samples using fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1437, 116–126.
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Integrated sampling and analysis unit for the determination of sexual pheromones in environmental air using fabric phase sorptive extraction and headspace-gas chromatography-mass spectrometry. J. Chromatogr. A 2017, 1488, 17–25.
- Heena Kaur, R.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Determination of cobalt (II), nickel (II) and palladium (II) Ions via fabric phase sorptive extraction in combination with high-performance liquid chromatography-UV detection. Sep. Sci. Technol. 2017, 52, 81–90.
- Furton, K.G.; Rani, S.; Malik, A.K.; Kabir, A.; Furton, K.G. Speciation of Cr (III) and Cr (VI) ions via fabric phase sorptive extraction for their quantification via HPLC with UV detection speciation of Cr (III) and Cr (VI) ions via fabric phase sorptive extraction for their quantification via HPLC with UV. J. Chromatogr. Sep. Tech. 2016.
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70.
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150.
- García-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207.
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marcé, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography-tandem mass spectrometry. Talanta 2015, 144, 1342–1351.
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26.
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Sieira, B.J.; Kabir, A.; Furton, K.G.; Cela, R. Fabric phase sorptive extraction: A new sorptive microextraction technique for the determination of non-steroidal anti-inflammatory drugs from environmental water samples. Anal. Chim. Acta 2015, 865, 22–30.
- Santana-Viera, S.; Guedes-Alonso, R.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Kabir, A.; Furton, K.G. Optimization and application of fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry for the determination of cytostatic drug residues in environmental waters. J. Chromatogr. A 2017, 1529, 39–49.
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45.
- Huang, G.; Dong, S.; Zhang, M.; Zhang, H.; Huang, T. Fabric phase sorptive extraction: Two practical sample pretreatment techniques for brominated flame retardants in water. Water Res. 2016, 101, 547–554.
- Aznar, M.; Úbeda, S.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction as a reliable tool for rapid screening and detection of freshness markers in oranges. J. Chromatogr. A 2017, 1500, 32–42.
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436.
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138.
- Samanidou, V.; Filippou, O.; Marinou, E.; Kabir, A.; Furton, K.G. Sol-gel-graphene-based fabric-phase sorptive extraction for cow and human breast milk sample cleanup for screening bisphenol A and residual dental restorative material before analysis by HPLC with diode array detection. J. Sep. Sci. 2017, 40, 2612–2619.
- Yang, M.; Gu, Y.; Wu, X.; Xi, X.; Yang, X.; Zhou, W.; Zeng, H.; Zhang, S.; Lu, R.; Gao, H.; et al. Rapid analysis of fungicides in tea infusions using ionic liquid immobilized fabric phase sorptive extraction with the assistance of surfactant fungicides analysis using IL-FPSE assisted with surfactant. Food Chem. 2018, 239, 797–805.
- Locatelli, M.; Kabir, A.; Innosa, D.; Lopatriello, T.; Furton, K.G. A fabric phase sorptive extraction-High performance liquid chromatography-Photo diode array detection method for the determination of twelve azole antimicrobial drug residues in human plasma and urine. J. Chromatogr. B 2017, 1040, 192–198.
- Samanidou, V.; Kaltzi, I.; Kabir, A.; Furton, K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2016, 30, 829–836.
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Tartaglia, A.; Di Donato, V.; D’Ovidio, C.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B 2018, 1084, 53–63.
- Quintana, J.B.; Rodil, R.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Multiresidue analysis of acidic and polar organic contaminants in water samples by stir-bar sorptive extraction-liquid desorption-gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1174, 27–39.
- Rodríguez, I.; Carpinteiro, J.; Quintana, J.B.; Carro, A.M.; Lorenzo, R.A.; Cela, R. Solid-phase microextraction with on-fiber derivatization for the analysis of anti-inflammatory drugs in water samples. J. Chromatogr. A 2004, 1024, 1–8.
- Noche, G.G.; Laespada, M.E.F.; Pavón, J.L.P.; Cordero, B.M.; Lorenzo, S.M. Microextraction by packed sorbent for the analysis of pharmaceutical residues in environmental water samples by in situ derivatization-programmed temperature vaporizer-gas chromatography-mass spectrometry. J. Chromatogr. A 2011, 1218, 9390–9396.




