Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Victoria Samanidou | -- | 2992 | 2023-07-17 18:17:44 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bitas, D.; Samanidou, V. Molecularly Imprinted Polymers in Sample Preparation. Encyclopedia. Available online: https://encyclopedia.pub/entry/46883 (accessed on 07 February 2026).
Bitas D, Samanidou V. Molecularly Imprinted Polymers in Sample Preparation. Encyclopedia. Available at: https://encyclopedia.pub/entry/46883. Accessed February 07, 2026.
Bitas, Dimitrios, Victoria Samanidou. "Molecularly Imprinted Polymers in Sample Preparation" Encyclopedia, https://encyclopedia.pub/entry/46883 (accessed February 07, 2026).
Bitas, D., & Samanidou, V. (2023, July 17). Molecularly Imprinted Polymers in Sample Preparation. In Encyclopedia. https://encyclopedia.pub/entry/46883
Bitas, Dimitrios and Victoria Samanidou. "Molecularly Imprinted Polymers in Sample Preparation." Encyclopedia. Web. 17 July, 2023.
Copy Citation
Molecularly Imprinted Polymers (MIPs) are synthetic polymeric materials with imprinted sites complementary to a specific molecule and high affinity over analytes with analogous molecular structure. Extraction can benefit from the production of MIPs that can be applied as sorbents for the extraction of specific antibiotics.
molecularly imprinted polymers
MIPs
antibiotics
extraction
1. Introduction
Molecularly Imprinted Polymers (MIPs) are synthetic polymeric materials with imprinted sites complementary to a specific molecule and high affinity over analytes with analogous molecular structure. Sample preparation protocols can benefit from the selectivity of the MIPs over specific molecule or group of molecules for improved clean-up efficiency. MIPs have already been reviewed as sorbents in sample preparation techniques [1][2][3] such as solid-phase extraction (SPE) [4][5][6], solid-phase microextraction (SPME) [7][8] and on-line extraction techniques [9] for bioanalysis [10], pharmaceutical [11], environmental [12], food [13], and forensic analysis [14]. MIPs have also been reviewed for the extraction of non-steroidal anti-inflammatory drugs and analgesics [15], drugs of abuse [16], personal care products [17] and proteins [18]. Apart from sample preparation purposes, MIPs have already been reviewed as monolithic columns in high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE) [19][20], in biosensors [21] and gas sensors [22], in drug delivery [23], for diagnostic purposes [24][25], in wastewater treatment [26] and for biomolecule identification and separation [27].
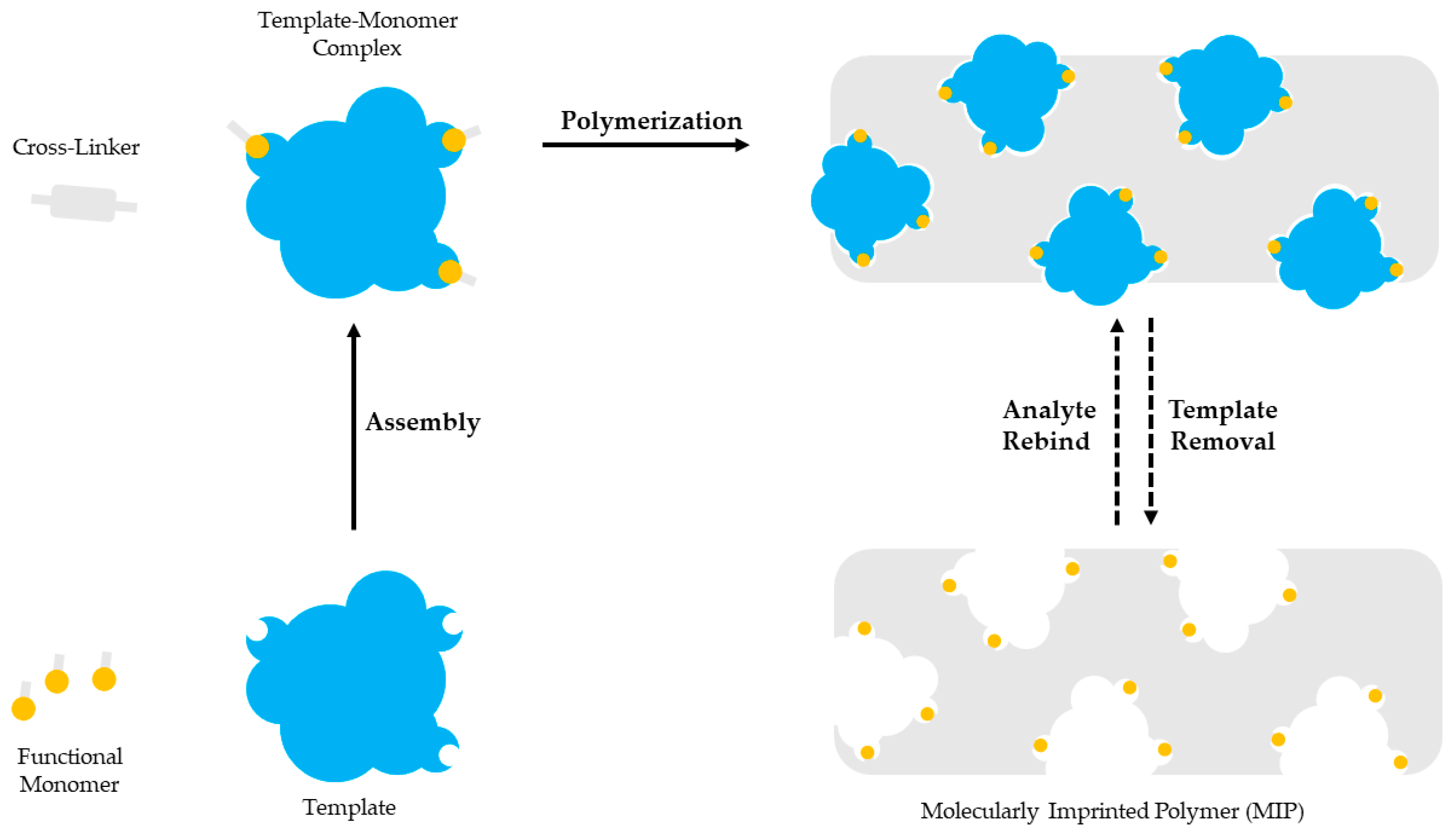
2. Molecular Imprinting
Molecular imprinting is based on the polymerization of a functional monomer and a cross-linker around a template molecule. Briefly, a selected template molecule and a functional monomer interact covalently or non-covalently and develop complexes. Then, polymerization takes place between the developed template-functional monomer complexes and a cross-linker. After the polymerization the template molecule is removed, leaving a polymer with imprinted sites complementary to the molecular structure and the functional groups of the template. The imprinted sites are available for binding the molecules with the same or similar structure as the template [28][29][30]. Molecular imprinting is schematically presented in Figure 1.

Figure 1. The template molecule and the functional monomer interact. The template-monomer complexes and a cross-linker polymerize. The template molecule is removed to provide a polymer with imprinted sites.
The essential components of a molecular imprinting process are the template molecule, the functional monomer and the cross-linker. The polymerization initiator and the porogenic solvent are also important. Furthermore, polymerization is conducted under a stream of an inert gas such as nitrogen to ensure oxygen removal.
For the selection of the template molecule specific criteria should be met. The functional groups of the template should be able to interact with the functional monomers and not hinder the polymerization reaction, while the template molecule should be chemically stable during polymerization. Usually, the templates are small organic molecules, such as pesticides, pharmaceuticals, sugars, amino acids and peptides [31][32].
Functional monomers consist of a recognition unit that interacts with the template and a polymerizable unit that is polymerized with the cross-linker. The recognition site of the selected functional monomer should develop strong interactions with the functional groups of the template molecule to form a complex before polymerization takes place. Functional monomers such as acrylic acid, methacrylic acid (MAA), methyl methacrylate, 2-(trifluoromethyl)acrylic acid (TFMAA), styrene, 4-vinylpyridine (4-VP), acrylamide (AM), methacrylamide, 2-hydroxyethyl methacrylate (HEMA) and 3-aminopropyltriethoxysilane (APTES) are used in non-covalent imprinting [28][31][32].
The cross-linker polymerizes with the polymerizable unit of the functional monomer to form a highly cross-linked polymer around the template-monomer complex that remains firm after the template removal. The selectivity and the binding capacity of the developed MIP depend on the amount of the selected cross-linker. Insufficient amount of the cross-linker results in mechanically unstable poorly cross-linked MIPs, one the other hand increased amount results in MIPs with reduced imprinted sites. Cross-linkers such as ethylene glycol dimethacrylate (EGDMA), trimethylopropane methacrylate (TRIM), divinylbenzene (DVB) and tetramethylene dimethacrylate are used in non-covalent imprinting [28][31][32].
The selection of the porogenic solvent is also important in molecular imprinting. The porogen influences the interaction between the template and the monomer, serves as the dispersion media and affects the pore forming during the polymerization reaction. In non-covalent imprinting less polar or non-polar solvents such as toluene, acetonitrile and chloroform are preferred [31][32].
Most MIPs are prepared by free radical polymerization that is initiated either thermally or photochemically with the addition of a peroxy or an azo compound. The most frequently used azo compound is azobisisobutyronitrile (AIBN) that is used at temperature range of 50–70 °C [31].
After the polymerization reaction is complete, template removal is essential before the prepared MIPs can be used in any application. Template removal should be meticulously performed in order to ensure that the maximum number of imprinted sites are free of the template molecule, especially in analytical applications where template bleeding can affect the results. Complete removal of the template often requires extreme conditions that can impair the imprinted sites. Template removal can be performed by three main approaches, solvent extraction, physically assisted extraction or the use of subcritical/supercritical solvents (subcritical water/supercritical CO2). Physically assisted extraction includes ultrasound-assisted, microwave-assisted and pressurized liquid extraction. The most common approach is the use of organic solvents, either by incubating the prepared MIPs in organic solvents or by extraction in a Soxhlet apparatus. MIP incubation is a mild template removal method that requires several hours to be complete but does not impair the imprinted sites. Incubation in organic solvents causes swelling of the MIP structure and favors template removal, while heating and stirring can reduce the duration of template removal. Extraction in a Soxhlet apparatus is a more drastic method where the template is extracted for several hours with heated organic solvent and is more commonly used for MIPs prepared by bulk polymerization. The heated solvent increases template solubility, thus template removal. However, Soxhlet extraction is a time-consuming method (up to 24 h) that requires increased volumes of organic solvent and high temperatures can cause template degradation [33].
There are two main approaches for the preparation of MIPs based on the interactions between the template molecule and the functional monomer that can be either covalent or non-covalent. In the covalent approach, reversible covalent interactions are developed between the functional groups of the template and the recognition unit of the monomer. Covalent imprinting is stoichiometric and functional monomer residues can only be found in the imprinted sites. Template removal is usually achieved by treating the prepared MIPs with organic solvents or Soxhlet extraction, while molecule binding is based on covalent interactions. However, there are limited reversible covalent interactions available and the strong nature of covalent interactions does not favor fast binding and removal on the imprinted sites [30][32][34].
In the non-covalent approach, hydrogen bonding, van der Waals forces and ionic or π-π interactions are developed for the formation of the template-monomer complex in a pre-polymerization step, with hydrogen bonding being the most common interaction. Template removal is achieved by washing the prepared MIPs with an organic solvent or a mixture of solvents, while molecule binding is based on non-covalent interactions. Non-covalent imprinting is a simple MIP preparation process that offers fast binding and removal on the imprinted sites and is the most preferred approach. However, the interactions forming the template-monomer are not as stable and prone to disruption [30][32][34].
Classic molecular imprinting involves a single template for the preparation of a MIP with imprinted sites that are not able to recognize more than one target molecule. With multi-template imprinting more templates are used for the preparation of MIPs with more than one type of imprinted sites able for simultaneous recognition of multiple target molecules. The multi-template MIPs enable the extraction and determination of multiple analytes; however, they have reduced selectivity compared with one-template MIPs [31].
Like multi-template imprinting, MIPs can be prepared by employing more than one functional monomer. Each functional monomer is able to recognize a different functional group of the template molecule and the resulting in MIPs presents increased selectivity. Multi-functional monomer imprinting can be helpful for the imprinting of macromolecules. However, thorough study is required for the selection of the appropriate monomers and their combining synergy in preparing MIPs with multiple functional monomers [31].
Sometimes in molecular imprinting the desirable template molecule can be chemically unstable or has low solubility during polymerization, difficult to handle, or even an expensive compound, thus dummy imprinting employs a surrogate structurally analogue molecule as an alternative template for MIP preparation. Furthermore, the drawback of template bleeding when MIPs are used for SPE applications can be removed by the employment of dummy imprinted MIPs [28][30][31].
2.1. MIP Characterization
Morphological evaluation of the prepared MIPs can be accomplished by scanning electron microscopy (SEM) and transmission electron microscopy (TEM), while structure analysis by X-ray photoelectron spectroscopy (XPS), extended X-ray absorption fine structures (EXAFS) and X-ray diffraction studies. Template molecule-functional monomer complex interactions can be screened by infrared (IR), nuclear magnetic resonance (NMR) and UV-Vis spectroscopy. In the case of MMIPs, the magnetic properties of MMIPs can be analyzed by vibrating sample magnetometer (VSM) [28][31].
In addition to the MIP, a non-imprinted polymer (NIP) is usually prepared without the presence of the template molecule. Although NIP has no imprinted sites, NIP preparation is carried out under the same conditions as the MIP and interactions between the NIP and the target molecule can be observed. This acts as control to characterize the quality of imprinted sites on the MIP surface and measure how strong are the interactions between the MIP and the target molecule in comparison with those between the NIP and the template. Binding tests are conducted by applying both the prepared MIP and NIP in solutions of predetermined concentration of the target molecule and measuring the retained amount. Binding tests can be conducted in solutions consisting of the same solvent used in MIP preparation or a solvent that simulates the nature of the analyzed sample. By doing these tests, the best type of functional monomer or template/monomer ratio can be chosen to optimize MIP preparation, while the best solvent for analyte extraction or analyte elution can be selected to optimize the sample preparation protocol [28].
3. Polymerization Techniques for MIP Preparation
There are two main approaches applied for the preparation of MIPs, free radical polymerization, controlled radical polymerization and sol-gel process.
3.1. Free Radical Polymerization (FRP)
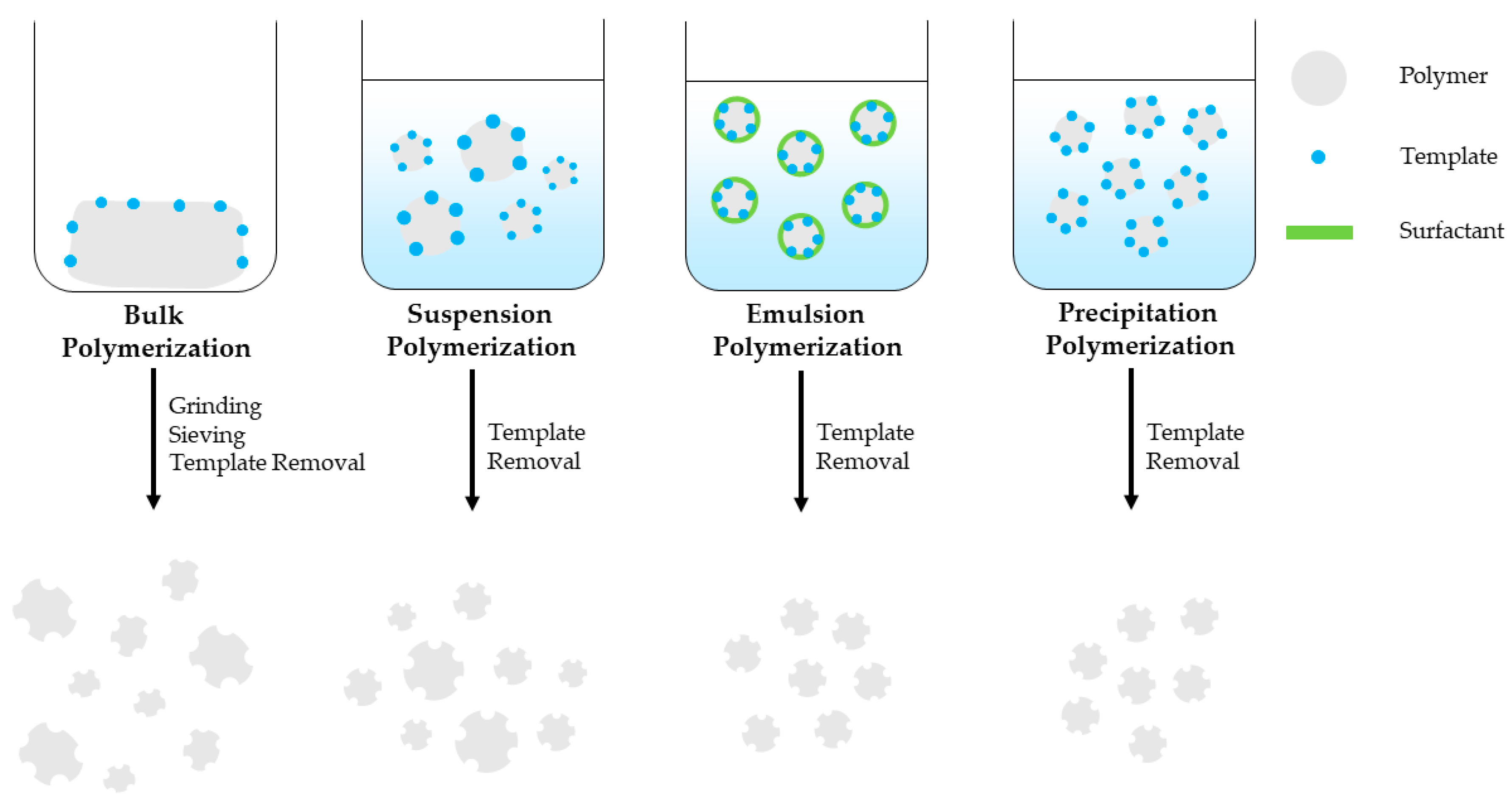
Bulk polymerization is a fast and simple polymerization method that provides pure MIPs with no special instrumentation requirements. It is the most widely used free radical polymerization method for preparing MIPs applied in sample extraction techniques. However, this method requires increased template amount and the prepared MIP bulk should be grinded, sieved and sedimented with the use of a solvent in order to remove the finer particles and obtain particles of the preferred size. This is time consuming and the resulting particles have irregular shape and size, decreased number of imprinted sites and binding capacity, while template bleeding can be observed [28][29][30][31]. Suspension polymerization is a simple, single-step polymerization method where polymerization mixture is suspended in a continuous aqueous, mineral oil or perfluorocarbon liquid phase and provides spherical porous MIP particles. However, the developed MIP particles size ranges between μm-mm with decreased recognition capability, unsuitable for solid-phase extraction applications [30][31]. Emulsion polymerization takes place in an oil/water diphasic system with the addition of surfactants that prevents diffusion and favors the formation of small, homogeneous emulsion droplets. Emulsion polymerization can provide mono-dispersed MIP particles, in high yield, however surfactant residues can interfere with the imprinted sites, thus reduce binding capacity of the MIPs [29][30][31]. Precipitation polymerization is a single-step polymerization method that provides high-quality, spherical MIP particles with homogenous size in good yield. As the polymerization takes place spherical MIP particles precipitate from the reaction solution. However, this method requires increased amount of organic solvent and meticulous control over the polymerization conditions such as solvent polarity, temperature and stirring speed that affect the MIP particle size [28][29][30][32]. MIP monoliths can be prepared inside a confined space such as a chromatographic column by in-situ polymerization. It is a simple, one-stem process and unlike bulk polymerization it does not require grinding and sieving of the prepared MIP bulk [30][31][32]. MIPS prepared by bulk, suspension, emulsion and precipitation polymerization are schematically presented in Figure 2.

Figure 2. Free radical polymerization techniques used for MIP preparation.
3.2. Controlled Radical Polymerization (CRP)
Polymerization usually involves reaction initiation, polymer chain propagation and reaction termination. In free radical polymerization, reaction initiation is slow while chain propagation is fast. Additionally, side reactions such as chain transfer reactions between the components of the polymerization mixture or reactions with impurities can occur. As a result, polymerization is prematurely terminated and polymer chains with variable lengths are formed. For this reason, MIP size, structure and molecular weight cannot be controlled, while heterogenous cross-linked network and imprinted site distribution can be observed. Controlled radical polymerization offers control over the polymerization reaction with the use of capping agents that prevent premature termination. Reaction initiation is fast, while chain propagation is slow and simultaneous for all polymer chains. The resulting polymer chains have homogenously distributed size and molecular weight.
The most common controlled radical polymerization approaches are atom transfer radical polymerization (ATRP), nitroxide-mediated polymerization (NMP) and reversible addition-fragmentation chain transfer (RAFT) polymerization. ATRP involves a reversible redox reaction catalyzed by a metal-ligand complex and the resulting MIPs have functional groups that enable surface modification. However, ATRP uses in MIP synthesis are limited because the functional groups of the template molecule and the recognition unit of the functional monomer can inhibit the metal-ligand complex. NMP involves a thermally reversible termination reaction that produces a nitroxyl radical that allows control over the polymerization reaction. Although NMP can be used in the presence of various functional groups, it requires temperatures over 100 °C that can affect the non-covalent interactions between the template molecule and the functional monomer, thus it is limited to covalent imprinting approaches. RAFT polymerization involves reversible addition-fragmentation sequences initiated by a FRP initiator and chain propagation is achieved with the help of a chain transfer agent. It can be used with a variety of functional groups and is suitable for non-covalent imprinting purposes [35][36].
Surface Imprinting
In surface imprinting, the template molecule is immobilized on the surface of a suitable material, where polymerization takes place, in order to prepare MIPs with controllable imprinted sites. The template can be completely removed and the resulting imprinted sites have improved accessibility that is important for imprinting macromolecules such as proteins. However, the number of the imprinted sites is proportionate to the surface area of the substrate material [29][30][31].
Core-shell MIPs can be prepared by surface imprinting on nanomaterials such as chitosan, polystyrene, SiO2, TiO2, and Fe3O4 combining the properties of both MIPs and nanomaterials. For the preparation of magnetic MIPs (MMIPs), appropriately prepared Fe3O4 nanoparticles undergo functionalization or surface modification and then molecular imprinting takes place on the surface of the magnetite nanoparticles, either with a free radical polymerization method or the sol-gel process. MMIPs can be collected from a solution with the application of an external magnet, thus they can be used in sample preparation providing an easier, less time-consuming and efficient analyte extraction procedure [29][30][37][38].
3.3. Sol-Gel Synthesis
Highly cross-linked materials can be prepared by the sol-gel synthesis that involves the hydrolysis of a tetraalkylosilane such as tetraethoxysilane (TEOS) and tetramethoxysilane (TMOS) into a colloidal solution and the polycondensation of the solution into a silica-based material. Compared with free radical polymerization, sol-gel process can take place in room temperature, is resistant to chemical and thermal decomposition and requires non-toxic solvents such as methanol and ultrapure water Fabric [28][39]. Although the sol-gel process is convenient and results in porous MIPs, the binding-rebinding process on the imprinted sites is slow and hard to be achieved [9]. Furthermore, there are limited polymerization reactions and precursors utilized in sol-gel synthesis of MIPs [31].
4. MIPs in Sample Preparation
The ability of the imprinted sites to recognize and bind with a specific molecule, makes MIPs excellent sorbent materials for sample preparation techniques such as solid-phase extraction (SPE), dispersive solid-phase extraction (DSPE), matrix solid-phase dispersion (MSPD) and solid-phase microextraction (SPME).
4.1. Molecularly Imprinted Solid-Phase Extraction (MISPE)
Following the classic SPE approach, MIP particles are packed inside an empty cartridge. The MIP SPE cartridges are preconditioned and loaded with a sample. Before analyte elution the MIPs are washed with a solvent or a mixture of solvents such as methanol, acetonitrile or water that will remove all interferences and not the bound analyte. Elution is carried out by a solvent that is able to release the bound analyte from the imprinted sites. MIPs prepared by bulk polymerization are very common in SPE applications. Furthermore, MIP packed pre-columns or monolithic columns can be used in on-line with an analytical instrument [29][30][32].
4.2. Dispersive Solid-Phase Extraction (DSPE)
In DSPE, MIP particles can be dispersed directly into the sample solution and after the extraction is complete they can be collected by centrifuging or filtration. This technique requires reduced MIPs amount in comparison with SPE and offers improved contact of the sorbent with the sample components, while eliminating the tedious packing the MIPs inside an SPE cartridge and the preconditioning step [30][31].
4.3. Matrix Solid-Phase Dispersion (MSPD)
Most sample preparation techniques are usually applied in sample solutions that result from a sample pre-treatment step, thus they cannot be applied directly to solid, semi-solid or samples with increased viscosity. With MSPD the MIP particles are added directly to the sample, mechanically mixed and the resulting mixture is packed inside a cartridge, washed and the analytes are eluted with the appropriate solvent. This technique has decreased organic solvent requirements and reduces matrix interferences [31].
4.4. Solid-Phase Microextraction (SPME)
In SPME, a syringe-like instrument with a sorbent-coated fiber at the end of the needle is applied for the analyte extraction from sample solutions. MIP-coated SMPE fibers can be prepared by synthesizing MIPs on the surface of a stainless steel or silica fiber. The coated fibber can be applied directly to the sample solution and after extraction it is injected directly to the analytical instrument and the analyte is desorpted either by an appropriate organic solvent in the case of an HPLC application or thermally in the case of a GC application. Apart from MIP-coated fibers, MIP monolith fibers can be prepared and used in an SPME protocol. Polymerization is performed inside a sealed capillary and after completion a part of the capillary is removed so that the MIP monolith is exposed [29][30][32][34][38].
References
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for sample preparation: A review. Anal. Chim. Acta 2010, 668, 87–99.
- Augusto, F.; Carasek, E.; Silva, R.G.C.; Rivellino, S.R.; Batista, A.D.; Martendal, E. New sorbents for extraction and microextraction techniques. J. Chromatogr. A 2010, 1217, 2533–2542.
- Tamayo, F.G.; Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for solid-phase extraction and solid-phase microextraction: Recent developments and future trends. J. Chromatogr. A 2007, 1152, 32–40.
- Yi, L.X.; Fang, R.; Chen, G.H. Molecularly Imprinted Solid-Phase Extraction in the Analysis of Agrochemicals. J. Chromatogr. Sci. 2013, 51, 608–618.
- Lasáková, M.; Jandera, P. Molecularly imprinted polymers and their application in solid phase extraction. J. Sep. Sci. 2009, 32, 799–812.
- He, C.; Long, Y.; Pan, J.; Li, K.; Liu, F. Application of molecularly imprinted polymers to solid-phase extraction of analytes from real samples. J. Biochem. Biophys. Methods 2007, 70, 133–150.
- Moein, M.M.; Said, R.; Bassyouni, F.; Abdel-Rehim, M. Solid phase microextraction and related techniques for drugs in biological samples. J. Anal. Methods Chem. 2014, 2014, 1–24.
- Abdulra’uf, L.B.; Chai, M.K.; Tan, G.H. Applications of solid-phase microextraction for the analysis of pesticide residues in fruits and vegetables: A review. J. AOAC Int. 2012, 95, 1272–1290.
- Moein, M.M.; Abdel-Rehim, M. Molecularly imprinted polymers for on-line extraction techniques. Bioanalysis 2015, 7, 2145–2153.
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Polymer–nanoparticles composites in bioanalytical sample preparation. Bioanalysis 2015, 7, 1723–1730.
- Kubo, T.; Otsuka, K. Recent progress for the selective pharmaceutical analyses using molecularly imprinted adsorbents and their related techniques: A review. J. Pharm. Biomed. Anal. 2016, 130, 68–80.
- Pichon, V.; Chapuis-Hugon, F. Role of molecularly imprinted polymers for selective determination of environmental pollutants-A review. Anal. Chim. Acta 2008, 622, 48–61.
- Núñez, O.; Gallart-Ayala, H.; Martins, C.P.B.; Lucci, P. New trends in fast liquid chromatography for food and environmental analysis. J. Chromatogr. A 2012, 1228, 298–323.
- Yılmaz, E.; Garipcan, B.; Patra, H.K.; Uzun, L. Molecular imprinting applications in forensic science. Sensors 2017, 17, 691.
- Madikizela, L.M.; Tavengwa, N.T.; Chimuka, L. Applications of molecularly imprinted polymers for solid-phase extraction of non-steroidal anti-inflammatory drugs and analgesics from environmental waters and biological samples. J. Pharm. Biomed. Anal. 2018, 147, 624–633.
- Vogliardi, S.; Tucci, M.; Stocchero, G.; Ferrara, S.D.; Favretto, D. Sample preparation methods for determination of drugs of abuse in hair samples: A review. Anal. Chim. Acta 2015, 857, 1–27.
- Figueiredo, L.; Erny, G.L.; Santos, L.; Alves, A. Applications of molecularly imprinted polymers to the analysis and removal of personal care products: A review. Talanta 2016, 146, 754–765.
- Stevenson, D.; EL-Sharif, H.F.; Reddy, S.M. Selective extraction of proteins and other macromolecules from biological samples using molecular imprinted polymers. Bioanalysis 2016, 8, 2255–2263.
- Zheng, C.; Huang, Y.P.; Liu, Z.S. Recent developments and applications of molecularly imprinted monolithic column for HPLC and CEC. J. Sep. Sci. 2011, 34, 1988–2002.
- Tóth, B.; Horvai, G. Chromatography, solid-phase extraction, and capillary electrochromatography with MIPs. In Topics in Current Chemistry; Springer: New York, NY, USA; Berlin, Germany, 2012; Volume 325, pp. 267–306. ISBN 9783642284205.
- Ertürk, G.; Mattiasson, B. Molecular imprinting techniques used for the preparation of biosensors. Sensors 2017, 17, 288.
- Zhang, Y.; Zhang, J.; Liu, Q. Gas Sensors Based on Molecular Imprinting Technology. Sensors 2017, 17, 1567.
- Puoci, F.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Iemma, F.; Picci, N. Molecularly imprinted polymers in drug delivery: State of art and future perspectives. Expert Opin. Drug Deliv. 2011, 8, 1379–1393.
- Bedwell, T.S.; Whitcombe, M.J. Analytical applications of MIPs in diagnostic assays: Future perspectives. Anal. Bioanal. Chem. 2016, 408, 1735–1751.
- Selvolini, G.; Marrazza, G. MIP-based sensors: Promising new tools for cancer biomarker determination. Sensors 2017, 17, 718.
- Huang, D.-L.; Wang, R.-Z.; Liu, Y.-G.; Zeng, G.-M.; Lai, C.; Xu, P.; Lu, B.-A.; Xu, J.-J.; Wang, C.; Huang, C. Application of molecularly imprinted polymers in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2015, 22, 963–977.
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly imprinted polymers for the identification and separation of chiral drugs and biomolecules. Polymers 2016, 8, 216.
- Boulanouar, S.; Mezzache, S.; Combès, A.; Pichon, V. Molecularly imprinted polymers for the determination of organophosphorus pesticides in complex samples. Talanta 2018, 176, 465–478.
- Ashley, J.; Shahbazi, M.-A.; Kant, K.; Chidambara, V.A.; Wolff, A.; Bang, D.D.; Sun, Y. Molecularly imprinted polymers for sample preparation and biosensing in food analysis: Progress and perspectives. Biosens. Bioelectron. 2017, 91, 606–615.
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26.
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211.
- Gama, M.R.; Bottoli, C.B.G. Molecularly imprinted polymers for bioanalytical sample preparation. J. Chromatogr. B 2017, 1043, 107–121.
- Lorenzo, R.A.; Carro, A.M.; Alvarez-Lorenzo, C.; Concheiro, A. To remove or not to remove? The challenge of extracting the template to make the cavities available in molecularly imprinted polymers (MIPs). Int. J. Mol. Sci. 2011, 12, 4327–4347.
- Ansari, S.; Karimi, M. Recent progress, challenges and trends in trace determination of drug analysis using molecularly imprinted solid-phase microextraction technology. Talanta 2017, 164, 612–625.
- Haupt, K.; Linares, A.V.; Bompart, M.; Bui, B.T.S. Molecularly Imprinted Polymers. In Topics in Current Chemistry; Springer: New York, NY, USA; Berlin, Germany, 2011; Volume 325, pp. 1–28. ISBN 9783642284205.
- Ye, L. Synthetic Strategies in Molecular Imprinting. In Advances in Biochemical Engineering/Biotechnology; Springer: New York, NY, USA; Berlin, Germany, 2015; Volume 150, pp. 1–24. ISBN 978-3-319-20728-5.
- Ansari, S.; Karimi, M. Recent configurations and progressive uses of magnetic molecularly imprinted polymers for drug analysis. Talanta 2017, 167, 470–485.
- Filippou, O.; Bitas, D.; Samanidou, V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043.
- Kazantzi, V.; Anthemidis, A. Fabric Sol–gel Phase Sorptive Extraction Technique: A Review. Separations 2017, 4, 20.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revision:
1 time
(View History)
Update Date:
17 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




