Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Covaliu | -- | 2165 | 2023-07-17 03:08:31 | | | |
| 2 | Jessie Wu | + 4 word(s) | 2169 | 2023-07-17 03:21:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Covaliu-Mierlă, C.I.; Păunescu, O.; Iovu, H. Nanofiltration Membranes Characteristics. Encyclopedia. Available online: https://encyclopedia.pub/entry/46851 (accessed on 03 March 2026).
Covaliu-Mierlă CI, Păunescu O, Iovu H. Nanofiltration Membranes Characteristics. Encyclopedia. Available at: https://encyclopedia.pub/entry/46851. Accessed March 03, 2026.
Covaliu-Mierlă, Cristina Ileana, Oana Păunescu, Horia Iovu. "Nanofiltration Membranes Characteristics" Encyclopedia, https://encyclopedia.pub/entry/46851 (accessed March 03, 2026).
Covaliu-Mierlă, C.I., Păunescu, O., & Iovu, H. (2023, July 17). Nanofiltration Membranes Characteristics. In Encyclopedia. https://encyclopedia.pub/entry/46851
Covaliu-Mierlă, Cristina Ileana, et al. "Nanofiltration Membranes Characteristics." Encyclopedia. Web. 17 July, 2023.
Copy Citation
The presence of heavy metal ions in polluted wastewater represents a serious threat to human health, making proper disposal extremely important. The utilization of nanofiltration (NF) membranes has emerged as one of the most effective methods of heavy metal ion removal from wastewater due to their efficient operation, adaptable design, and affordability. NF membranes created from advanced materials are becoming increasingly popular due to their ability to depollute wastewater in a variety of circumstances.
membranes
nanofiltration
heavy metal
removal
wastewater
1. Nanofiltration Membranes
Initially, nanofiltration (NF) was developed as an offshoot of reverse osmosis (RO) and ultrafiltration (UF); hence, it was initially called open RO or tight UF, depending on its application. The obtaining of Loeb-Sourirajan (L-S) asymmetric or anisotropic cellulose acetate membranes in the late 1950s for seawater desalination provided the foundation for the development of NF membranes, as well as pressure-driven membranes in the RO and UF sectors in the early 1960s [1]. These membranes served as the basis for the development of today’s membranes in the UF and RO sectors. Lately, an asymmetric UF was developed, supported by RO composites with a submicron coating on a selective layer. Advances in RO and UF technologies led to the emergence of a new field known as nanofiltration (NF), which was researched and developed for approximately 15 years beginning in 1960. In the 1970s, a range of cellulose acetate asymmetric membranes covering the whole spectrum from RO to UF were available [2]. Limitations of cellulose acetate as a membrane material were observed, and thus NF could not be widely applied [3]. Thus, cellulose acetate (Figure 1) has been replaced by other materials such as polyether sulfone (PES) (Figure 2), polysulfone (PSU) (Figure 3), chlorinated polyvinyl chloride (PVC) (Figure 4), polyamide (PA) (Figure 5), or polyvinylidene fluoride (PVDF) (Figure 6). Polymers, such as PVC, can be leached into the treated water due to continuous exposure to high pressure [4]. Even so, the NF membranes were not good enough to achieve the required selectivity/flux balance [5][6]. Composite membranes based on interfacial polymerization (IP) of UF supports having a submicron selective barrier were then developed [7]. Another alternative was the development of ceramic and inorganic NF membranes [8].
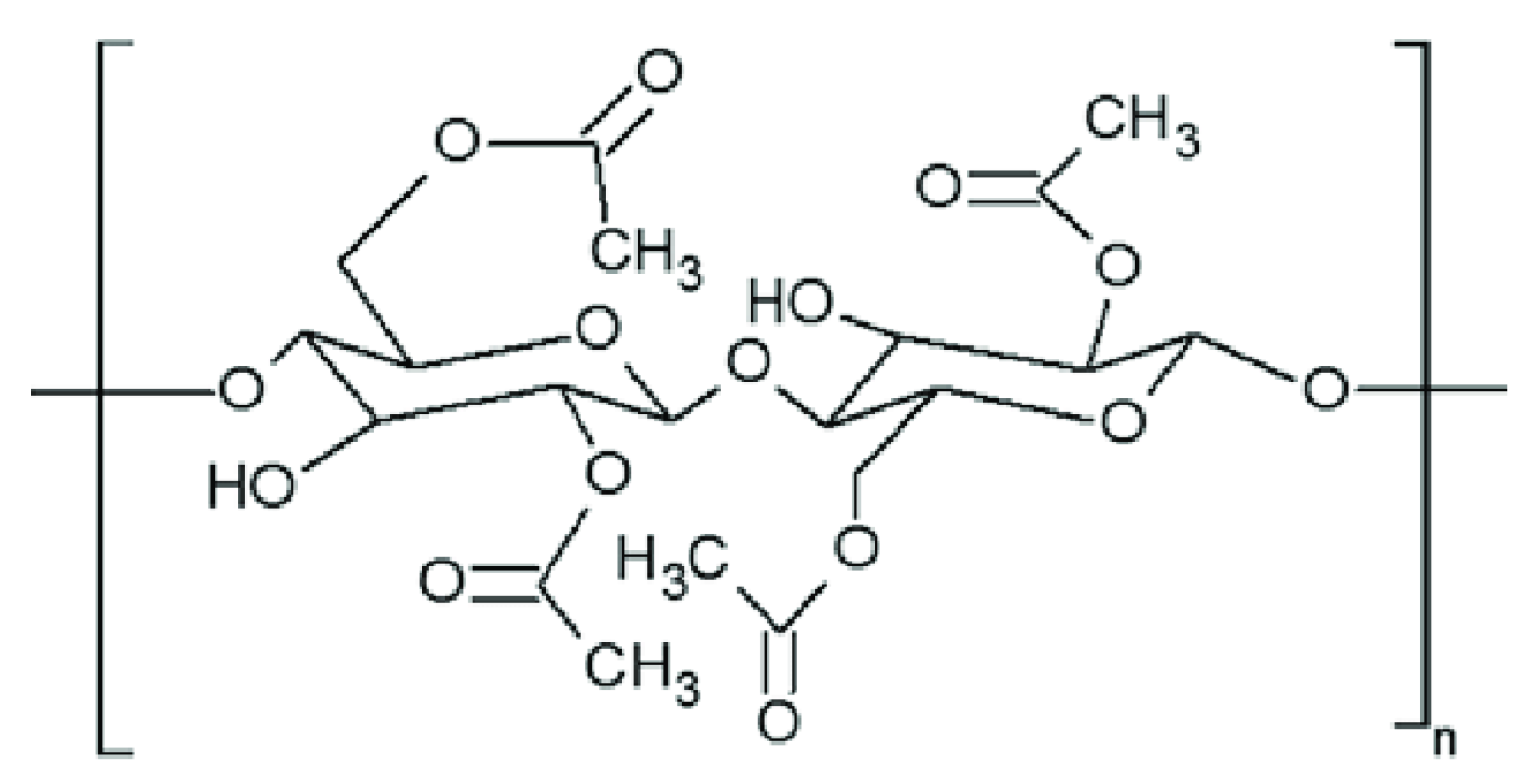
Figure 1. Cellulose acetate chemical structure.
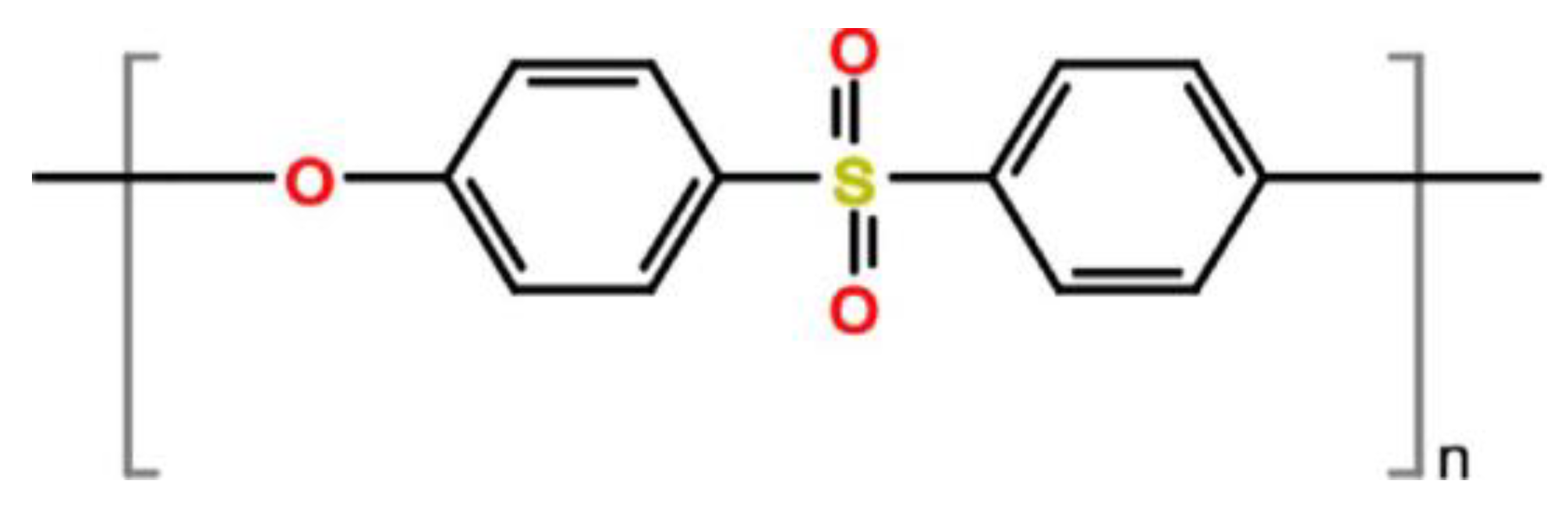
Figure 2. Polyether sulfone chemical structure.
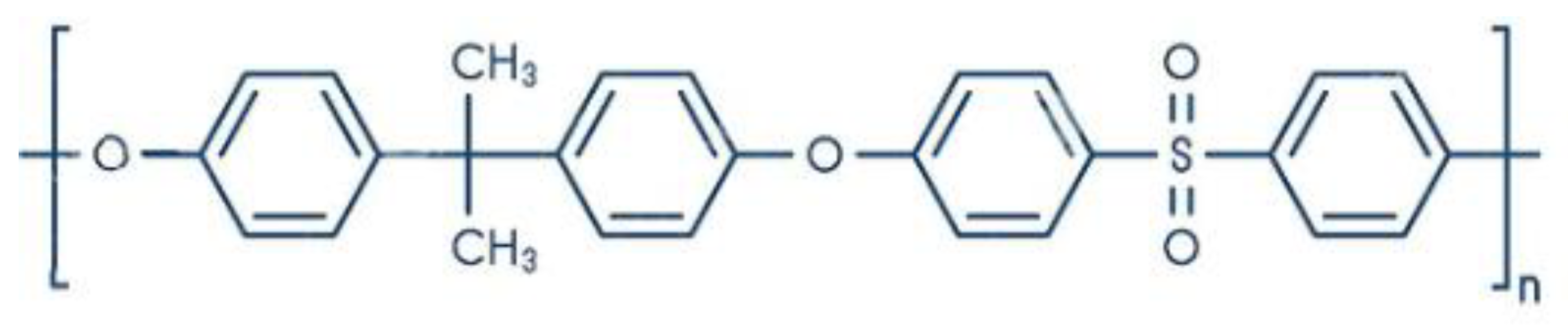
Figure 3. Polysulfone chemical structure.
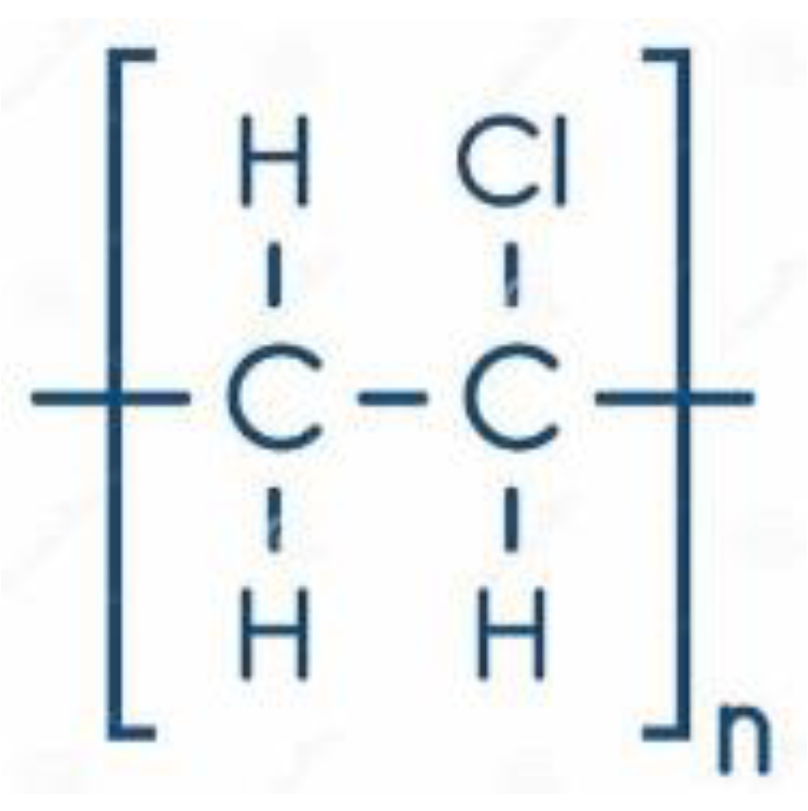
Figure 4. Polyvinyl chloride chemical structure.
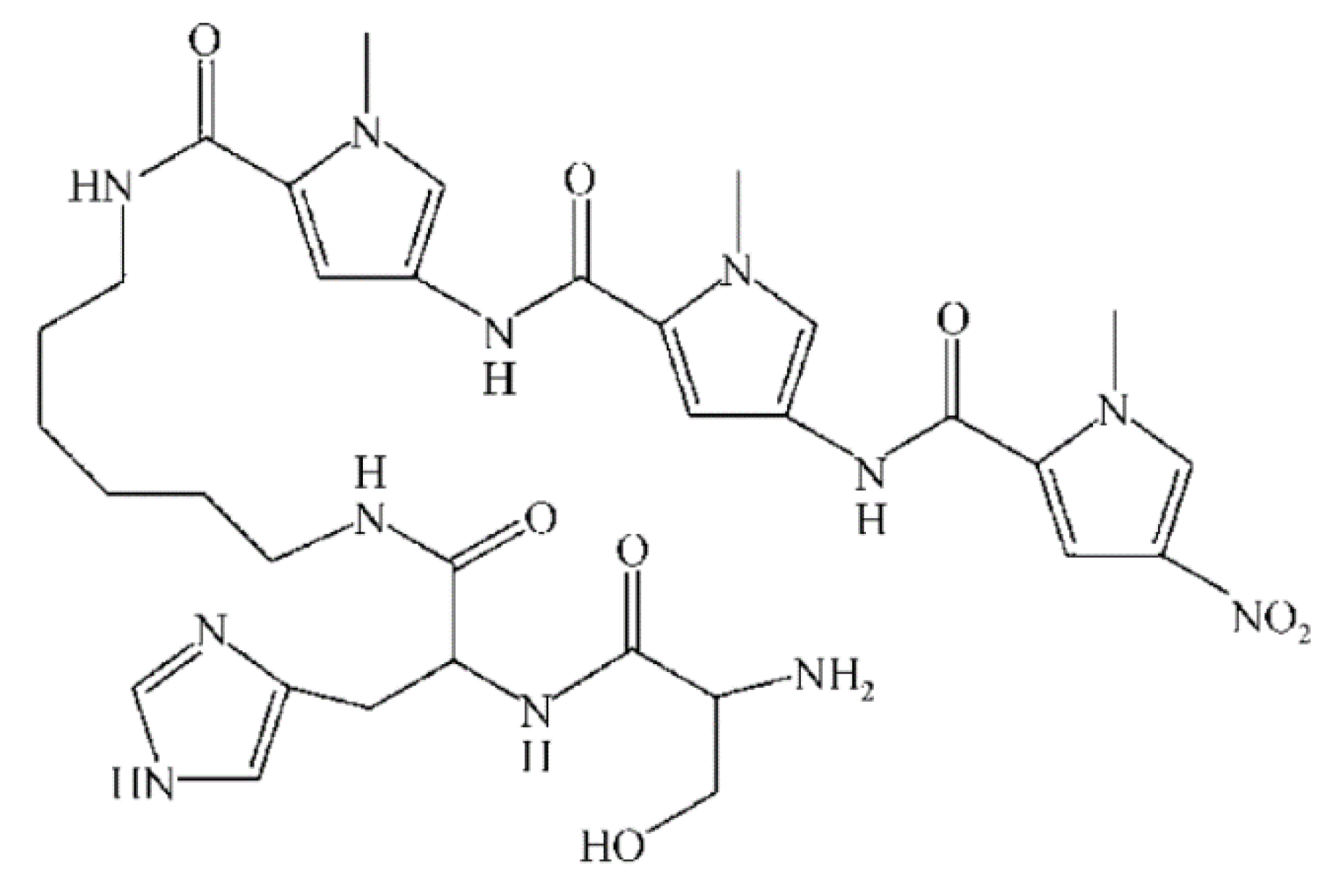
Figure 5. Polyamide chemical structure.
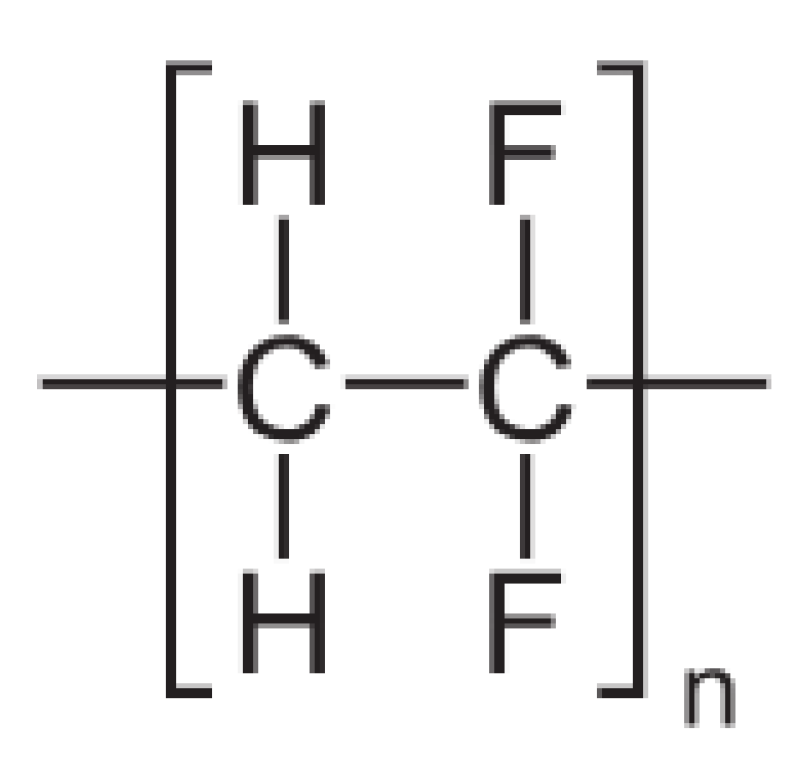
Figure 6. Polyvinylidene fluoride chemical structure.
NF membranes, which are commonly composed of three TFC layers, have a support layer on top that facilitates mass transportation. The second layer acts as a UF or MF membrane and supports the first layer. By this mechanism, the third active layer support layer controls the hydrophilicity, membrane charge, and surface features. Regenerated cellulose and polyvinyl alcohol are two common hydrophilic materials used to manufacture NF membranes [9], but other synthetic polymers have also gained popularity since the 2010s due to their suitability for specific applications [10]. However, membrane technology has some drawbacks, such as membrane fouling and high initial investment costs, which necessitate additional treatment procedures [11]. A major problem is also the membranes’ biofouling with bacteria and soluble microbial products. Biofouling can create significant problems in terms of removal efficiency during filtration and flux [12]. To enhance the performance of NF membranes, various methods such as plasma and chemical treatment, UV radiation, additive blending, grafting, crosslinking, and adsorbed coatings are used to modify the membrane surface. Cross-linking with hydroxyl compounds, for example, is used to improve membrane stability, increase the hydrophilicity of PA surfaces, and decrease molecular cut-off [13]. Recently, the development of positively charged NF membranes to remove heavy metals using the IP approach has attracted significant attention. Studies have demonstrated that the addition of nanoparticles and the creation of interlayers and surfactants can enhance the permeation flux of NF membranes [14]. The timeline for the discovery and use of nanofiltration membranes is shown in Figure 7.
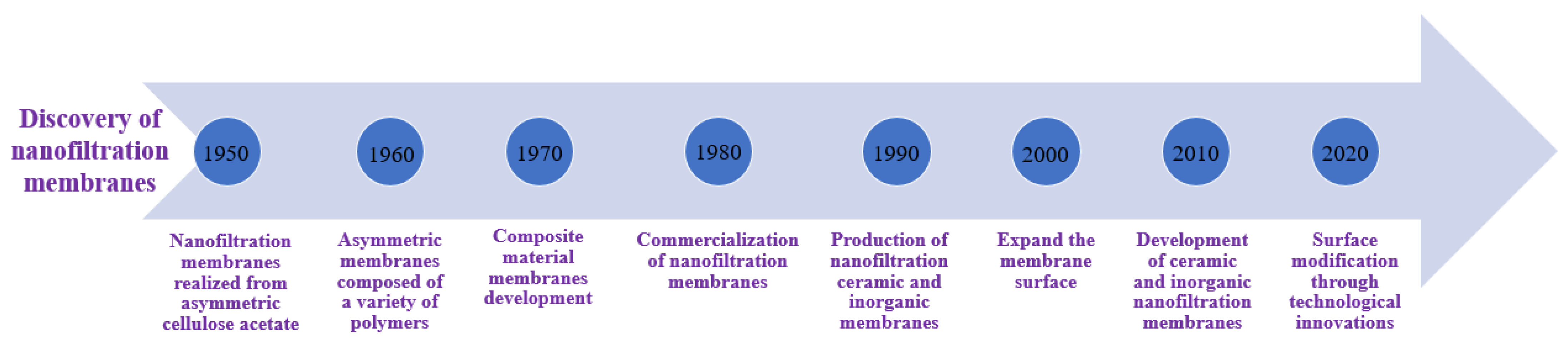
Figure 7. Timeline representing the use of membranes over time since their discovery in 1950.
Commercially available NF membranes are known for their pore size of approximately 1 nm. They have a molecular weight cutoff between 300 and 500 Da. While NF membranes exhibit low salt rejection (10–30%) for monovalent salts (e.g., NaCl), they exhibit high salt rejection (80–100%) for divalent salts (e.g., Na2SO4). These inherent characteristics distinguish NF membranes from RO membranes, giving NF membranes superior selectivity for various classes of ions and small molecules. As a result, NF membranes find extensive utility in specialized applications in various industries, including water and wastewater treatment, biotechnology, food engineering, and pharmaceuticals [15].
2. Characteristics
2.1. Hydrophilicity
To evaluate a membrane’s hydrophilicity, the water contact angle is utilized. Increasing a membrane’s hydrophilicity can enhance its permeability and antifouling properties. Although most solids possess natural roughness, this roughness is usually not enough to maintain a superhydrophilic state on the material’s surface. In theory, any natural or artificial substance can be chemically processed or mechanically roughened to create a super hydrophilic surface, or it can be broken down into sub-microscopic particles and stored to create a super hydrophilic coating. Titanium dioxide (TiO2) and zinc oxide (ZnO) are two inorganic substances that are commonly used because of their photoinduced self-cleaning capacities [16]. Silicon dioxide (SiO2) is extensively studied for its low cost and hydrophilicity. Various processes, such as electron beam, X-ray or ion surface irradiation, microwave treatment, and plasma, can be used to modify the surface chemistry of a polymer and increase its hydrophilicity. In order for a polymer to become super hydrophilic, the treatment must affect surface roughness or be applied simultaneously with surface roughening [17]. Increasing the surface roughness will decrease the strength of the NF membrane due to the thinning of the outer separation layer and, at the same time, the NF membrane [18]. Various coating techniques have been employed to modify the surface wetting, including dip coating, sol-gel, thermal, layer-by-layer assembly, electrospinning, electrodeposition, ion beam irradiation, femtosecond laser irradiation, spin coating, plasma irradiation, chemical vapor deposition, and spray coating. The production of super hydrophilic surfaces typically involves the use of low surface energy materials, surface roughness, or a combination of both. The membrane’s performance depends on properties such as surface energy, pore size, and wettability [17].
In recent years, interest has been growing in coatings that exhibit switchable wetting properties. Some coatings have been developed that can transition between superhydrophobic and superhydrophilic states, such as those produced using the sol-gel technique [19]. Graft polymerization has emerged as a practical alternative for improving the polymeric membranes’ hydrophilicity and enhancing their antifouling properties [20]. This technique involves attaching hydrophilic polymer chains to the surface of the membrane and offers advantages such as long-term hydrophilicity maintenance and high water flux [17].
2.2. Permeability and Selectivity
The energy consumption and effectiveness of NF processes are determined by the selectivity of NF membranes, which is largely influenced by pore size distribution and Donnan effects. A narrow pore-size distribution can play a crucial role in obtaining high efficiency of selective solute separation, while the steric resistance and charge interactions of NF membranes determine their selective properties. Manipulating the membrane surface charge can increase selectivity, specifically for charged solutes. Membrane fouling, which causes the membrane pore diameters to become smaller, can decrease the water flux through NF membranes during operation. Heavy metals can have a significant impact on membrane fouling by altering sludge properties or causing inorganic fouling [21]. Inorganic fouling by heavy metal compounds can be permanent, requiring cleaning with acids like citric acid [22]. Researchers have made various attempts to improve membrane selectivity by creating NF membranes with uniform pore diameters or modifying the charges on or in the selective layer or hydrophilicity, with some success in minimizing the fouling of membranes [23]. Increasing the selectivity of NF membranes can lead to improved membrane permeance while maintaining high rejection of ions/molecules. One approach for enhancing NF membrane selectivity is by incorporating nanofillers into the polymer matrix, creating new molecular transport channels. Metal-organic frameworks and covalent organic frameworks are promising materials for increasing membrane selectivity due to their high surface area, controllable pore structure, strong thermochemical stability, and functionalized pore walls. Metal-organic frameworks and covalent organic frameworks can tailor their pore shape and size and chemical design versatility through post-functionalization or by combining their ligands. Additionally, their cavities can be customized for specific applications and can facilitate beneficial interactions with polymers [24].
2.3. Surface Morphology
The effectiveness of membrane filtration in heavy metal removal depends heavily on the surface coating and morphology of the membrane, as they can impact both fouling and anti-fouling performance. The surface topography of a membrane, which includes its roughness, lay, waviness, and flow, can be affected by a variety of factors such as vibrations, manufacturing processes, work deflections, stresses, and the material’s internal structure [25].
The effect of surface roughness on membrane performance remains a significant challenge. It was observed that a commercial thin film composite membrane fouled more quickly than a cellulose acetate membrane, attributing the effect to the thin film composite membrane’s rougher surface [26]. It was demonstrated that, for certain NF membranes, fouling is closely related to surface roughness [27], as colloidal particles tend to accumulate in the valleys of a rough membrane surface due to increased interactions between them. This obstructs the valleys and leads to increased fouling on rougher surfaces [28]. Thus, the effect of surface roughness on membrane performance remains a complex issue. However, different results have been observed for organic particles [29][30]. Adhesive forces, which refer to the interaction between the membrane surface and the organic particles, are thought to be crucial factors in fouling [31]. In the fouling process, particles directly contact the membrane surface, and the interaction between the membrane surface and the organic particles determines the extent of fouling. On the other hand, after the formation of the gel layer, the interaction between organic particles becomes very important. It is plausible that a smoother surface would present less adsorption for the organic molecules, while a more heterogeneous and rougher surface would have a greater surface area and be more effective at adsorption [32].
Chemical surface modification is a technique used to decrease surface roughness by allowing chemicals in the liquid phase to enter pores more effectively, providing a smoother surface [33]. Atomic layer deposition is an alternative method used to manage roughness. It is a process of self-limiting surface reaction that produces uniform thin coatings with flawless intactness and an atomic-scale-controllable thickness [34]. By adjusting the total number of atomic layer deposition cycles, the thickness of the thin film can be precisely controlled at the atomic scale during deposition [35][36]. Surface hydrophilicity can be achieved using atomic layer deposition of alumina [37][38]. TFC-PA (thin film composite—polyamide) membranes were treated with atomic layer deposition to enhance their hydrophilicity and anti-fouling ability [39]. Additionally, low-temperature plasma discharges offer a flexible and controllable method for homogenous surface treatments, allowing for a wide range of conceivable surface functionality and minimizing damage [40].
2.4. Surface Charge
NF membranes can acquire an electric charge through various mechanisms when they come in contact with an aqueous electrolyte solution. For example, potential mechanisms include the adsorption of ions from solutions, ionic surfactants, and charged macromolecules on surfaces; the adsorption of polyelectrolytes; and the separation of functional groups [41][42][43][44]. This process can occur on the membrane’s external surface as well as its internal pore surface. Because the system must maintain electroneutrality, the distribution of ions is affected by surface charges. Thus, the surface becomes charged, leading to the development of an electrical double layer and the neutralization of excess counterions present in the surrounding solution. In alkaline or neutral settings, NF membranes tend to be negatively charged, and in highly acidic settings, positively charged. The surface charge of the NF membrane is helpful for selectively intercepting multivalent ions. Due to the set negative charge of the polymer backbone (which usually contains sulfonic acid and carboxylic acid), commercially available NF membranes typically have a negative charge [41][42][43][44][45][46][47].
Negatively charged NF membranes have been found to have better retention for divalent or multivalent anions having the same charge as the membrane surface due to the steric hindrance and Donnan effect [48]. The retention of heavy metal cations is poor in commercially available NF membranes, with rejection rates reported to be as low as 12% for PbCl2 and up to 90% for CdCl2, depending on the membrane and conditions used [49]. A lower pH in the feed solution relative to the isoelectric point of the membrane can improve the retention of heavier metal cations. The selectivity of the membrane improves with greater charge density, and a positively charged surface on an NF membrane can facilitate the retention of divalent or multivalent cations because of electrostatic repulsion [50].
Three main methods have been developed for producing positively charged NF membranes, namely phase inversion, interfacial polymerization, and surface modification (which includes surface grafting, surface deposition, and surface cross-linking) [51]. Producing composite membranes that are positively charged using surface modification and interfacial polymerization often necessitates the use of harmful or carcinogenic chemicals, as well as many preparation stages [52]. As an alternative, integrally skinned asymmetric membranes can be created using a simpler cross-linking approach and phase inversion methodology [53]. By introducing nitrogen groups or quaternary amines to the surface and interior pores of the membrane, it is reported that positively charging the integrally skinned asymmetric membrane can be possible [54].
References
- Singh, R. Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification: Application, Systems Design and Operation, 2nd ed.; Butterworth-Heinemann: Colorado Springs, CO, USA, 2015; pp. 1–80.
- Linder, C.; Kedem, O. History of Nanofiltration Membranes from 1960 to 1990. In Nanofiltration: Principles, Applications, and New Materials, 2nd ed.; Schäefer, A.I., Fane, A.G., Eds.; WILEY-VCH GmbH: Weinheim, Germany, 2021; Chapter 1; pp. 1–34.
- Liu, L.; Yu, L.; Borjigin, B.; Liu, Q.; Zhao, C.; Hou, D. Fabrication of thin-film composite nanofiltration membranes with improved performance using β-cyclodextrin as monomer for efficient separation of dye/salt mixtures. Appl. Surf. Sci. 2021, 539, 148284.
- Zhu, J.; Yuan, S.; Wang, J.; Zhang, Y.; Tian, M.; Van der Bruggen, B. Microporous organic polymer-based membranes for ultrafast molecular separations. Prog. Polym. Sci. 2020, 110, 101308.
- Alenazi, N.A.; Hussein, M.A.; Alamry, K.A.; Asiri, A.M. Modified polyether-sulfone membrane: A mini review. Des. Monomers Polym. 2017, 20, 532–546.
- Qian, X.; Ostwal, M.; Asatekin, A.; Geise, G.M.; Smith, Z.P.; Phillip, W.A.; Lively, R.P.; McCutcheon, J.R. A critical review and commentary on recent progress of additive manufacturing and its impact on membrane technology. J. Membr. Sci. 2021, 645, 120041.
- Shen, L.; Cheng, R.; Yi, M.; Hung, W.S.; Japip, S.; Tian, L.; Zhang, X.; Jiang, S.; Li, S.; Wang, Y. Polyamide-based membranes with structural homogeneity for ultrafast molecular sieving. Nat. Commun. 2022, 13, 500.
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80.
- Bandehali, S.; Parvizian, F.; Ruan, H.; Moghadassi, A.; Shen, J.; Figoli, A.; Adeleye, A.S.; Hilal, N.; Matsuura, T.; Drioli, E.; et al. A planned review on designing of high performance nanocomposite nanofiltration membranes for pollutants removal from water. J. Ind. Eng. Chem. 2021, 101, 78–125.
- Oatley-Radcliffe, D.L.; Walters, M.; Ainscough, T.J.; Williams, P.M.; Mohammad, A.W.; Hilal, N. Nanofiltration membranes and processes: A review of research trends over the past decade. J. Water Process Eng. 2017, 19, 164–171.
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean Water 2021, 4, 36.
- Park, N.; Kwon, B.; Kim, I.S.; Cho, J. Biofouling potential of various NF membranes with respect to bacteria and their soluble microbial products (SMP): Characterizations, flux decline, and transport parameters. J. Membr. Sci. 2005, 258, 43–54.
- Farahbakhsh, J.; Vatanpour, V.; Khoshnam, M.; Zargar, M. Recent advancements in the application of new monomers and membrane modification techniques for the fabrication of thin film composite membranes: A review. React. Funct. Polym. 2021, 166, 105015.
- Fallahnejad, Z.; Bakeri, G.; Ismail, A.F. Overcoming the trade off between the permeation and rejection of TFN nanofiltration membranes through embedding magnetic inner surface functionalized nanotubes. Process Saf. Environ. Prot. 2022, 165, 815–840.
- Ahmad, N.N.R.; Ang, W.L.; Teow, Y.H.; Mohammad, A.W.; Hilal, N. Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: A review. J. Water Process Eng. 2022, 45, 102478.
- Rafique, M.S.; Tahir, M.B.; Rafique, M.; Shakil, M. Photocatalytic nanomaterials for air purification and self-cleaning. In Nanotechnology and Photocatalysis for Environmental Applications; Tahir, M.B., Rafique, M., Rafique, M.S., Eds.; In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–219.
- Samavati, Z.; Samavati, A.; Goh, P.S.; Ismail, A.F.; Abdullah, M.S. A comprehensive review of recent advances in nanofiltration membranes for heavy metal removal from wastewater. Chem. Eng. Res. Des. 2023, 189, 530–571.
- Maguire, N.A.P.; Ebrahimi, M.; Fan, R.; Gießelmann, S.; Ehlen, F.; Schütz, S.; Czermak, P. Influence of Ceramic Membrane Surface Characteristics on the Flux Behavior of a Complex Fermentation Broth. Membranes 2021, 11, 402.
- Gu, J.; Ji, L.; Xiao, P.; Zhang, C.; Li, J.; Yan, L.; Chen, T. Recent progress in superhydrophilic carbon-based composite membranes for oil/water emulsion separation. ACS Appl. Mater. Interfaces 2021, 13, 36679–36696.
- Pinem, J.A.; Wardani, A.K.; Aryanti, P.T.P.; Khoiruddin, K.; Wenten, I.G. Hydrophilic Modification of Polymeric Membrane using Graft Polymerization Method: A Mini Review. IOP Conf. Ser. Mater. Sci. Eng. 2019, 547, 012054.
- Jamil, T.S.; Mansor, E.S.; Abdallah, H.; Shaban, A.M.; Souaya, E.R. Novel anti fouling mixed matrix CeO2/Ce7O12 nanofiltration membranes for heavy metal uptake. J. Environ. Chem. Eng. 2018, 6, 3273–3282.
- Agnihotri, B.; Sharma, A.; Gupta, A.B. Characterization and analysis of inorganic foulants in RO membranes for groundwater treatment. Desalination 2020, 491, 114567.
- Mkpuma, V.O.; Moheimani, N.R.; Fischer, K.; Schulze, A.; Ennaceri, H. Membrane surface zwitterionization for an efficient microalgal harvesting: A review. Algal Res. 2022, 66, 102797.
- Guo, C.; Duan, F.; Zhang, S.; He, L.; Wang, M.; Chen, J.; Zhang, J.; Jia, Q.; Zhang, Z.; Du, M. Heterostructured hybrids of metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs). J. Mater. Chem. A 2022, 10, 475–507.
- Pakizeh, M.; May, P.; Matthias, M.; Ulbricht, M. Preparation and characterization of polyzwitterionic hydrogel coated polyamide-based mixed matrix membrane for heavy metal ions removal. J. Appl. Polym. Sci. 2020, 137, 49595.
- Elimelech, M.; Zhu, X.; Childress, A.E.; Hong, S. Role of membrane surface morphology in colloidal fouling of cellulose acetate and composite aromatic polyamide reverse osmosis membranes. J. Membr. Sci. 1997, 127, 101–109.
- Vrijenhoek, E.M.; Hong, S.; Elimelech, M. Influence of membrane surface properties on initial rate of colloidal fouling of reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2001, 188, 115–128.
- Zhang, W.; Jiang, F. Membrane fouling in aerobic granular sludge (AGS)-membrane bioreactor (MBR): Effect of AGS size. Water Res. 2019, 157, 445–453.
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167.
- Li, Y.S.; Yan, L.; Xiang, C.B.; Hong, L.J. Treatment of oily wastewater by organic–inorganic composite tubular ultrafiltration (UF) membranes. Desalination 2006, 196, 76–83.
- Huisman, I.H.; Prádanos, P.; Hernández, A. The effect of protein–protein and protein–membrane interactions on membrane fouling in ultrafiltration. J. Membr. Sci. 2000, 179, 79–90.
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471.
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface modification of polymers: Methods and applications. Adv. Mater. Interfaces 2018, 5, 1801247.
- Lee, J.; Kim, I.S.; Hwang, M.H.; Chae, K.J. Atomic layer deposition and electrospinning as membrane surface engineering methods for water treatment: A short review. Environ. Sci. Water Res. Technol. 2020, 6, 1765–1785.
- Yasmeen, S.; Ryu, S.W.; Lee, S.H.; Lee, H.B.R. Atomic layer deposition beyond thin film deposition technology. Adv. Mater. Technol. 2022, 2200876.
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Progress in powder coating technology using atomic layer deposition. Adv. Mater. Interfaces 2018, 5, 1800581.
- Hyde, G.K.; Scarel, G.; Spagnola, J.C.; Peng, Q.; Lee, K.; Gong, B.; Roberts, K.G.; Roth, K.M.; Hanson, C.A.; Devine, C.K.; et al. Atomic layer deposition and abrupt wetting transitions on nonwoven polypropylene and woven cotton fabrics. Langmuir 2010, 26, 2550–2558.
- Waldman, R.Z.; Yang, H.C.; Mandia, D.J.; Nealey, P.F.; Elam, J.W.; Darling, S.B. Janus membranes via diffusion-controlled atomic layer deposition. Adv. Mater. Interfaces 2018, 5, 1800658.
- Nikkola, J.; Sievänen, J.; Raulio, M.; Wei, J.; Vuorinen, J.; Tang, C.Y. Surface modification of thin film composite polyamide membrane using atomic layer deposition method. J. Membr. Sci. 2014, 450, 174–180.
- Ataeefard, M.; Moradian, S.; Mirabedini, M.; Ebrahimi, M.; Asiaban, S. Surface properties of low density polyethylene upon low-temperature plasma treatment with various gases. Plasma Chem. Plasma Process. 2008, 28, 377–390.
- Teixeira, M.R.; Rosa, M.J.; Nystrom, M. The role of membrane charge on nanofiltration performance. J. Membr. Sci. 2005, 265, 160–166.
- Zhang, Y.; Hudson-Smith, N.V.; Frand, S.D.; Cahill, M.S.; Davis, L.S.; Feng, Z.V.; Haynes, C.L.; Hamers, R.J. Influence of the spatial distribution of cationic functional groups at nanoparticle surfaces on bacterial viability and membrane interactions. J. Am. Chem. Soc. 2020, 142, 10814–10823.
- DuChanois, R.M.; Epsztein, R.; Trivedi, J.A.; Elimelech, M. Controlling pore structure of polyelectrolyte multilayer nanofiltration membranes by tuning polyelectrolyte-salt interactions. J. Membr. Sci. 2019, 581, 413–420.
- Dickhout, J.M.; Virga, E.; Lammertink, R.G.; de Vos, W.M. Surfactant specific ionic strength effects on membrane fouling during produced water treatment. J. Colloid Interface Sci. 2019, 556, 12–23.
- Nafi, A.W.; Taseidifar, M. Removal of hazardous ions from aqueous solutions: Current methods, with a focus on green ion flotation. J. Environ. Manag. 2022, 319, 115666.
- Jeon, M.Y.; Yoo, S.H.; Kim, C.K. Performance of negatively charged nanofiltration membranes prepared from mixtures of various dimethacrylates and methacrylic acid. J. Membr. Sci. 2008, 313, 242–249.
- Tanninen, J.; Platt, S.; Weis, A.; Nyström, M. Long-term acid resistance and selectivity of NF membranes in very acidic conditions. J. Membr. Sci. 2004, 240, 11–18.
- Azizi, N.; Goudarzi, S.; Eslami, R.; Zarrin, H. Polymer-based bioinspired, biomimetic, and stimuli-responsive nanofiltration membranes. In Advancement in Polymer-Based Membranes for Water Remediation; Elsevier: Toronto, ON, Canada, 2022; pp. 237–271.
- Al-Rashdi, B.A.M.; Johnson, D.J.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17.
- Wang, C.; Chen, Y.; Hu, X.; Guo, P. Engineering novel high flux thin-film composite (TFC) hollow fiber nanofiltration membranes via a facile and scalable coating procedure. Desalination 2022, 526, 115531.
- Yadav, D.; Karki, S.; Ingole, P.G. Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. J. Environ. Chem. Eng. 2022, 10, 108109.
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. Int. J. Biol. Macromol. 2022, 206, 954–976.
- Tashvigh, A.A.; Feng, Y.; Weber, M.; Maletzko, C.; Chung, T.S. 110th anniversary: Selection of cross-linkers and crosslinking procedures for the fabrication of solvent-resistant nanofiltration membranes: A review. Ind. Eng. Chem. Res. 2019, 58, 10678–10691.
- Nozad, E.; Marjani, A.P.; Mahmoudian, M. A novel and facile semi-IPN system in fabrication of solvent resistant nanofiltration membranes for effective separation of dye contamination in water and organic solvents. Sep. Purif. Technol. 2022, 282, 120121.
More
Information
Subjects:
Environmental Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
17 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





