Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daria A. Kondratieva | -- | 7020 | 2023-07-13 13:27:04 | | | |
| 2 | Rita Xu | -3 word(s) | 7017 | 2023-07-14 03:21:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shleeva, M.O.; Kondratieva, D.A.; Kaprelyants, A.S. Bacillus licheniformis. Encyclopedia. Available online: https://encyclopedia.pub/entry/46763 (accessed on 14 January 2026).
Shleeva MO, Kondratieva DA, Kaprelyants AS. Bacillus licheniformis. Encyclopedia. Available at: https://encyclopedia.pub/entry/46763. Accessed January 14, 2026.
Shleeva, Margarita O., Daria A. Kondratieva, Arseny S. Kaprelyants. "Bacillus licheniformis" Encyclopedia, https://encyclopedia.pub/entry/46763 (accessed January 14, 2026).
Shleeva, M.O., Kondratieva, D.A., & Kaprelyants, A.S. (2023, July 13). Bacillus licheniformis. In Encyclopedia. https://encyclopedia.pub/entry/46763
Shleeva, Margarita O., et al. "Bacillus licheniformis." Encyclopedia. Web. 13 July, 2023.
Copy Citation
Bacillus licheniformis produces several classes of antimicrobial substances, including bacteriocins, which are peptides or proteins with different structural composition and molecular mass: ribosomally synthesized by bacteria (1.4–20 kDa), non-ribosomally synthesized peptides and cyclic lipopeptides (0.8–42 kDa) and exopolysaccharides (>1000 kDa). Different bacteriocins act against Gram-positive or Gram-negative bacteria, fungal pathogens and amoeba cells.
Bacillus licheniformis
Mycobacterium tuberculosis
bacteriocin
1. Introduction
The spread of bacterial strains that cause severe infectious diseases but are now resistant to known antibiotics necessitates the search for and development of new approaches to combat these diseases [1]. The most known and medically important example that illustrates this problem is the growing number of cases of multidrug-resistant strains of Mycobacterium tuberculosis (Mtb), which is the causative agent of tuberculosis. In addition to drug resistance, Mtb is able to asymptomatically persist in the host organism for many years, causing latent forms of tuberculosis. In this dormant state, Mtb cells are also resistant to known antibiotics [2][3][4].
The search for and study of substances that have bactericidal or bacteriostatic properties against human and animal pathogens are also required for the development of new antibiotic therapy or disinfectants for objects and surfaces that have been in close contact with patients and therefore may carry pathogenic bacteria. Currently, in addition to the synthesis of new chemical substances, considerable attention has focused on the analysis of the potential of natural products from different origins as antimicrobials. The discovery of antibiotics with activity against human pathogens is often based on the observation of the interaction between microorganisms, called antagonism. This antagonism manifests through the synthesis and release of substances that inhibit or completely suppress the growth of other species. Under natural conditions, a microorganism-secreted substance(s) that inhibits the growth of another organism gains a competitive advantage in the struggle for environmental resources. Most of the antibiotics used for medical applications are secreted products or derivatives of microorganisms belonging to the order Actinomycetalis (among them, the most well-known are Streptomyces). The bacterial world represents a huge reservoir of not-yet-discovered and used substances that have antibacterial potential. In this regard, representatives of the genus Bacillus are known as producers of many enzymes and antimicrobial compounds. For example, Bacillus amyloliquefaciens is a source of the natural antibiotic barnase (ribonuclease), alpha-amylase, which is used in starch hydrolysis; protease subtilisin, which is used in combination with detergents; and the restriction enzyme, BamH1, used in DNA research [5]. Bacillus subtilis produces 66 derived antimicrobials, and Bacillus brevis produces 23 peptide antibiotics [6]. There is a growing interest in considering these substances, including bacteriocins, as alternative antimicrobials for the treatment of human and animal infections [7][8][9][10][11].
Currently, the use of bacterial probiotic strains and their metabolic products is considered a new approach for the control and prevention of various infectious diseases [12]. Animal studies have demonstrated that probiotics from the Bacillus genus have antimicrobial properties. This conclusion also applies to humans [13][14]. The use of bacteriocins and antimicrobial peptides produced by probiotic strains is a suitable alternative to antibiotics because their production is inexpensive and resistance to them is rare [15]. They exhibit a broad spectrum of activity against many Gram-positive and Gram-negative bacteria and fungi. Owing to the efficacy and cost-effectiveness of many of these compounds, they are attractive for clinical use [16]. A few natural peptides have shown potential because of their desirable therapeutic properties, including antimicrobial, antiviral, anticancer, and contraceptive activities. Additionally, they have been shown to protect against topical and systemic infections in combination with conventional antibiotics [17].
Among the organisms belonging to the Bacillus genus, Bacillus licheniformis is a unique specie that which produces wide variety of antimicrobial substances. This bacterium shows promise for use as a probiotic in the treatment of dysbacteriosis, which is caused by various diseases [13]. The effectiveness of B. licheniformis as a probiotic is associated with its ability to produce a large amount of substances with antimicrobial, antioxidant and immunomodulatory activities [13], for example, a phosphorus-containing triene antibiotic called proticin [18][19]. B. licheniformis shows a protective effect in zebrafish (Danio rerio) against Vibrio parahaemolyticus infection. Due to the antagonistic activity of this probiotic, the complete survival of infected fish was observed in contrast with untreated fish [20]. This probiotic, in combination with Bifidobacterium breve, significantly inhibited the adhesion of the pathogen Kocuria rhizophila in vitro [21] and showed antivibrio activity against Vibrio parahaemolyticus [22]. The use of a crude extract from B. licheniformis resulted in marked antiviral activity against porcine epidemic diarrhea virus in Vero cells and reduced virus shedding in piglets [23]. After the administration of fermented B. licheniformis products, the number of pathogenic bacteria including Clostridium perfringens significantly decreased in cats with chronic diarrhea [24]. In piglets, B. licheniformis treatment showed positive effects against Salmonella [25]. Probiotic B. licheniformis produces antimicrobial substances and has a strong ability to auto- and coaggregate against pathogenic bacteria [26]. Approaches are being developed to combat bacterial biofilms using silver nanoparticles and the probiotic B. licheniformis [27].
The bacteriocins from B. licheniformis are being considered as natural preparations as a preservative in the food industry [28][29].
In general, bacteriocins are a group of antimicrobial peptides that represent a potential alternative to classical antibiotics in the fight against antimicrobial resistance in pathogenic microorganisms. Several reports have been published in the literature about numerous bacteriocins, many of which currently remain undiscovered due to the wide variety of their natural sources; hence, further research in this area is required [11].
Considering the medical and industrial application of Bacillus licheniformis, a thorough description and characterization of the variety of antimicrobial compounds produced and their use against resistant pathogens, such as mycobacteria, are required.
2. Antibacterial Substances Secreted by Bacillus licheniformis
The endospore-forming bacterium Bacillus licheniformis is capable of producing a large amount of substances with different structures and different antibacterial activities [30] (Figure 1). When grown on identical media, different strains of B. licheniformis produce different sets of substances with antibacterial activities [31]. It is plausible that all strains of B. licheniformis potentially are able to produce variety of antimicrobial substances, however, the synthesis and production of particular substance can be differently regulated on the transcriptional or translational level for certain strains grown in identical media. As a result, the amount of secreted antimicrobial compounds may substantially vary from strain to strain allowing to consider them as unique producers of defined set of antimicrobials. The secreted antimicrobial substances have molecular masses ranging from 1.4 to 20 kDa [28][29][32][33][34][35][36][37][38].
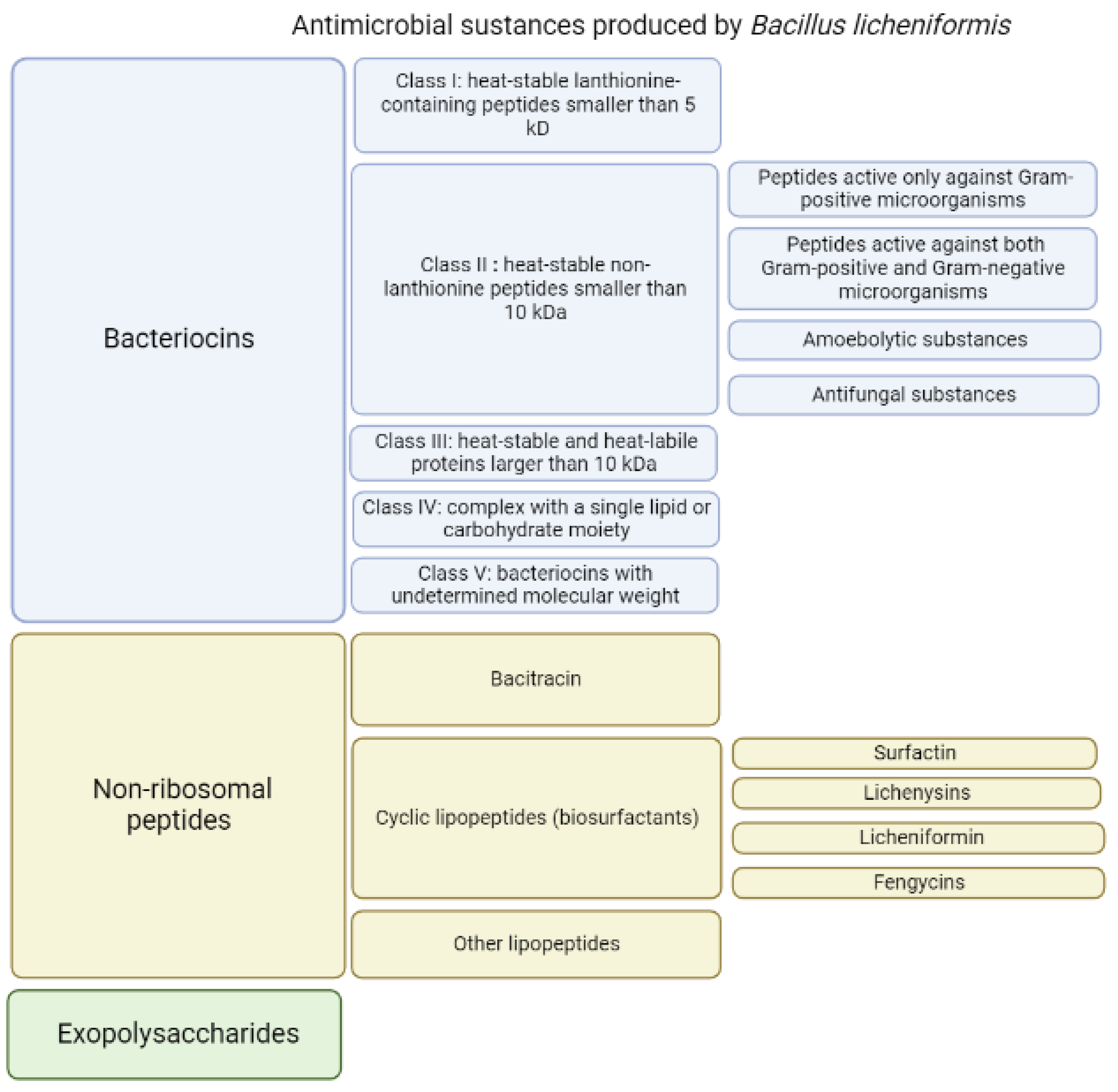
Figure 1. Antibacterial substances produced by Bacillus licheniformis.
Changing the composition of the medium used for B. licheniformis growth results in the alterations in the secreted substances. Thus, on media containing iron, B. licheniformis can synthesize the red pigment pulcherrimin [39]. When grown on a medium with lactate and a high ratio of nitrogen and carbon, B. licheniformis strain N.C.T.C. 7072 produces licheniformins; when grown on a medium with glucose and a low nitrogen/carbon ratio, this strain produces bacitracins [37]. Several substances synthesized by B. licheniformis have been investigated as antibiotics against various types of bacteria. They are listed and characterized below. Some of them (bacitracin) are used in combined antibacterial preparations intended for topical use. Others are used as oral antibiotics, but only in animals due to their toxic effects.
Among the antimicrobial components (Figure 1) produced by various strains of B. licheniformis in a nutrient medium, several groups differ in properties and structure.
2.1. Bacteriocins
Bacteriocins are substances represented by an amino acid sequence (peptides or proteins) that act against other strains of bacteria or closely related species. They demonstrate both bactericidal and bacteriostatic effects. Bacteriocins are natural antimicrobial peptides that are ribosomally synthesized by bacteria [10][11][40]. Genes whose expression leads to the synthesis of bacteriocins are organized into clusters of operons and can be located in the genome, plasmids, or other mobile genetic elements. These genes are inducible; peptide secretion and accumulation outside the cell are required for their induction. More details regarding bacteriocins biosynthesis are described in the review by Nishie et al. [9]. Bacteriocins are heterogeneous substances that demonstrate various biochemical properties, molecular weights, inhibitory spectra and mechanisms of action [10][41]. Due to the wide spectrum of antagonistic activity inherent to the bacteriocins of some strains of microorganisms, they have the potential to be used as a component of antibacterial drugs. The resistance to enzyme activity of the antimicrobial peptides produced by Bacillus spp. differs with stability over a wide range of pH and temperature. Most of these peptides have high specificity against microbial pathogens and low cytotoxicity against human cells [42]. The related bactericidal mechanisms include the pore-forming type, nuclease type with DNase and RNase functions, and the peptidoglycanase type [10] (Figure 2).
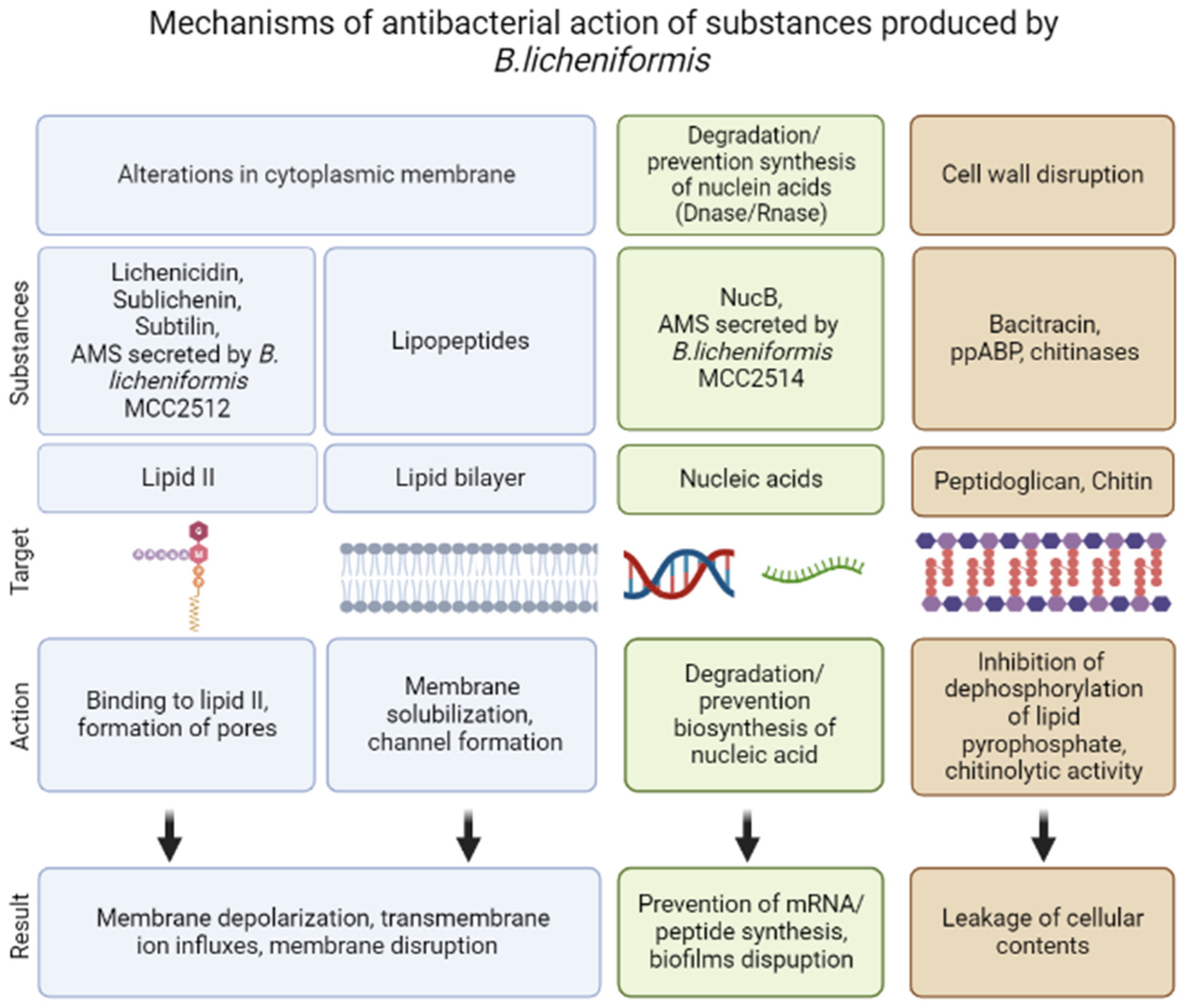
Figure 2. Mechanisms of antibacterial action of substances produced by Bacillus licheniformis.
As a result, pores are formed, which leads to the rapid removal of small cytoplasmic molecules, ions from target cells, and the collapse of the protonmotive force, leading to the death of bacterial cells [9][43]. However, other antimicrobial mechanisms of bacteriocins were also identified [11]. Despite the recent popularity of research on the properties of bacteriocins and their use in medicine, veterinary sciences, and food industry [10][11], many bacteriocins have not yet been studied.
The production of several bacteriocin-like substances with different characteristics and a wide spectrum of activity against pathogenic bacteria was recorded in the strains of B. licheniformis [44]. For example, B. licheniformis SMIA-2, a thermophilic and thermostable enzyme-producing strain, is active against some strains of Staphylococcus aureus and Bacillus sp. The genome annotation of this strain detected gene clusters responsible for antimicrobial component production (lichenysin, fengycin, lichenicidin and bacillibactin biosynthetic gene clusters) [45].
B. licheniformis produces various bacteriocins ranging in molecular weight from 1.4 to 55 kDa, but the expression of a particular antimicrobial agent may depend on environmental conditions, growth period, and the specific bacterial strain [28][32][33][36][46].
In general, based on their thermostability, size and chemical moieties, bacteriocins are classified into four major groups [47]: class I, heat-stable lanthionine-containing peptides smaller than 5 kDa; class II, heat-stable non-lanthionine peptides smaller than 10 kDa; class III, heat-labile proteins larger than 30 kDa; and class IV, complex with a single lipid or carbohydrate moiety [48]. To describe the antimicrobial substances produced by different strains of B. licheniformis, Cotter’s classification is used, with a slight modification: heat-stable and heat-labile proteins larger than 10 kDa are assigned to class III, and class V was added, which includes proteins with undetermined molecular weight (Figure 1).
2.1.1. Class I: Heat-Stable Lanthionine-Containing Peptides Smaller Than 5 kDa
Lantibiotics are antimicrobial peptides that undergo post-translational modification. They contain non-standard amino acids: lanthionine, β-methyl lanthionine and dehydrated residues (dehydrated amino acids) [49]. Their molecular weight does not exceed 5 kDa. Lantibiotics are active at low concentrations and are therefore attractive antimicrobials. They mainly target lipid II. A number of lantibiotics interact with the cell wall precursor lipid II (undecaprenyl-pyrophosphoryl-MurNAc-(pentapeptide)-GlcNAc), which prevents cell wall biosynthesis and contributes to the destruction of the bacterial membrane [50]. Thus, the most well-studied lantibiotic nisin interacts with the pyrophosphate fragment of lipid II. Critical to this binding are the two N-terminal rings of the lantibiotic [51]. The formation of the pore complex results in cell membrane permeabilization and dissipation of the proton motive force [50].
In general, lantibiotics are synthesized and secreted by Gram-positive microorganisms, and their activity is most often manifested in connection with closely related Gram-positive bacteria. For Gram-negative bacteria, their activity is rather limited [49] because the cell wall of Gram-negative bacteria is an effective permeability barrier due to the presence of an outer membrane, which prevents access to the peptidoglycan layer (localization of lipid II) and the cytoplasmic membrane (Figure 2). Moreover, the anionic cell surface of Gram-negative bacteria promotes the binding of cationic lantibiotics, where such an interaction potentially increases the stability of the outer membrane through electrostatic interactions [52].
The first one is sublichenin, which is a subtilin-like lantibiotic of probiotic bacterium Bacillus licheniformis MCC 2512 that has a molecular weight of 3.348 kDa and the succinylated form has a molecular weight of 3.448 kDa [31][53]. Subtilin is a natural variant of nisin that refers to linear pentacyclic class I antibiotics [54]. The sublichenins from B. licheniformis are almost identical to the JS-4 subtilin from B. subtilis. Subtilin JS-4 retained >90% and 86.1% of its antibacterial activity even after a 30 min exposure to 80–100 °C and 121 °C, respectively, indicating considerable thermostability. Subtilin JS-4 was also rapidly inactivated by proteolytic enzymes including proteinase K, trypsin, papain and pepsin [55]. It also showed a broad antimicrobial spectrum against Gram-positive bacteria. Subtilin JS-4 inhibited the growth of foodborne bacterium L. monocytogenes by increasing cell membrane permeability, triggering pore formation and K+ leakage, and damaging cell membrane integrity, which eventually disrupted the membrane and cellular structure [55] (Figure 2).
The second antibiotic is lichenicidin, a dipeptide lantibiotic consisting of a synergetic lantibiotic pair, Licα (3.251kDa) and Licβ (3.021 kDa), which was described for B. licheniformis DSM 13. This substance demonstrated activity against the growth of Gram-positive bacteria, such as Bacillus subtilis, Micrococcus luteus, Staphylococcus aureus, Streptococcus pyogenes, Staphylococcus simulans and enterococci but neither caused hemolysis nor inhibited the growth of Gram-negative bacteria. Lichenicidin is associated with the cell surface and shows stability against trypsin, chymotrypsin, and proteases [56]. Moreover, lichenicidin can be produced by other strains of B. licheniformis, and the structure of its peptides may differ depending on the strain. Lichenicidin was not cytotoxic to human erythrocytes and fibroblasts [57]. B licheniformis strain ATCC 14580 produced lichenicidin with activity against a range of pathogenic microorganisms including Listeria monocytogenes, Staphylococcus aureus, vancomycin-resistant enterococci, Bacillus cereus, Streptococcus pneumoniae and Streptococcus mutants [58]. Lichenicidin has been produced by B. licheniformis strain VK21 [59], and WIT 562, 564 and 566 [60]. Additionally, lichenicidin production was found in B. licheniformis isolates (isolated from retail infant milk formulae): strains IMF20, IMF66, IMF69 and IMF80. These strains demonstrated antimicrobial activity against target Gram-positive organisms. No activity was observed against the Gram-negative bacteria E. coli or S. typhimurium [61]. For Lchα and Lchβ, lichenicidin subunits produced by VK21 strain, described tertiary structures and details of mode of action [59].
Another variant of lichenicidin comprises two mature peptides, Bliα and Bliβ, produced by the I89 strain; their synergistic activity is required for full activity [62][63].
Lichenicidin acts through a dual mode of action that involves the α subunit recognition of lipid II, providing specificity and stability for the interaction of β subunit, which induces leakage of the intracellular contents of bacteria [64][65] (Figure 2).
2.1.2. Class II: Heat-Stable Non-Lanthionine Peptides Smaller Than 10 kDa
B. licheniformis Secreted Peptides Active Only against Gram-Positive Microorganisms
Because antagonism provides a survival advantage in the suppression of related species of microorganisms, most bacteriocins secreted by different strains of B. licheniformis are active only against Gram-positive bacteria. Peptides may be insensitive or sensitive to the action of proteolytic enzymes. However, the vast majority of the identified bacteriocins that are active only against Gram-positive microorganisms are sensitive to the action of proteinases.
Bacillocin 490, a bacteriocin with a low molecular mass (2 kDa) and produced by a thermophilic strain (B. licheniformis 490/5) isolated from dairy foods, shows high thermal stability, with 46.4% residual activity after 1 h of exposure to 100 °C. This bacteriocin was inactivated by pronase E and proteinase K. Bactericidal activity was maintained during storage at 4 °C and was remarkably stable over a wide pH range. The activity range of bacillocin 490 was limited to some Gram-positive bacteria. The highest antimicrobial activity was observed against Bacillus stearothermophilus, B. smithii, B. subtilis and B. anthracis. Moderate inhibition of B. cereus, very low inhibition of Listeria innocua and S. aureus, and no inhibition of B. thuringensis and Streptococcus thermophilus were noted. This activity spectrum shows that bacillocin 490 is principally active against species phylogenetically related to the producer strain. The incubation of B. smithii in the presence of bacillocin 490 resulted in 96% death in 30 min, indicating that bacteriocin has a bactericidal effect [28].
The supernatant of thermophilic strain B. licheniformis H1 exhibited antagonistic activity against various species of Gram-positive bacteria, such as Listeria monocytogenes but not against Gram-negative bacteria, except for Pseudomonas fluorescens. The inactivation of this bacteriocin-like activity by a-chymotrypsin, trypsin, and papain was highly significant. No significant decrease was found in antimicrobial activity after the incubation of a bacteriocin-containing supernatant from B. licheniformis H1with pepsin or lipase. The bacteriocin-like substance was stable at temperatures up to 75 °C for 60 min, but activity was lost after being autoclaved at 121 °C for 15 min. Concentrated antimicrobial activity was found in the protein fraction obtained with 60% ammonium sulfate saturation. The results of sodium dodecyl sulfate–polyacrylamide electrophoresis analysis of concentrated partially purified supernatants collected after resting bacterial cells at 55 °C revealed a bacteriocin-like protein with a molecular mass of approximately 3.5 kDa [66].
B. licheniformis AnBa9 produced antibacterial bacteriocin-type peptides with a molecular mass of <10 kDa. The production of these peptides was 25-fold higher under optimized conditions for producer growth compared with nonoptimized condition. The level of bacteriocin production and its specific activity gradually decreased with increasing concentrations of lactose and NH4NO3. F high concentration of yeast extract, an alkaline pH and an elevated temperature improved the production of antibacterial peptides by B. licheniformis AnBa9. B. licheniformis AnBa9 inhibited several Gram-positive bacteria, including Staphylococcus aureus, Bacillus cereus, Staphylococcus epidermidis, Kurthia gibsonii, Micrococcus luteus, Streptococcus mitis, Bacillus subtilis, L. lactis, Bacillus smithii, Lactobacillus acidophilus, Pediococcus acidilactici, and Leuconostoc mesenteriodes. However, these bacteriocins did not inhibit Listeria strains or Gram-negative bacteria. The loss of antibacterial activity of the permeate after treatment with proteinase K, pronase E, and trypsin, suggested that these bacteriocins are sensitive to proteolytic enzymes. They were resistant to temperature up to 100 °C for 30 min and over a wide range of pH from 4 to 12 [35].
Under anaerobic conditions, B. licheniformis 26L10/3RA produced an inhibitory bacteriocin-like component called lichenin. This peptide was purified to homogeneity, and the molecular mass was estimated at approximately 1.4 kDa. Lichenin was found to be hydrophobic, was sensitive to atmospheric oxygen, retained biological activity even after boiling for 10 min, and was active over a pH range of 4.0–9.0. It was active against Streptococcus bovis, Ruminococcus albus, Ruminococcus avefaciens, and Eubacterium ruminantium. The biological activity of this peptide was completely inactivated by proteinase K treatment, but this peptide was resistant to trypsin. Heat treatment at 80 °C and boiling for 10 min at pH 4.0 and 9.0 resulted in a significant reduction in biological activity. Lichenin production was observed only upon B. licheniformis anaerobic growth, the antibacterial activity of which was also demonstrated for the reference strains grown under anaerobic conditions only. The inability of lichenin to inhibit aerobically grown bacteria was explained either by its inactivation by atmospheric oxygen or the target bacteria due to oxidative respiration. No N-terminal block was observed in the sequence and the peptide did not show any characteristics of cyclicity. However, the seventh amino acid residue could not be identified and it did not belong to any of the natural amino acids [32].
Strain BTHT8, identified as B. licheniformis, inhibited the growth of Gram-positive test organisms. The active component, labeled as bacteriocin BL8, was purified from the supernatant of B. licheniformis strain BTHT8. The molecular mass was determined as 1.4 kDa. The results of N-terminal amino acid sequencing of BL8 identified a 13 amino acid sequence stretch. Bacteriocin BL8 was stable even after boiling at 100 °C for 30 min and over a wide pH range of 1–12 [67].
A bacteriocin from B. licheniformis cy2, named BSCY2, was stable at pH 2.5–9.5, showing activity against B. subtilis. BSCY2 was stable below 40 °C and retained its antimicrobial activity during long tern storage at −20 °C and −70 °C. BSCY2 was inactivated after 15 min exposure to temperatures over 80 °C and lost 50% of its antimicrobial activity within 2 h at 70 °C. BSCY2 was inactivated by proteinase K treatment, which indicated its proteinous nature. The direct detection of the BSCY2 band showing antimicrobial activity on Tricine–SDS-PAGE suggested an apparent molecular mass of about 6.5 kDa [68].
In contrast to the above-mentioned bacteriocins of this group, some bacteriocins retain their activity after treatment with proteolytic enzymes.
B. licheniformis strain VPS50.2 produced bacteriocin licheniocin 50.2 (molecular mass of approximately 3.25 kDa) and was effective against Gram-positive bacteria, including Listeria monocytogenes, methicillin-resistant Staphylococcus aureus, and b-haemolytic streptococci. Bacteriocin activity was insensitive to lysozyme and proteinase K, being heat stable after incubation at 100 °C for 30 min and over a wide range of pH (2–12). The inhibitory spectrum considered in this study was limited to Gram-positive bacteria only. The maximum antagonistic activity was found in the precipitate with 60% saturation of ammonium sulfate [69].
Despite the varying degrees of sensitivity to the action of proteolytic enzymes, bacteriocins of this group are resistant to elevated temperatures and wide pH values, so they are especially suitable for medical applications.
B. licheniformis Secreted Peptides Active against Both Gram-Positive and Gram-Negative Microorganisms
The bacteriocins secreted by B. licheniformis that show activity against both Gram-positive and Gram-negative microorganisms are common and are sensitive to the action of proteolytic enzymes but resistant to elevated temperatures. They show different sensitivities to a wide range of pH values. All bacteriocins of this sub-group are sensitive to the action of proteinases.
B. licheniformis strain IITRHR2 produced a bacteriocin-like inhibitory substance (~1.2 kDa), which was thermostable (up to 80 °C but showed decreased activity at higher temperatures) and pH-resistant but lost activity when subjected to proteinase treatment (proteinase K and pronase E). This bacteriocin inhibited various Gram-positive bacterial strains, such as B. subtilis, B. cereus, Streptococcus thermophilus, Pediococcus pentosaceus, Leuconostoc mesenteroides, L. monocytogenes, Bifidobacterium bifidum, and Enterococcus faecalis. The growth of Gram-negative bacteria Shigella flexneri, Shigella sonnei and Pseudomonas aeruginosa was also inhibited by this compound [70].
A culture supernatant of B. licheniformis MKU3 exhibited bacteriocin-like activity against several type strains of Gram-positive bacteria, such as Bacillus subtilis, Bacillus smithii, Staphylococcus epidermidis, Micrococcus luteus, Leuconostoc mesenteriodes and Pediococus acidilactici, B. cereus, B. megaterium, K. gibsonii, Staphyloccus sp., Streptococcus sp., and Micrococcus caseolyticus but not Listeria sp. However, Gram-negative bacteria, such as Serratia marcescens and Pseudomonas fluorescens B10 were not inhibited by this bacteriocin, excluding Escherichia coli. The extract showed strong activity against different fungi including Aspergillus niger, A. versicolor, A. fischeri, and A. fumigatus and the yeast Candida albicans. The active substance apparently is a bacteriocin-like protein with a molecular mass of 1.5 kDa. The activity of this bacteriocin was stable at pH 3.0–10.0 and temperatures up to 100 °C for 60 min but was inactivated by proteinase K, trypsin or pronase E. The bacteriocin lost its activity after incubation at 121 °C for 15 min. The composition of the medium affected the production of this bacteriocin [34].
B. licheniformis strain B116 showed strong antimicrobial activity against Staphylococcus aureus and Salmonella enterica ser. Pullorum. Bacteriocin was precipitated by ammonium sulfate, and its molecular mass was determined as ~4 kDa. The culture supernatant of this strain exhibited antimicrobial activity against both Gram-positive and Gram-negative bacteria, including Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes, Micrococcus luteus, Escherichia coli, Streptococcus equi, and Salmonella spp. The bacteriocin was resistant to heat, acid, and alkaline treatments. The activity of this bacteriocin was totally lost after digestion by pronase and activity was partially lost after digestion by papain and lipase. The inactivation by lipase indicated that this bacteriocin may have contained a lipid moiety [71].
B. licheniformis MCC 2016 (strain was also named Me1) produced the antibacterial peptide ppABP that was completely abolished by proteinase K. The culture, which was isolated from milk, was able to produce a proteinaceous antibacterial peptide with a low molecular weight between 3.0 and 3.5 kDa. It exhibited a broad spectrum of inhibitory activity and was stable over a wide range of temperature and pH. ppABPs were found to be thermally stable for 15 min at 80 °C. The SN of this strain exhibited inhibitory activity against both Gram-positive and Gram-negative food-borne and human pathogens [72][73]. Films activated with ppABP from B. licheniformis Me1 showed a zone of inhibition that was not confined to the film area, indicating that the ppABP diffused from the films into the medium [74].
B. licheniformis strain N.C.T.C. 7072 produced licheniformins, which are antibacterial agents, with in vitro bacteriostatic activity against many organisms, including Mycobacterium tuberculosis. In addition to inhibiting the growth of mycobacteria, they showed efficacy against Staphylococcus aureus and Escherichia coli [75]. The peptides had a molecular mass of 3.8, 4.4 and 4.8 kDa, respectively [37].
B. licheniformis strains MCC2512 and MCC2514 exhibited inhibitory activity against Micrococcus luteus, Staphylococcus aureus, Klebsiella sp., and Aeromonas hydrophila. In addition to these pathogenic strains, B. licheniformis strain MCC2512 also showed inhibitory activity against Listeria monocytogenes and Salmonella typhimurium. The activities of the bacteriocins from both cultures were completely lost upon exposure to proteinase K, indicating the proteinaceous nature of the compounds. After treating the sample with trypsin and pepsin, 100% of the activity was retained; however, with a-amylase, 50% of the activity was lost. The isolated bacteriocins varied in their mechanisms of action and stability. The molecular weights of the inhibitor components from MCC2514 and MCC2512 were 6.5 and 3.5 kDa, respectively. B. licheniformis MCC2512 produced a subtilin-type antimicrobial compound that acted on cell wall synthesis. Strain MCC2514 inhibited RNA synthesis [31]. The active substance produced by B. licheniformis MCC2512 was identified as sublichenin [53] (Figure 2).
B. licheniformis Peptide Activity against Fungal Pathogens
An important characteristic of some bacteriocins is antifungal activity, which substantially expands the horizons of their application in medicine, agriculture, and the food industry.
The cell-free supernatant of B. licheniformis, ZJU12, isolated from soil exhibited pronounced antibacterial (for Gram-positive bacteria) activity. The bacteriocin-like peptides produced by B. licheniformis ZJU12 showed no activity against Gram-negative bacteria, but exhibited activity against fungi (Xanthomonas oryzae pv.oryzae, Alternaria brassicae, Fusarium oxysporum, and others). After treatment with proteinase K and trypsin, the antagonistic activity was completely lost. The molecular mass estimated via Tricine–SDS-PAGE of the antagonistic compound was approximately 3 kDa. These characteristics indicated that the antagonistic substances produced by this strain had the property of bacteriocin. The activity was stable following exposure up to 100 °C for 30 min but was completely lost at 121 °C for 15 min. The maximum antagonistic activity was found in the resolved precipitate of supernatant with 60% saturation of ammonium sulfate. The toxicity of the substance was low because no adverse effects on mice were detected at a dose of up to 0.8 mg/20 g in acute toxicity tests [33].
B. licheniformis strain MGrP1 produced antibiotics in liquid media containing soyabean meal and mannitol that inhibited the growth of plant fungal pathogens of agricultural importance, namely Colletotrichum lindemuthianum (bean anthracnose), Colletotrichum kahawae (coffee berry disease), Fusarium oxysporum f.sp. phaseoli (fusarium yellow), and Alternaria solani (early blight). The results of paper chromatography combined with bioautography revealed two thermostable active compounds whose activity was optimal at pH 6. Low pH ranges and autoclaving temperatures significantly reduced the activities of the antibiotics [76].
Fungicin M-4, produced by B. licheniformis M-4, is composed of 34 amino acid residues of 7 different amino acids, including 4 residues of ornithine per molecule. The same strain showed inhibitory activity against the human pathogenic amoeba Naegleria fowleri. Purified fungicin M-4 demonstrated antifungal activity against the pathogenic fungi Sporothrix schenckii and Microsporum canis. Fungicin M-4 was resistant to proteolytic enzymes and lipase. Its antifungal activity was fairly resistant to heat, although incubation at 80 °C for 30 min caused 30% inactivation. The activity was stable in the pH range from 2.5 to 9.0. Its molecular weight was 3.6 kDa. Attempts to deduce an amino acid sequence were unsuccessful, suggesting that fungicin may be a cyclic peptide or it is blocked at its amino-terminal end [77].
Peptide A12-C from B. licheniformis A12 showed a pronounced antifungal effect, being an acidic hydrophilic peptide with a mass of 0.77 kDa, containing only six different amino acids. Peptide A12-C was resistant to proteolytic enzymes, such as trypsin, pronase, and proteinase K, as well as to carboxypeptidase A, alkaline phosphatase, lipase, lysozyme, β-glucosidase, and β-glucuronidase. Peptide A12-C showed resistance to heat (100 °C for 30 min at pH 7.0) and incubation at room temperature under acidic conditions (pH 2.5), but 75% of the activity was lost after incubation at pH 9.5 for 30 min at room temperature. Peptide A12-C was active against several fungi (Microsporum canis, Mucor mucedo, M. plumbeus, Sporothrix schenckii, and Trichophyton mentagrophytes) and bacteria (Bacillus megaterium, Corynebacteriurn glutamicum, Sarcina and Mycobacterium phlei) [46].
B. licheniformis NCIMB 8874 produced peptide ComX with antifungal activity against the fungal leaf pathogen Alternaria alternata. ComX comprises 13 amino acid residues: Glu-Ala-Gly-Trp-Gly-Pro-Tyr-Pro-Asn-Leu-Trp-Phe-Lys [78].
Amoebolytic Substances from B. licheniformis
Bacteriocins with amoebolytic activity have been identified, all of which showed resistance to the action of proteolytic enzymes and elevated temperatures.
B. licheniformis A12 produced two amoebolytic substances (amoebicins A12-A and A12-B) in liquid media during sporulation. Both substances were heat- and protease-resistant peptides containing aspartic acid, glutamic acid, serine, proline, and tyrosine in a molar ratio of 5:2:2:2:2. No fatty acids or carbohydrates were detected. Both amoebicins retained 100% of their activity after being heated at 100 °C for 30 min at pH 7.0. They were also resistant to incubation at room temperature under acidic conditions (pH 2.5) but lost 75% of their activity upon incubation at pH 9.5 for 30 min. The crude supernatants, as well as the purified substances, retained 100% of their activity after storage for 1 month at 4 °C or for 6 months at −20 °C. Amoebicins A12-A and A12-B were resistant to the enzymes trypsin, pronase, proteinase K, alkaline phosphatase, lipase, lysozyme, α-glucosidase, and 3-glucuronidase. They were also resistant to carboxy peptidase A, suggesting that a free carboxyl terminus was not present. Their molecular weight was found to be 1.43–1.60 kDa. Purified amoebicins A12-A and A12-B exhibited amoebolytic action against Naegleria fowleri. They also exhibited antibiotic action against yeasts (Saccharomyces heterogenicus and Cryptococcus neoformans) and several fungal species (Aspergillus niger, Microsporum canis, Mucor plumbeus, and Trychophyton mentagrophytes). Their antibacterial spectrum appears to be restricted to Bacillus megaterium, Corynebacterium glutamicum, and Sarcina sp. The amoebolytic effect was studied via electron microscopy. At 10 min after addition, the characteristic shape of the cells changed. First, they developed abnormal globular pseudopodia, and then they became rounded. After 30 min of incubation, the cell membrane ruptured, with the release of the cytoplasmic material. All of this was followed by complete cellular destruction within 1 h [79].
B. licheniformis M-4 produced three antibiotic peptides (m4-A, m4-B, and m4-C) with amoebolytic activity. They were active against human pathogenic and non-pathogenic strains of Naegleria fowleri, which is the causative agent of primary amoebic meningoencephalitis. The amoebicidal activity of these peptides was resistant to the actions of trypsin, proteinase K, and carboxypeptidase A. They were cyclic peptides with molecular weights ranging from 3.0 to 3.2 kDa. These peptides were composed of six different amino acids (Asp, Glu, Ser, Thr, Pro, and Tyr), and they differed only in the number of Asp residues. The three amoebicins had a broad antifungal spectrum, although peptide m4-C showed a two-fold higher specific activity against a variety of fungi and yeasts than others. The three peptides showed a narrow antibacterial spectrum, but Bacillus megaterium (not spores) was highly sensitive [80]. The amoebicins from B. licheniformis M-4 differ from those produced by strain A12 in molecular weight, in their amino acid composition (A12-A and A12-B contained threonine), in the number of residues per molecule, and in their solubility in water (A12-A and A12-B are not water-soluble) [79][80].
B. licheniformis D-13 produced three hydrophobic peptides (amoebicins d13-A, d13-B, and d13-C) that showed anti-amoebic activity against human-pathogenic and non-pathogenic species of Naegleria and had a broad spectrum of antibacterial activity. The three amoebicins showed the same amino acid composition and molecular weight of 1.87 kDa. The three amoebicins were stable at pH 2.5 to 9.5, and they retained 100% of their activity after being heated at 100 °C for 30 min and after being stored at −20 °C for 6 months. Because the purified amoebicins were not soluble in aqueous buffers, a mixture of partially purified amoebicins in 20 mM Tris-HCl (pH 7.2) was tested for sensitivity to various enzymes. The mixture retained 100% of its activity after being treated for 1 h with proteases (trypsin, pronase, and proteinase K), lipase, or β-glucuronidase. Amoebicin d13-B caused the lysis of amoebae through the disorganization of the cell membrane (Figure 2). No amino acid residues were detected after the N-terminal sequence of amoebicin d13-B, suggesting that this peptide is cyclic or blocked at its amino terminus [81].
2.1.3. Class III: Heat-Stable and Heat-Labile Proteins Larger Than 10 kDa
This class includes unmodified peptides with a molecular weight larger than 10 kDa. In most cases, these are thermostable membrane-active peptides that are sensitive to proteinase treatment.
B. licheniformis SXAU06 produced a bacteriocin-like substance (BLIS) with an approximate molecular weight of 14 kDa designated as BLIS_SXAU06. It was active against Escherichia coli, Salmonella enterica, Staphylococcus aureus, Staphylococcus epidermidis, Micrococcus luteus, and Listeria monocytogenes. BLIS_SXAU06 exhibited high resistance to treatment at high temperature, high acidity and alkalinity, and proteinase K, but it was fully inactivated by pronase E and partially inactivated by trypsin and pepsin. BLIS_SXAU06 was heterologously expressed in E. coli, and the recombinant BLIS_SXAU06 exhibited effective antibacterial activity against S. aureus, S. epidermidis, M. luteus, and L. monocytogenes [82].
When a tropical marine strain of B. licheniformis D1 was grown in Luria–Bertani (LB) broth-containing tryptone medium, it produced a 14 kDa protein BL-DZ1 (BL00275) with antimicrobial activity against pathogenic Candida albicans BH, Pseudomonas aeruginosa PAO1, and biofouling Bacillus pumilus TiO1 cultures. The antimicrobial activity was lost after treatment with trypsin and proteinase K. The protein was stable at 75 °C for 30 min and over a pH range of 3.0 to 11.0. The BL-DZ1 protein was able to inhibit both biofilm growth and disrupt pre-formed biofilms of C. albicans, P. aeruginosa, and B. pumilus [83].
B. licheniformis HS10 produced an antifungal protein with a molecular weight of approximately 55 kDa, identified as carboxypeptidase. It showed significant inhibition activity of eight different kinds of plant pathogenic fungi, and it was stable with strong biological activity at as high as 100 °C for 30 min and in pH values ranging from 6 to 10. The biological activity was negatively affected by protease K. The protein had broad spectrum antifungal activity against seven kinds of plant pathogenic fungi [84].
Isolated from seaweed, B. licheniformis produced a protein with antibacterial activity against methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and Listeria monocytogenes. The antibacterial activity was strongest in cultures grown under shaking at 210 to 230 rpm. No antibacterial activity was found in cultures grown statically or at other rotary shaking speeds. The antibacterial compound was sensitive to proteinase K, pronase, and trypsin, but was not affected by Tween-20, -40, -60, or -80, or a- or b-amylase. Its activity was not adversely affected by heating up to 40 °C or treatment at pH from 5 to 14. The bioactive compound was determined to be associated with a 30.7 kDa protein, that showed homology to the secreted YbdN protein of B. licheniformis ATCC 14580 [85].
B. licheniformis MY75 secreted high levels of extracellular chitinase with a molecular weight of 55 kDa and inhibited the growth of pathogenic fungi Gibberella saubinetii and Aspergillus niger. The secretion of this protein was induced by chitin powder [86]. Chitinase proteins were present in the culture supernatant of B. licheniformis Mb-2 [87], B. licheniformis TP–1 [88], B. licheniformis S213 [89], B. licheniformis SSCL-10 [90], B. licheniformis B307 [91].
B. licheniformis BS-3 produced an antifungal 31 kDa protein, F2, that inhibited the growth of Aspergillus niger, Magnaporthe oryzae, and Rhizoctonia solani. The F2 protein was moderately resistant to hydrolysis by trypsin and proteinase K. Higher F2 antifungal activity was observed from pH 6.0 to pH 10.0 and at a temperature below 70 °C for 30 min [92].
As in the other cases, this group of bacteriocins contains some proteinase-resistant members. Owing to this property, these proteins may be applicable for administration through the digestive system.
B. licheniformis strain JS produced 16 kDa antimicrobial protein (AMP), which demonstrated more activity against Gram-positive bacteria Bacillus cereus and less activity against Gram-negative bacteria (S. dysenteriae and S. typhimurium). The purified peptide also increased the effectiveness of antibiotics, such as kanamycin, neomycin, and streptomycin. Hence, it could be important because the AMPs produced by B. licheniformis may facilitate the entry of these antibiotics inside the pathogens and increase their efficiency. The antimicrobial activity was 100% after AMP incubation between 10 and 90 °C. The trypsin digestion study revealed that AMP retained 100% of its activity [93].
B. licheniformis T6-5 inhibited more than 65% of the 40 Bacillus strains and of the sulfate-reducing bacteria Desulfovibrio alaskensis. The treatment of the supernatant with organic solvents led to total (acetone, ethanol, and methanol) or partial (chloroform) inactivation of the inhibitor component. The inhibitor probably contained a lipidic portion as a part of its structure. This substance was heat-stable after incubation at 100 °C for 1 h and maintained its activity after being autoclaved at 121 °C for 15 min. It was active in a wide range of pH (3.5–9.5). The inhibitory component was resistant to the action of pronase E, proteinase K, trypsin, RNase, chitinase, b-galactosidase, a-galactosidase, and manosidase. The substance produced by strain T6-5 was estimated via dialysis to be bigger than 12 kDa. According to the results of SDS-PAGE analysis, strain T6-5 showed an inhibitory zone of ca. 20 kDa, corresponding to the molecular weight suggested by the dialysis membrane approach [36]. The substance inhibitory zones of B. licheniformis H2O-1 antimicrobial were related to a region of high molecular mass (90–120 kDa) [36]. B. licheniformis strains T6-5 and H2O-1 prevented the formation of B. pumilus LF4 biofilm and eliminated pre-established LF4 biofilm [94]. The nature and precise structure of the above inhibitory substances are still unclear.
2.1.4. Class IV: Complex with a Lipid Moiety or Carbohydrate Moiety
B. licheniformis BFP011 isolated from papaya (Thailand), produced extracellular antimicrobial substances that were active against some important phytopathogens, pathogenic and spoilage microorganisms, such as Colletotrichum capsici, and Escherichia coli O157: H7, and Salmonella typhi ATCC 5784. The three types of antimicrobial substances (F4, F5, and F6) produced by B. licheniformis BFP011 were not sensitive to pronase as revealed in stationary phase cultures. The antimicrobial substances of this bacterium were stable at 37 and 70 °C and partly resistant at 121 °C. Most of the antimicrobial protein substances from the culture supernatant were extracellular compounds having low molecular weights of less than 45 kDa. The antimicrobial substances of B. licheniformis BFP011 contained peptides and unsaturated fatty acids; however, the precise structural organizations of these compounds are not known. They exhibited a broad spectrum of antimicrobial activity against both Gram-positive and Gram-negative bacteria and the fungus C. capsici. These substances differed from iturin A (commercial), bacitracin (commercial), and a bacteriocin-like substance of B. licheniformis P40 [95].
Two glycolipopeptides, ieodoglucomides A and B, were isolated from marine-derived Bacillus licheniformis 09IDYM23. They consisted of an amino acid, a new fatty acid, a succinic acid, and a sugar. These glycolipopeptides showed moderate antimicrobial activity when tested against both Gram-positive and Gram-negative bacteria and fungi, such as S. aureus, P. aeruginosa, E. coli, B. cereus, and A. niger. The molecular formula of ieodoglucomides A and B were assigned as C30H53NO12 and –C29H51NO12, respectively [96].
The same strain, 09IDYM23, produced a glycolipopeptide, ieodoglucomide C, and a new monoacyldiglycosylglycerolipid, ieodoglycolipid. These compounds showed antimicrobial activity against fungi C. albicans, A. niger, R. solani, C. acutatum, and B. cenerea and bacteria S. aureus, B. subtilis, B. cereus, S. typhi, E. coli, and P. aeruginosa. The molecular formulae of each isolated component were determined to be C29H51NO12 and C30H56O14, respectively [97].
2.1.5. Class V: Bacteriocins with Undetermined Molecular Weight
A skin isolate of B. licheniformis showed the most potent antibacterial activity at pH 7, with an incubation period of 48 h, at an incubation temperature of 25 °C. The antipathogenic metabolites were then detected as bacteriocin-like substances, which demonstrated heat stability up to 80 °C for 30 min. The papain-treated cell-free supernatant did not show any bacteriocin activity, suggesting that the substances were antimicrobial peptides. This bacteriocin inhibited the growth of Staph. aureus and Kl. pneumoniae subsp. Pneumonia [98].
A skin isolate B. licheniformis UpA was observed to produce antimicrobial metabolite that was effective against Klebsiella pneumoniae subsp. pneumoniae. It was detected as a bacteriocin-like substance and was further confirmed as an antimicrobial peptide through papain treatment. The produced bacteriocin was stable with heat treatment up to 80 °C for 30 min and up to pH 7 [99].
The supernatant of B. licheniformis A-1-5B-AP significantly reduced the growth of oral pathogenic strains Porphyromonas gulae 3/H, Prevotella intermedia 1/P, and Streptococcus mutans ATCC 35668. However, B. licheniformis A-2-11B-AP only significantly inhibited the growth of P. intermedia 1/P and S. mutans ATCC 35668. The enzyme-treated SN of B. licheniformis A-1-5B-AP did not lose its antimicrobial effect and significantly inhibited the growth of Micrococcus luteus DSM 1790. Proteinase K, lipase, or α-amylase did not affect the antimicrobial activity present in the SN of strain of B. licheniformis A-1-5B-AP. The presence of genes associated with the synthesis of lichenysin was detected, although their presence in the medium was not confirmed [100].
B. licheniformis HJ2020 MT192715.1 produced a bacteriocin active against many food spoilage microorganisms. The residual inhibition activity of bacteriocin varied according to the incubation conditions and treatment type. The inhibitory activity was 220 and 360 U mL−1 against the clinical isolates of pathogenic strains Escherichia coli and Salmonella typhi, respectively; the activity was 42, 60, and 80 U/mL against to B. subtilis, B. cereus and Candida albican, respectively [101]. No activity was detected against Lactobacillus or Bifidobacterium. These results are similar to those reported for B. licheniformis P40 [29]. The bacteriocin lost approximately 25–40% of its activity when incubated in acidic pH (between 3 and 5), it lost approximately 80% of its activity at pH 10, and no activity was observed at pH 12. The heat stability of the bacteriocin also was tested, and the results showed that it retained all activity when incubated at 5–35 °C for 30 min. It lost approximately 25–50% of its activity after incubation at 50–80 °C and lost all activity when incubated at 100 °C for 30 min or autoclaved at 121 °C for 15 min at 15 psi. The reduction in bacteriocin activity and the loss of all of its activity at high temperature were attributed to denaturation, indicating the proteinaceous nature of bacteriocin. The results also revealed that the bacteriocin was stable when treated with α-amylase and lipase, pointing to the absence of glycosidic or lipidic residuals [101].
The bacteriocins produced by B. licheniformis are characterized by resistance to various pH ranges, thermal stability, and, in some cases, sensitivity to proteolytic enzymes. However, they differ in the spectrum of antibacterial activity for different strains of B. licheniformis. For example, a bacteriocin produced by B. licheniformis MKU3 isolated from slaughterhouse sediments did not inhibit L. monocytogenes, P. fluorescens or S. marcescens, but inhibited E. coli [34]. A bacteriocin-like peptide produced by B. licheniformis ZJU12 isolated from soil exhibited antagonistic activity against S. aureus [33]; B. licheniformis P40 inhibited E. aerogenes but did not inhibit P. fluorescens [29]. Anaerobiosis specifically expressing lichenin demonstrated a narrow spectrum of activity against ruminal anaerobes [32].
Bacteriocins have different structures and different mechanisms of action against bacteria. Their ability to reach their targets is crucial for their effectiveness. Due to variations in cell wall structure, certain bacteriocins, particularly those designed to target intracellular components, may encounter challenges in penetrating the cell walls of mycobacteria or Gram-negative bacteria. Conversely, pore-forming bacteriocins have demonstrated a broader spectrum of activity against various types of bacteria.
References
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal Transfer of Antibiotic Resistance Genes in Clinical Environments. Can. J. Microbiol. 2019, 65, 34–44.
- Shleeva, M.O.; Kudykina, Y.K.; Vostroknutova, G.N.; Suzina, N.E.; Mulyukin, A.L.; Kaprelyants, A.S. Dormant Ovoid Cells of Mycobacterium Tuberculosis Are Formed in Response to Gradual External Acidification. Tuberculosis 2011, 91, 146–154.
- Trutneva, K.A.; Shleeva, M.O.; Demina, G.R.; Vostroknutova, G.N.; Kaprelyans, A.S. One-Year Old Dormant, “Non-Culturable” Mycobacterium Tuberculosis Preserves Significantly Diverse Protein Profile. Front. Cell Infect. Microbiol. 2020, 10, 26.
- Kaprelyants, A.; Salina, E.; Makarov, V. How to Kill Dormant Mycobacterium Tuberculosis. Int. J. Mycobacteriol. 2018, 7, 399–400.
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the Use of Bacillus Species for Industrial Production. Can. J. Microbiol. 2004, 50, 1–17.
- Stoica, R.-M.; Moscovici, M.; Tomulescu, C.; Casarica, A.; Babeanu, N.; Popa, O.; Kahraman, H.A. Antimicrobial Compounds of the Genus Bacillus: A Review. Rom. Biotechnol. Lett. 2019, 24, 1111–1119.
- Lawton, E.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Two-Peptide Lantibiotics: A Medical Perspective. Mini-Rev. Med. Chem. 2007, 7, 1236–1247.
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A Viable Alternative to Antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105.
- Nishie, M.; Nagao, J.I.; Sonomoto, K. Antibacterial Peptides “Bacteriocins”: An Overview of Their Diverse Characteristics and Applications. Biocontrol Sci. 2012, 17, 1–16.
- Yang, S.C.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 241.
- Magashi, A.M.; Bukar, A.; Omola, E.M.; Halima, B.A.; Hadiza, M.S. Bacteriocin and its application—A review. Int. J. Adv. Acad. Res. Sci. Technol. Eng. 2019, 5, 242–256.
- Yang, H.; Sun, Y.; Cai, R.; Chen, Y.; Gu, B. The Impact of Dietary Fiber and Probiotics in Infectious Diseases. Microb. Pathog. 2020, 140, 103931.
- Ramirez-Olea, H.; Reyes-Ballesteros, B.; Chavez-Santoscoy, R.A. Potential Application of the Probiotic Bacillus Licheniformis as an Adjuvant in the Treatment of Diseases in Humans and Animals: A Systematic Review. Front. Microbiol. 2022, 13, 993451.
- Hallaj-Nezhadi, S.; Hamdipour, R.; Shahrvirani, M.; Zare tin, R.; Chapeland-leclerc, F.; Ruprich-Robert, G.; Esnaashari, S.; Elyasi Far, B.; Dilmaghani, A. Antimicrobial Activity of Bacillus sp. Isolated Strains of Wild Honey. BMC Complement. Med. Ther. 2022, 22, 78.
- Joerger, R.D. Alternatives to Antibiotics: Bacteriocins, Antimicrobial Peptides and Bacteriophages. Poult. Sci. 2003, 82, 640–647.
- Seal, B.S.; Drider, D.; Oakley, B.B.; Brüssow, H.; Bikard, D.; Rich, J.O.; Miller, S.; Devillard, E.; Kwan, J.; Bertin, G.; et al. Microbial—Derived Products as Potential New Antimicrobials. Vet. Res. 2018, 49, 66.
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial Peptides: Premises and Promises. Int. J. Antimicrob. Agents 2004, 24, 536–547.
- Todorov, S.D.; Ivanova, I.V.; Popov, I.; Weeks, R.; Chikindas, M.L. Bacillus Spore-Forming Probiotics: Benefits with Concerns? Crit. Rev. Microbiol. 2022, 48, 513–530.
- Nesemann, G.; Präve, P.; Sukatsch, D.; Vértesy, L. Ein Polyen-Antibiotikum Aus Bakterien . Naturwissenschaften 1972, 59, 81–82.
- Girija, V.; Malaikozhundan, B.; Vaseeharan, B.; Vijayakumar, S.; Gopi, N.; Del, M.; Herrera, V.; Chen, J.; Santhanam, P. In Vitro Antagonistic Activity and the Protective Effect of Probiotic Bacillus Licheniformis Dahb1 in Zebrafish Challenged with GFP Tagged Vibrio Parahaemolyticus Dahv2. Microb. Pathog. 2018, 114, 274–280.
- Rohith, H.S.; Halami, M.P. In Vitro Validation Studies for Adhesion Factor and Adhesion Efficiency of Probiotic Bacillus Licheniformis MCC 2514 and Bifidobacterium Breve NCIM 5671 on HT—29 Cell Lines. Arch. Microbiol. 2021, 203, 2989–2998.
- Sekar, A.; Kim, M.; Jeon, H.; Kim, K. Screening and Selection of Bacteria Inhibiting White Spot Syndrome Virus Infection to Litopenaeus Vannamei. Biochem. Biophys. Rep. 2019, 19, 100663.
- Peng, J.-Y.; Horng, Y.-B.; Wu, C.-H.; Chang, C.-Y.; Chang, Y.-C.; Tsai, P.-S.; Jeng, C.-R.; Cheng, Y.-H.; Chang, H.-W. Evaluation of Antiviral Activity of Bacillus Licheniformis—Fermented Products against Porcine Epidemic Diarrhea Virus. AMB Express 2019, 9, 191.
- Lee, T.-W.; Chao, T.-Y.; Chang, H.-W.; Cheng, Y.-H.; Wu, C.-H.; Chang, Y.-C. The Effects of Bacillus Licheniformis—Fermented Products on the Microbiota and Clinical Presentation of Cats with Chronic Diarrhea. Animals 2022, 12, 2187.
- Barba-Vidal, E.; Roll, V.F.B.; Castillejos, L.; Guerra-Ordaz, A.A.; Manteca, X.; Mallo, J.J.; Martin-Orúe, S.M. Response to a Salmonella Typhimurium Challenge in Piglets Supplemented with Protected Sodium Butyrate or Bacillus Licheniformis: Effects on Performance, Intestinal Health and Behavior. Transl. Anim. Sci. 2017, 1, 186–200.
- Pahumunto, N.; Dahlen, G.; Teanpaisan, R. Evaluation of Potential Probiotic Properties of Lactobacillus and Bacillus Strains Derived from Various Sources for Their Potential Use in Swine Feeding. Probiotics Antimicrob. Proteins 2021, 15, 479–490.
- Shanthi, S.; Jayaseelan, B.D.; Velusamy, P.; Vijayakumar, S.; Chih, C.T.; Vaseeharan, B. Biosynthesis of Silver Nanoparticles Using a Probiotic Bacillus Licheniformis Dahb1 and Their Antibiofilm Activity and Toxicity Effects in Ceriodaphnia Cornuta. Microb. Pathog. 2016, 93, 70–77.
- Luca, M.; Mario, V.; Gino, N.; Felice, M. De Purification and Partial Characterization of Bacillocin 490, a Novel Bacteriocin Produced by a Thermophilic Strain of Bacillus Licheniformis. Microb. Cell Fact. 2002, 91, 1–5.
- Cladera-Olivera, F.; Caron, G.R.; Brandelli, A. Bacteriocin-like Substance Production by Bacillus Licheniformis Strain P40. Lett. Appl. Microbiol. 2004, 38, 251–256.
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological Applications of Bacillus Licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627.
- Shobharani, P.; Padmaja, R.J.; Halami, P.M. Diversity in the Antibacterial Potential of Probiotic Cultures Bacillus Licheniformis MCC2514 and Bacillus Licheniformis MCC2512. Res. Microbiol. 2015, 166, 546–554.
- Pattnaik, P.; Kaushik, J.K.; Grover, S.; Batish, V.K. Purification and Characterization of a Bacteriocin-like Compound (Lichenin) Produced Anaerobically by Bacillus Licheniformis Isolated from Water Buffalo. J. Appl. Microbiol. 2001, 91, 636–645.
- He, L.; Chen, W.L.; Liu, Y. Production and Partial Characterization of Bacteriocin-like Pepitdes by Bacillus Licheniformis ZJU12. Microbiol. Res. 2006, 161, 321–326.
- Kayalvizhi, N.; Gunasekaran, P. Production and Characterization of a Low-Molecular-Weight Bacteriocin from Bacillus Licheniformis MKU3. Lett. Appl. Microbiol. 2008, 47, 600–607.
- Anthony, T.; Rajesh, T.; Kayalvizhi, N.; Gunasekaran, P. Influence of Medium Components and Fermentation Conditions on the Production of Bacteriocin(s) by Bacillus Licheniformis AnBa9. Bioresour. Technol. 2009, 100, 872–877.
- Korenblum, E.; Rosado, A.S.; Sebastia, G.V.; De Paiva, M.M.; Seldin, L. Production of Antimicrobial Substances by Bacillus Subtilis LFE-1, B. Firmus H 2 O-1 and B. Licheniformis T6-5 Isolated from an Oil Reservoir in Brazil. J. Appl. Microbiol. 2005, 98, 667–675.
- Callow, R.K.; Work, T.S. Antibiotic Peptides from Bacillus Licheniformis; Licheniformins A, B and C. Biochem. J. 1952, 51, 558–568.
- Präve, P.; Sukatsch, D.; Vértesy, L. Proticin, a New Phosphorus-Containing Antibiotic. I. Taxonomy, Fermentation, Isolation, and Biological Properties. J Antibiot 1972, 25, 1–3.
- Li, X.; Wang, D.; Cai, D.; Zhan, Y.; Wang, Q.; Chen, S. Identification and High-Level Production of Pulcherrimin in Bacillus Licheniformis DW2. Appl. Biochem. Biotechnol. 2017, 183, 1323–1335.
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20.
- O’Sullivan, L.; Ross, R.P.; Hill, C. Potential of Bacteriocin-Producing Lactic Acid Bacteria for Improvements in Food Safety and Quality. Biochimie 2002, 84, 593–604.
- Mercado, V.; Olmos, J. Bacteriocin Production by Bacillus Species: Isolation, Characterization, and Application. Probiotics Antimicrob. Proteins 2022, 14, 1151–1169.
- Jack, R.W.; Tagg, J.R.; Ray, B. Bacteriocins of Gram-Positive Bacteria. Microbiol. Rev. 1995, 59, 171–200.
- Abriouel, H.; Franz, C.M.A.P.; Omar, N.B.; Galvez, A. Diversity Andapplications of Bacillus Bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232.
- Bernardo, S.P.C.; Rosana, A.R.R.; de Souza, A.N.; Chiorean, S.; Martins, M.L.L.; Vederas, J.C. Draft Genome Sequence of the Thermophilic Bacterium Bacillus Licheniformis SMIA-2, an Antimicrobial- and Thermostable Enzyme-Producing Isolate from Brazilian Soil. Microbiol. Resour. Announc. 2020, 9, e00106-20.
- Gálvez, A.; Maqueda, M.; Martínez-Bueno, M.; Lebbadi, M.; Valdivia, E. Isolation and Physico-Chemical Characterization of an Antifungal and Antibacterial Peptide Produced by Bacillus Licheniformis A12. Appl. Microbiol. Biotechnol. 1993, 39, 438–442.
- Cotter, P.D.; Hill, C.; Ross, R.P. Food Microbiology: Bacteriocins: Developing Innate Immunity for Food. Nat. Rev. Microbiol. 2005, 3, 777–788.
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally Synthesized and Post-Translationally Modified Peptide Natural Products: Overview and Recommendations for a Universal Nomenclature. Nat. Prod. Rep. 2013, 30, 108–160.
- Du, A.; Staden, P.V.; van Zyl, W.F.; Trindade, M.; Dicks, L.M.T.; Smith, C. Therapeutic Application of Lantibiotics and Other Lanthipeptides: Old and New Findings. Appl. Environ. Microbiol. 2021, 87, e00186-21.
- Wiedemann, I.; Breukink, E.; van Kraaij, C.; Kuipers, O.P.; Bierbaum, G.; de Kruijff, B.; Sahl, H.-G. Specific Binding of Nisin to the Peptidoglycan Precursor Lipid II Combines Pore Formation and Inhibition of Cell Wall Biosynthesis for Potent Antibiotic Activity. J. Biol. Chem. 2001, 276, 1772–1779.
- Hsu, S.D.; Breukink, E.; Tischenko, E.; Lutters, M.A.G.; Kruijff, B.D.; Kaptein, R.; Bonvin, A.M.J.J.; van Nuland, N.A.J. The Nisin—Lipid II Complex Reveals a Pyrophosphate Cage That Provides a Blueprint for Novel Antibiotics. Nat. Struct. Mol. Biol. 2004, 11, 963–967.
- Helander, I.M.; Mattila-Sandholm, T. Permeability Barrier of the Gram-Negative Bacterial Outer Membrane with Special Reference to Nisin. Int. J. Food Microbiol. 2000, 60, 153–161.
- Halami, P.M. Sublichenin, a New Subtilin-like Lantibiotics of Probiotic Bacterium Bacillus Licheniformis MCC 2512 T with Antibacterial Activity. Microb. Pathog. 2019, 128, 139–146.
- Banerjee, S.; Hansen, J.N. Structure and Expression of a Gene Encoding the Precursor of Subtilin, a Small Protein Antibiotic. J. Biol. Chem. 1988, 263, 9508–9514.
- Wei, Z.; Shan, C.; Zhang, L.; Ge, D.; Wang, Y.; Xia, X.; Liu, X.; Zhou, J. A Novel Subtilin-like Lantibiotics Subtilin JS-4 Produced by Bacillus Subtilis JS-4, and Its Antibacterial Mechanism against Listeria Monocytogenes. LWT-Food Sci. Technol. 2021, 142, 110993.
- Dischinger, J.; Josten, M.; Szekat, C.; Sahl, H.G.; Bierbaum, G. Production of the Novel Two-Peptide Lantibiotic Lichenicidin by Bacillus Licheniformis DSM 13. PLoS ONE 2009, 4, e0006788.
- Barbosa, J.C.; Silva, Í.C.; Caetano, T.; Mösker, E.; Seidel, M.; Lourenço, J.; Süssmuth, R.D.; Santos, N.C.; Gonçalves, S.; Mendo, S. Assessing the Potential of the Two-Peptide Lantibiotic Lichenicidin as a New Generation Antimicrobial. World J. Microbiol. Biotechnol. 2022, 38, 18.
- Begley, M.; Cotter, P.D.; Hill, C.; Ross, R.P. Identification of a Novel Two-Peptide Lantibiotic, Lichenicidin, Following Rational Genome Mining for LanM Proteins. Appl. Environ. Microbiol. 2009, 75, 5451–5460.
- Shenkarev, Z.O.; Finkina, E.I.; Nurmukhamedova, E.K.; Balandin, S.V.; Mineev, K.S.; Nadezhdin, K.D.; Yakimenko, Z.A.; Tagaev, A.A.; Temirov, Y.V.; Arseniev, A.S.; et al. Isolation, Structure Elucidation, and Synergistic Antibacterial Activity of a Novel Two-Component Lantibiotic Lichenicidin from Bacillus Licheniformis VK21. Biochemistry 2010, 49, 6462–6472.
- Prieto, M.L.; O’Sullivan, L.; Tan, S.P.; McLoughlin, P.; Hughes, H.; O’Connor, P.M.; Cotter, P.D.; Lawlor, P.G.; Gardiner, G.E. Assessment of the Bacteriocinogenic Potential of Marine Bacteria Reveals Lichenicidin Production by Seaweed-Derived Bacillus spp. Mar. Drugs 2012, 10, 2280–2299.
- Alvarez-Ordóñez, A.; Begley, M.; Clifford, T.; Deasy, T.; Considine, K.; O’Connor, P.; Paul Ross, R.; Hill, C. Investigation of the Antimicrobial Activity of Bacillus Licheniformis Strains Isolated from Retail Powdered Infant Milk Formulae. Probiotics Antimicrob. Proteins 2014, 6, 32–40.
- Mendo, S.; Faustino, N.A.; Sarmento, A.C.; Amado, F.; Moir, A.J.G. Purification and Characterization of a New Peptide Antibiotic Produced by a Thermotolerant Bacillus Licheniformis Strain. Biotechnol. Lett. 2004, 26, 115–119.
- Caetano, T.; Krawczyk, J.M.; Mösker, E.; Süssmuth, R.D.; Mendo, S. Heterologous Expression, Biosynthesis, and Mutagenesis of Type II Lantibiotics from Bacillus Licheniformis in Escherichia Coli. Chem. Biol. 2011, 18, 90–100.
- Barbosa, J.C.; Goncalves, S.; Makowski, M.; Silva, I.C.; Caetano, T.; Schneider, T.; Mosker, E.; Süssmuth, R.D.; Santos, N.C.; Mendo, S.; et al. Insights into the Mode of Action of the Two-Peptide Lantibiotic Lichenicidin. Colloids Surfaces B Biointerfaces 2022, 211, 112308.
- Panina, I.S.; Balandin, S.V.; Tsarev, A.V.; Chugunov, A.O.; Tagaev, A.A.; Finkina, E.I.; Antoshina, D.V.; Sheremeteva, E.V.; Paramonov, A.S.; Rickmeyer, J.; et al. Specific Binding of the α-Component of the Lantibiotic Lichenicidin to the Peptidoglycan Precursor Lipid II Predetermines Its Antimicrobial Activity. Int. J. Mol. Sci. 2023, 24, 1332.
- Abdel-Mohsein, H.S.; Sasaki, T.; Tada, C.; Nakai, Y. Characterization and Partial Purification of a Bacteriocin-like Substance Produced by Thermophilic Bacillus Licheniformis H1 Isolated from Cow Manure Compost. Anim. Sci. J. 2011, 82, 340–351.
- Smitha, S.; Bhat, S.G. Thermostable Bacteriocin BL8 from Bacillus Licheniformis Isolated from Marine Sediment. J. Appl. Microbiol. 2013, 114, 688–694.
- Chang, J.Y.; Lee, H.H.; Kim, I.C.; Chang, H.C. Characterization of bacteriocin produced by Bacillus licheniformis cy2. J. Korean Soc. Food Sci. Nutr. 2001, 30, 410–414.
- Berić, T.; Stanković, S.; Draganić, V.; Kojić, M.; Lozo, J.; Fira, D. Novel Antilisterial Bacteriocin Licheniocin 50.2 from Bacillus Licheniformis VPS50.2 Isolated from Soil Sample. J. Appl. Microbiol. 2014, 116, 502–510.
- Sharma, S.; Singh, R.L.; Kakkar, P. Bacillus Licheniformis IITRHR2: A Novel Source of Antimicrobial Proteinaceous Food Substance. J. Microbiol. Antimicrob. 2010, 2, 127–133.
- Guo, Y.; Yu, Z.; Xie, J.; Zhang, R. Identification of a New Bacillus Licheniformis Strain Producing a Bacteriocin-like Substance. J. Microbiol. 2012, 50, 452–458.
- Nithya, V.; Halami, P.M. Antibacterial Peptides, Probiotic Properties and Biopreservative Efficacy of Native Bacillus Species Isolated from Different Food Sources. Probiotics Antimicrob. Proteins 2012, 4, 279–290.
- Vadakedath, N.; Halami, P.M. Characterization and Mode of Action of a Potent Bio-Preservative from Food-Grade Bacillus Licheniformis MCC 2016. Prep. Biochem. Biotechnol. 2019, 49, 334–343.
- Nithya, V.; Murthy, P.S.K.; Halami, P.M. Development and Application of Active Films for Food Packaging Using Antibacterial Peptide of Bacillus Licheniformis Me1. J. Appl. Microbiol. 2013, 115, 475–483.
- Callow, R.; Glover, R.; Hart, P.D.; Hills, G.M. Licheniformin, an Antibiotic Substance from Bacillus Licheniformis, Active against Mycobacterium Tuberculosis. Br. J. Exp. Pathol. 1947, 28, 418–440.
- Makumba, B.A.N.; Mwaura, F.B.; Mutitu, E.W. In Vitro and in Vivo Tests of Bacillus Licheniformis MGrP1 Antibiotics Culture Filtrate as a Potential Biocontrol Agent against Bean Anthracnose. E. Afr. J. Pure Appl. Sci. 2009, 2, 1–16.
- Lebbadi, M.; Gálvez, A.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Fungicin M4: A Narrow Spectrum Peptide Antibiotic from Bacillus Licheniformis M-4. J. Appl. Bacteriol. 1994, 77, 49–53.
- Esmaeilishirazifard, E.; Dariush, A.; Moschos, S.A.; Keshavarz, T. A Novel Antifungal Property for the Bacillus Licheniformis ComX Pheromone and Its Possible Role in Inter-Kingdom Cross-Talk. Appl. Microbiol. Biotechnol. 2018, 102, 5197–5208.
- Galvez, A.; Valdivia, E.; Gonzalez-segura, A.; Lebbadi, M.; Martinez-Bueno, M.; Maqueda, M. Purification, Characterization, and Lytic Activity against Naegleria Fowleri of Two Amoebicins Produced by Bacillus Licheniformis A12. Appl. Environ. Microbiol. 1993, 59, 1480–1486.
- Lebbadi, M.; Gálvez, A.; Valdivia, E.; Martínez-Blueno, M.; Maqueda, M. Purification of Amoebolytic Substances from Bacillus Licheniformis M-4. Arch. Microbiol. 1994, 162, 98–102.
- Galvez, A.; Maqueda, M.; Cordovilla, P.; Martinez-Bueno, M.; Lebbadi, M.; Valdivia, E. Characterization and Biological Activity against Naegleria Fowleri of Amoebicins Produced by Bacillus Licheniformis D-13. Antimicrob. Agents Chemother. 1994, 38, 1314–1319.
- Yu, X.; Han, X.; Li, Y.; Sun, Z.; Dong, C. Isolation, Identification and Prokaryotic Expression of a Bacteriocin-like Substance from Bacillus Licheniformis. Sheng Wu Gong Cheng Xue Bao 2021, 37, 2453–2462.
- Dusane, D.H.; Damare, S.R.; Nancharaiah, Y.V.; Ramaiah, N.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Disruption of Microbial Biofilms by an Extracellular Protein Isolated from Epibiotic Tropical Marine Strain of Bacillus Licheniformis. PLoS ONE 2013, 8, e0064501.
- Wang, Z.; Wang, Y.; Zheng, L.; Yang, X.; Liu, H.; Guo, J. Isolation and Characterization of an Antifungal Protein from Bacillus Licheniformis HS10. Biochem. Biophys. Res. Commun. 2014, 454, 48–52.
- Jamal, M.T.; Morris, P.C.; Hansen, R.; Jamieson, D.J.; Burgess, J.G.; Austin, B. Recovery and Characterization of a 30.7-KDa Protein from Bacillus Licheniformis Associated with Inhibitory Activity against Methicillin-Resistant Staphylococcus Aureus, Vancomycin-Resistant Enterococci, and Listeria Monocytogenes. Mar. Biotechnol. 2006, 8, 587–592.
- Xiao, L.; Xie, C.C.; Cai, J.; Lin, Z.J.; Chen, Y.H. Identification and Characterization of a Chitinase-Produced Bacillus Showing Significant Antifungal Activity. Curr. Microbiol. 2009, 58, 528–533.
- Toharisman, A.; Suhartono, M.T.; Spindler-Barth, M.; Hwang, J.K.; Pyun, Y.R. Purification and Characterization of a Thermostable Chitinase from Bacillus Licheniformis Mb-2. World J. Microbiol. Biotechnol. 2005, 21, 733–738.
- Tantimavanich, S.; Pantuwatana, S.; Bhumiratana, A.; Panbangred, W. Multiple Chitinase Enzymes from a Single Gene of Bacillus Licheniformis TP-1. J. Ferment. Bioeng. 1998, 85, 259–265.
- Slimene, I.B.; Tabbene, O.; Gharbi, D.; Mnasri, B.; Schmitter, J.M.; Urdaci, M.C.; Limam, F. Isolation of a Chitinolytic Bacillus Licheniformis S213 Strain Exerting a Biological Control Against Phoma Medicaginis Infection. Appl. Biochem. Biotechnol. 2015, 175, 3494–3506.
- Sasi, A.; Duraipandiyan, N.; Marikani, K.; Dhanasekaran, S.; Al-Dayan, N.; Venugopal, D. Identification and Characterization of a Newly Isolated Chitinase-Producing Strain Bacillus Licheniformis SSCL-10 for Chitin Degradation. Archaea 2020, 2020, 8844811.
- Akeed, Y.; Atrash, F.; Naffaa, W. Partial Purification and Characterization of Chitinase Produced by Bacillus Licheniformis B307. Heliyon 2020, 6, e03858.
- Cui, T.B.; Chai, H.Y.; Jiang, L.X. Isolation and Partial Characterization of an Antifungal Protein Produced by Bacillus Licheniformis BS-3. Molecules 2012, 17, 7336–7347.
- Waghmare, S.R.; Randive, S.A.; Jadhav, D.B.; Nadaf, N.H.; Parulekar, R.S.; Sonawane, K.D. Production of Novel Antimicrobial Protein from Bacillus Licheniformis Strain JS and Its Application against Antibiotic-Resistant Pathogens. J. Proteins Proteomics 2019, 10, 17–22.
- Korenblum, E.; Sebastián, G.V.; Paiva, M.M.; Coutinho, C.M.L.M.; Magalhães, F.C.M.; Peyton, B.M.; Seldin, L. Action of Antimicrobial Substances Produced by Different Oil Reservoir Bacillus Strains against Biofilm Formation. Appl. Microbiol. Biotechnol. 2008, 79, 97–103.
- Arbsuwan, N.; Sirithorn, P.; Daduang, S.; Dhiravisit, A.; Thammasirirak, S. Purification and Characterization of Antimicrobial Substances from Bacillus Licheniformis BFP011. Appl. Biochem. Microbiol. 2014, 50, 580–587.
- Tareq, F.S.; Kim, J.H.; Lee, M.A.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Erratum: Ieodoglucomides A and B from a Marine-Derived Bacterium Bacillus Licheniformis (Organic Letters (1466)). Org. Lett. 2013, 15, 2071.
- Tareq, F.S.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, New Glycolipids from a Marine-Derived Bacterium Bacillus Licheniformis 09IDYM23. Lipids 2015, 50, 513–519.
- Karim, R.; Mahmud, N.; Sharifuzzaman, M.; Islam, H. Production of Bacteriocin Like Substances as Antipathogenic Metabolites by Bacillus Licheniformis Isolated from Healthy Human Skin. Int. J. Sci. Basic Appl. Res. 2017, 36, 48–60.
- Karim, R.; Nuruddin Mahmud, M.A.H. Detection of Bacteriocin like Substances from Normal Skin Microflora as Alternative to Conventional Antibiotics. Asian J. Agric. Biol. 2019, 7, 531–537.
- Šurín Hudáková, N.; Kačírová, J.; Sondorová, M.; Šelianová, S.; Mucha, R.; Maďar, M. Inhibitory Effect of Bacillus Licheniformis Strains Isolated from Canine Oral Cavity. Life 2022, 12, 1238.
- Jebur, H.A.; Auda, J.M. Evalution of Antimicrobial Activity of Partial Purified Bacteriocin from Local Isolate of Bacillus Licheniforims HJ2020 MT192715.1. Iraqi J. Agric. Sci. 2020, 51, 1644–1652.
More
Information
Subjects:
Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
14 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




