
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Augusto Zanella | -- | 4306 | 2023-07-13 12:41:54 | | | |
| 2 | Dean Liu | -25 word(s) | 4281 | 2023-07-14 04:02:48 | | | | |
| 3 | Dean Liu | Meta information modification | 4281 | 2023-07-31 08:22:30 | | |
Video Upload Options
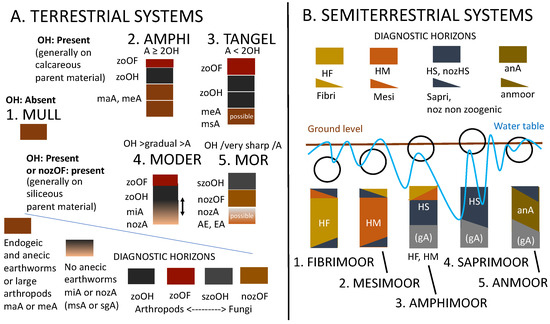
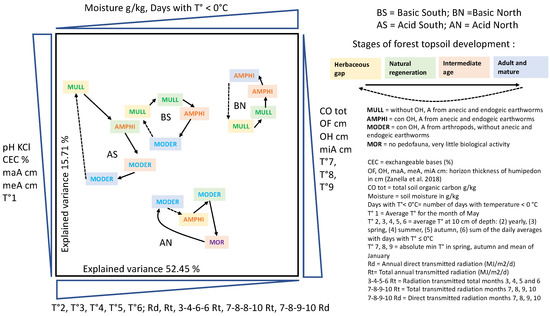
Three sections (Humipedon, Copedon and Lithopedon) were recognized in the soil profile. It was then possible to link the first and most biologically active section to the characteristics of the environment and soil genesis. In particular, it is now possible to distinguish organic horizons, mainly produced by arthropods and enchytraeids in cold and acidic or dry and arid environments, from organo-mineral horizons produced by earthworms in more temperate and mesotrophic environments. Each set of horizons can be associated with a humus system or form, with important implications for forestry. Anecic/endogeic earthworms and Mull or Amphi systems are more abundant in the early and late stages of sylvogenesis; by completely recycling litter, earthworms accelerate the availability of organic and inorganic soil nutrients to roots and pedofauna. On the other hand, arthropods and Moder or Tangel systems characterize the intermediate stages of sylvogenesis, where thickening in the organic horizons and the parallel impoverishment/reduction in the underlying organo-mineral horizons are observed. Recognizing the humus system at the right spatial and temporal scale is crucial for the biological management of a forest.
1. What Is Soil, and What, in Particular, Is Forest Soil?

2. The Humipedon in Mountain and High-Mountain Forest Environments

3. Humipedon and Forest Management
References
- Pavlis, R. Humus Does Not Exist—Says New Study. 2016. Available online: https://www.gardenmyths.com/humus-does-not-exist-says-new-study/ (accessed on 24 June 2023).
- Jenny, H. Alcohol or Humus? Science 1980, 209, 444.
- Kopnina, H.; Washington, H.; Taylor, B.; J Piccolo, J. Anthropocentrism: More than Just a Misunderstood Problem. J. Agric. Environ. Ethics 2018, 31, 109–127.
- Drosos, M.; Nebbioso, A.; Piccolo, A. Humeomics: A key to unravel the humusic pentagram. Appl. Soil Ecol. 2018, 123, 513–516.
- Orwin, K.H.; Dickie, I.A.; Wood, J.R.; Bonner, K.I.; Holdaway, R.J. Soil microbial community structure explains the resistance of respiration to a dry–rewet cycle, but not soil functioning under static conditions. Funct. Ecol. 2016, 30, 1430–1439.
- Zanella, A.; Ponge, J.-F.; Jabiol, B.; Van Delft, B.; De Waal, R.; Katzensteiner, K.; Kolb, E.; Bernier, N.; Mei, G.; Blouin, M.; et al. A Standardized Morpho-Functional Classification of the Planet’s Humipedons. Soil Syst. 2022, 6, 59.
- Gobat, J.-M.; Guenat, C. Sols et Paysages—Types de Sols, Fonctions et Usages en Europe Moyenne, 1st ed.; Science et ingénierie de l’environnement; EPFL Press: Lausanne, Switzerland, 2019; p. 576.
- Zanella, A.; Berg, B.; Ponge, J.-F.; Kemmers, R.H. Humusica 1, article 2: Essential bases—Functional considerations. Appl. Soil Ecol. 2018, 122, 22–41.
- Brandolese, A. Studio Preliminare di Suoli di Cengia. Esempi di Forme di Humus su Roccia Madre Acida—Preliminary Study of Ledge Soils. Examples of Humus Forms on Acid Parent Material; Prova finale di Laurea triennale in Tecnologie Forestali e Ambientali; Università degli Studi di Padova: Padova, Italy, 2020; pp. 1–40.
- Carollo, S. Studio Preliminare di Suoli di Cengia. Esempi di Forme di Humus su Roccia Madre Basica—Preliminary Study of Ledge Soils. Examples of Humus Forms on Basic Parent Material; Prova finale di Laurea triennale in Tecnologie Forestali e Ambientali; Università degli Studi di Padova: Padova, Italy, 2020; pp. 1–62.
- Artz, R.; Anastasiou, D.; Arrouays, D.D.; Bastos, A.C.; Bendetti, A.; Bispo, A.; Brandmayr, P.; Broll, G.; Bunning, S.; Castracani, C.; et al. European Atlas of Soil Biodiversity; European Commission: Ispra, Italy, 2010; pp. 1–136.
- Geisen, S.; Briones, M.J.I.; Gan, H.; Behan-Pelletier, V.M.; Friman, V.-P.; de Groot, G.A.; Hannula, S.E.; Lindo, Z.; Philippot, L.; Tiunov, A.V.; et al. A methodological framework to embrace soil biodiversity. Soil Biol. Biochem. 2019, 136, 107536.
- Bach, E.M.; Ramirez, K.S.; Fraser, T.D.; Wall, D.H. Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability 2020, 12, 2662.
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325.
- Zanella, A.; Ponge, J.-F.; Andreetta, A.; Aubert, M.; Bernier, N.; Bonifacio, E.; Bonneval, K.; Bolzonella, C.; Chertov, O.; De Nobili, M.; et al. Forest Biodiversity, Soil Functions and Human Behavior—A Case Study. The October 29, 2018 Catastrophe in Italian Alps. Available online: https://hal.archives-ouvertes.fr/hal-02342793 (accessed on 24 June 2023).
- Rees, M. Delayed Germination of Seeds: A Look at the Effects of Adult Longevity, the Timing of Reproduction, and Population Age/Stage Structure. Am. Nat. 1994, 144, 43–64.
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005; pp. 1–260.
- Miller, S.L.; Urey, H.C. Organic Compound Synthes on the Primitive Earth. Science 1959, 130, 245–251. Available online: http://science.sciencemag.org/content/130/3370/245.abstract (accessed on 24 June 2023).
- Wamelink, G.W.W.; Frissel, J.Y.; Krijnen, W.H.J.; Verwoert, M.R.; Goedhart, P.W. Can plants grow on mars and the moon: A growth experiment on mars and moon soil simulants. PLoS ONE 2014, 9, e103138.
- Ponge, J.-F. The soil as an ecosystem. Biol. Fertil. Soils 2015, 51, 645–648.
- Filotas, E.; Parrott, L.; Burton, P.J.; Chazdon, R.L.; Coates, K.D.; Coll, L.; Haeussler, S.; Martin, K.; Nocentini, S.; Puettmann, K.J.; et al. Viewing forests through the lens of complex systems science. Ecosphere 2014, 5, art1.
- Edwards, C.A.; Arancon, N.Q. The Influence of Environmental Factors on Earthworms; Springer: New York, NY, USA, 2022; pp. 191–232.
- Richardson, D.R.; Snyder, B.A.; Hendrix, P.F. Soil Moisture and Temperature: Tolerances and Optima for a Non-native Earthworm Species, Amynthas agrestis (Oligochaeta: Opisthopora: Megascolecidae). Southeast. Nat. 2009, 8, 325–334.
- Edwards, C.A.; Arancon, N.Q. Biology and Ecology of Earthworms; Springer: New York, NY, USA, 2022; p. XVI-567. Available online: https://link.springer.com/book/10.1007/978-0-387-74943-3?source=shoppingads&locale=en-it&gclid=EAIaIQobChMI5c7HnOiF_wIVKhGLCh1WnQVJEAQYASABEgLRq_D_BwE#toc (accessed on 24 June 2023).
- Darwin, C. The Formation of Vegetable Mould through the Action of Worms, with Observations on Their Habits; John Murray: London, UK, 1881; p. 326.
- Ponge, J.F.; André, J.; Bernier, N.; Gallet, C. La régénération naturelle: Connaissances actuelles. le cas de l’épicéa en forêt de Macot (Savoie). Rev. For. Française 1994, 46, 25–45.
- Bernier, N. Earthworm feeding activity and development of the humus profile. Biol. Fertil. Soils 1998, 26, 215–223.
- Barois, I. Mucus production and microbial activity in the gut of two species of amynthas (megascolecidae) from cold and warm tropical climates. Soil Biol. Biochem. 1992, 24, 1507–1510.
- Barois, I.; Villemin, G.; Lavelle, P.; Toutain, F. Transformation of the soil structure through Pontoscolex corethrurus (Oligochaeta) intestinal tract. Geoderma 1993, 56, 57–66.
- André, J.; Gensac, P.; Pellissier, F.; Trosset, L. Régénération des peuplements d’épicéa en altitude: Recherches préliminaires sur le rôle de l’allélopathie et de la mycorhization dans les premiers stades du développement. Rev. D’écologie Biol. Du Sol 1987, 24, 301–310.
- André, J.; Gauthier, P.; Gensac, M. La Régénération dans la pessière à myrtille: Description préliminaire de deux stations dans les Alpes septentrionales internes. Bull. D’ecologie 1990, 21, 51–61.
- Bernier, N.; André, J. Dynamique de la biodiversité en milieu subalpin: Exemple de diversité des processus et gestion de l’espace en moyenne vallée de Tarentaise (Savoie, France): {Biodiversity dynamics at the subalpine level: A case study of processes diversity and land manag. Ecologie 1998, 29, 547–555. Available online: https://www.researchgate.net/publication/233902840_Dynamique_de_la_biodiversite_en_milieu_subalpin_exemple_de_diversite_des_processus_et_gestion_de_l%27espace_en_moyenne_vallee_de_Tarentaise_Savoie_France_Biodiversity_dynamics_at_the_subalpine_level_a_c (accessed on 24 June 2023).
- Riera, B. Importance des buttes de déracinement dans la régénération forestière en Guyane française. Revue d’Écologie 1985, 40, 321–329.
- Brown, J.L. Étude de la perturbation des horizons du sol par un arbre qui se renverse et de son impact sur la pédogenèse. Can. J. Soil Sci. 1977, 57, 173–186.
- Romell, L.G. An Example of Myriapods as Mull Formers. Ecology 1935, 16, 67–71.
- Zackrisson, O.; Bernier, N.; Nilsson, M.-C.; Gallet, C. The Forest Regeneration Puzzle. BioScience 1998, 48, 523–530.
- Van der Meer, P.J.; Bongers, F. Patterns of Tree-Fall and Branch-Fall in a Tropical Rain Forest in French Guiana. J. Ecol. 1996, 84, 19–29.
- Galvan, P.; Ponge, J.-F.J.F.; Chersich, S.; Zanella, A. Humus Components and Soil Biogenic Structures in Norway Spruce Ecosystems. Soil Sci. Soc. Am. J. 2008, 72, 548–557.
- Bouché, M. Des vers de Terre et des Hommes. Découvrir nos Ecosystèmes Fonctionnant à L’énergie Solaire; Acte Sud Nature: Paris, France, 2014; p. 336.
- Selosse, M.-A. Jamais Seul—Ces Microbes qui Construisent les Plantes, les Animaux et les Civilisations; Actes Sud: Arles, France, 2017; p. 352.
- Lovelock, J.E. Hands up for the Gaia hypothesis. Nature 1990, 344, 100–102.
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736.
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590.
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841.
- Wang, Z.; Feng, B.; Zhang, D.; Ghosh, S.; Pan, B.; Xing, B. Role of NOM–hematite nanoparticle complexes and organic and inorganic cations in the coherence of silica and clay particles: Evaluation based on nanoscale forces and molecular self-assembly. Environ. Sci. Nano 2021, 8, 822–836.
- Mahmoodi, K.; West, B.J.; Grigolini, P. Self-organizing Complex Networks: Individual versus global rules. Front. Physiol. 2017, 8, 478.
- Feng, W.; Plante, A.F.; Aufdenkampe, A.K.; Six, J. Soil organic matter stability in organo-mineral complexes as a function of increasing C loading. Soil Biol. Biochem. 2014, 69, 398–405.
- Zanella, A.; Ponge, J.-F.; Jabiol, B.; Sartori, G.; Kolb, E.; Le Bayon, R.-C.; Gobat, J.-M.; Aubert, M.; De Waal, R.; Van Delft, B.; et al. Humusica 1, article 5: Terrestrial humus systems and forms—Keys of classification of humus systems and forms. Appl. Soil Ecol. 2018, 122, 75–86.
- Giannini, R.; Susmel, L. Forests, woods, forest plantations. —Riv. Selvic. Ecol. For. 2006, 3, 464–487.
- Oldeman, R.A.A. Forests: Elements of Silvology; Springer: Berlin/Heidelberg, Germany, 2012; p. 624.
- Stutz, K.P.; Lang, F. Forest ecosystems create pedogenic patchworks through woody debris, trees, and disturbance. Geoderma 2023, 429, 116246.
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463.
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237.
- Bernier, N.; Ponge, J.F. Humus form dynamics during the sylvogenetic cycle in a mountain spruce forest. Soil Biol. Biochem. 1994, 26, 183–220.
- Mori, K.; Bernier, N.; Kosaki, T.; Ponge, J.-F. Tree influence on soil biological activity: What can be inferred from the optical examination of humus profiles? Eur. J. Soil Biol. 2009, 45, 290–300.
- Bernier, N. Fonctionnement biologique des humus et dynamique des pessières alpines. Le cas de la forêt de Macot-La-Plagne (Savoie). . Ecologie 1997, 28, 23–44.
- Zampedri, R. Relazione tra Clima, Forme di Humus e Dinamica Forestale in Ambiente di Pecceta Altimontana; Tesi di Dottorato: Padova, Italy, 2005.
- Ponge, J.-F. Plant–soil feedbacks mediated by humus forms: A review. Soil Biol. Biochem. 2013, 57, 1048–1060.
- Motta, R.; Brang, P.; Frehner, M.; Ott, E. Copertura muscinale e rinnovazione di abete rosso (Picea abies L.) nella pecceta subalpina di Sedrum (Grigioni, Svizzera). Monti E Boschi 1994, 3, 49–56.
- Baier, R.; Ettl, R.; Hahn, C.; Göttlein, A. Early development and nutrition of Norway spruce (Picea abies (L.) Karst.) seedlings on different seedbeds in the Bavarian limestone Alps—A bioassay. Ann. For. Sci. 2006, 63, 339–348.
- Bütler, R.; Patty, L.; Le Bayon, R.-C.; Guenat, C.; Schlaepfer, R. Log decay of Picea abies in the Swiss Jura Mountains of central Europe. For. Ecol. Manag. 2007, 242, 791–799.
- Susmel, L. Normalizzazione Delle Foreste Alpine: Basi Ecosistemiche, Equilibrio, Modelli Colturali, Produttività: Con Applicazione Alle Foreste del Trentino; Liviana: Padova, Italy, 1980; p. 437.
- Martin, M.; Paillet, Y.; Larrieu, L.; Kern, C.C.; Raymond, P.; Drapeau, P.; Fenton, N.J. Tree-Related Microhabitats Are Promising Yet Underused Tools for Biodiversity and Nature Conservation: A Systematic Review for International Perspectives. Front. For. Glob. Change 2022, 5, 136.
- McMahen, K.; Anglin, C.D.; Lavkulich, L.M.; Grayston, S.J.; Simard, S.W. Small-volume additions of forest topsoil improve root symbiont colonization and seedling growth in mine reclamation. Appl. Soil Ecol. 2022, 180, 104622.
- Wohlleben, P. The Hidden Life of Trees: What They Feel, How They Communicate—Discoveries from a Secret World; Greystone Books: Vancouver, Canada, 2016; p. 288.




