
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tsun-Thai Chai | -- | 1908 | 2023-07-13 03:46:03 | | | |
| 2 | Lindsay Dong | -24 word(s) | 1884 | 2023-07-14 04:49:35 | | |
Video Upload Options
Lipoxygenases are non-heme iron-containing enzymes that catalyze the oxidation of polyunsaturated fatty acids, resulting in the production of lipid hydroperoxides, which are precursors of inflammatory lipid mediators. These enzymes are widely distributed in humans, other eukaryotes, and cyanobacteria. Lipoxygenases hold promise as therapeutic targets for several human diseases, including cancer and inflammation-related disorders. Inhibitors of lipoxygenase have potential applications in pharmaceuticals, cosmetics, and food. Bioactive peptides are short amino acid sequences embedded within parent proteins, which can be released by enzymatic hydrolysis, microbial fermentation, and gastrointestinal digestion. A wide variety of bioactivities have been documented for protein hydrolysates and peptides derived from different biological sources.
1. Introduction
2. Lipoxygenases (LOX)
3. Production of Anti-LOX Protein Hydrolysates and Peptides
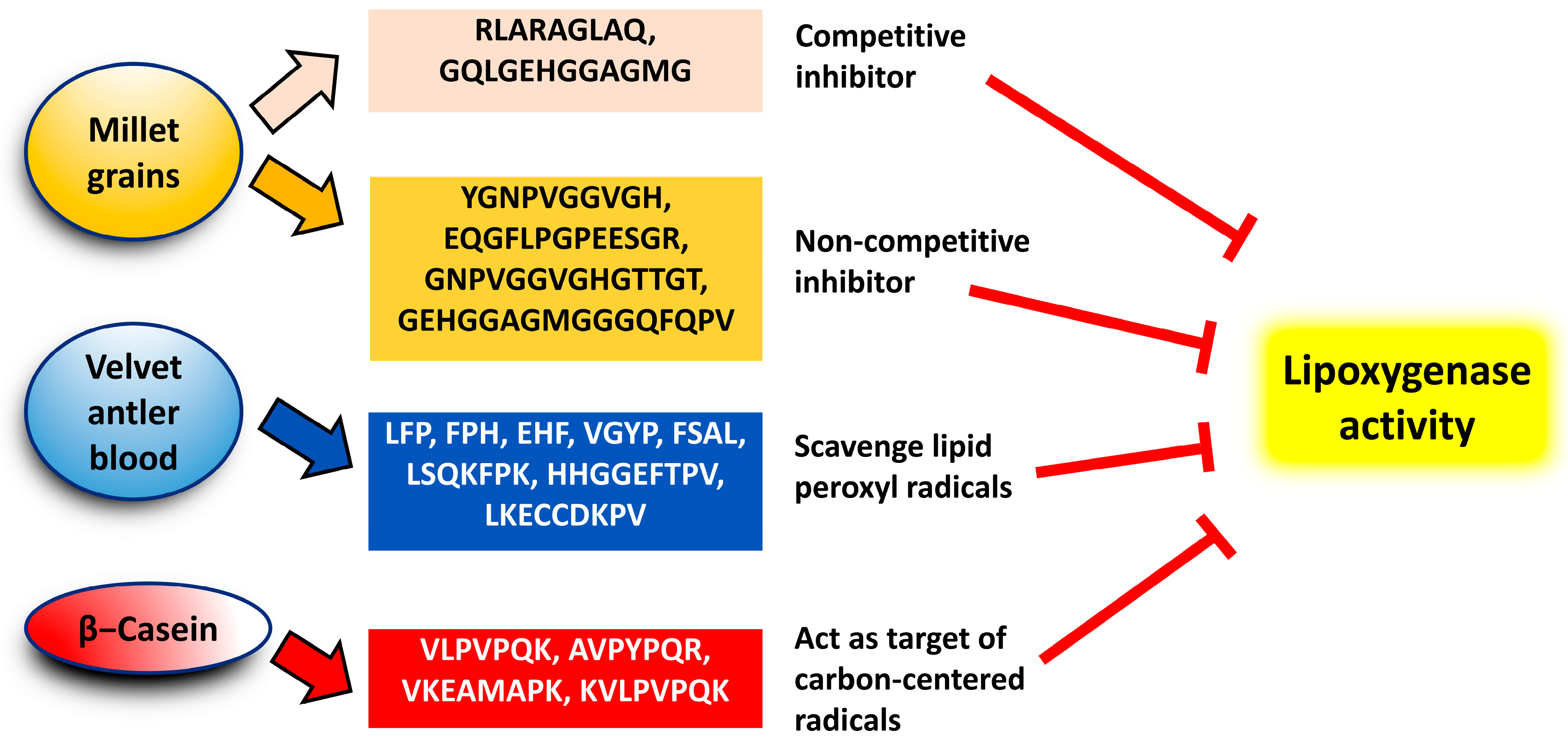
4. Potency and Modes of Action
References
- Chai, T.-T.; Ee, K.-Y.; Kumar, D.T.; Manan, F.A.; Wong, F.-C. Plant bioactive peptides: Current status and prospects towards use on human health. Protein Pept. Lett. 2021, 28, 623–642.
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42.
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57.
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978.
- Singh, B.P.; Aluko, R.E.; Hati, S.; Solanki, D. Bioactive peptides in the management of lifestyle-related diseases: Current trends and future perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 4593–4606.
- Złotek, U.; Jakubczyk, A.; Rybczyńska-Tkaczyk, K.; Ćwiek, P.; Baraniak, B.; Lewicki, S. Characteristics of new peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as inhibitors of enzymes involved in metabolic syndrome and antimicrobial potential. Molecules 2020, 25, 2492.
- Tawalbeh, D.; Al-U’datt, M.H.; Wan Ahmad, W.A.N.; Ahmad, F.; Sarbon, N.M. Recent advances in in vitro and in vivo studies of antioxidant, ace-inhibitory and anti-inflammatory peptides from legume protein hydrolysates. Molecules 2023, 28, 2423.
- Thaha, A.; Wang, B.-S.; Chang, Y.-W.; Hsia, S.-M.; Huang, T.-C.; Shiau, C.-Y.; Hwang, D.-F.; Chen, T.-Y. Food-derived bioactive peptides with antioxidative capacity, xanthine oxidase and tyrosinase inhibitory activity. Processes 2021, 9, 747.
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells: Via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906.
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Study on the structure–activity relationship of watermelon seed antioxidant peptides by using molecular simulations. Food Chem. 2021, 364, 130432.
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. 2021, 3, 100047.
- Ye, H.; Tao, X.; Zhang, W.; Chen, Y.; Yu, Q.; Xie, J. Food-derived bioactive peptides: Production, biological activities, opportunities and challenges. J. Future Foods 2022, 2, 294–306.
- Wong, F.-C.; Xiao, J.; Ong, M.G.L.; Pang, M.-J.; Wong, S.-J.; Teh, L.-K.; Chai, T.-T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622.
- Quah, Y.; Tong, S.-R.; Bojarska, J.; Giller, K.; Tan, S.-A.; Ziora, Z.M.; Esatbeyoglu, T.; Chai, T.-T. Bioactive peptide discovery from edible insects for potential applications in human health and agriculture. Molecules 2023, 28, 1233.
- Mohana, D.S.; Chai, T.-T.; Wong, F.-C. Antioxidant and protein protection potentials of fennel seed-derived protein hydrolysates and peptides. Mod. Food Sci. Technol. 2019, 35, 22–29+21.
- Chai, T.-T.; Xiao, J.; Mohana Dass, S.; Teoh, J.-Y.; Ee, K.-Y.; Ng, W.-J.; Wong, F.-C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876.
- Ding, C.; Hao, M.; Ma, S.; Zhang, Y.; Yang, J.; Ding, Q.; Sun, S.; Zhang, J.; Zhang, Y.; Liu, W. Identification of peptides with antioxidant, anti-lipoxygenase, anti-xanthine oxidase and anti-tyrosinase activities from velvet antler blood. LWT 2022, 168, 113889.
- Quah, Y.; Mohd Ismail, N.I.; Ooi, J.L.S.; Affendi, Y.A.; Abd Manan, F.; Wong, F.-C.; Chai, T.-T. Identification of novel cytotoxic peptide KENPVLSLVNGMF from marine sponge Xestospongia testudinaria, with characterization of stability in human serum. Int. J. Pept. Res. Ther. 2018, 24, 189–199.
- Kshetri, P.; Singh, P.L.; Chanu, S.B.; Singh, T.S.; Rajiv, C.; Tamreihao, K.; Singh, H.N.; Chongtham, T.; Devi, A.K.; Sharma, S.K.; et al. Biological activity of peptides isolated from feather keratin waste through microbial and enzymatic hydrolysis. Electron. J. Biotechnol. 2022, 60, 11–18.
- Chen, Y.P.; Liang, C.H.; Wu, H.T.; Pang, H.Y.; Chen, C.; Wang, G.H.; Chan, L.P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. 2018, 55, 2310–2317.
- Ong, J.-H.; Koh, J.-A.; Cao, H.; Tan, S.-A.; Manan, F.A.; Wong, F.-C.; Chai, T.-T. Purification, identification and characterization of antioxidant peptides from corn silk tryptic hydrolysate: An integrated in vitro-in silico approach. Antioxidants 2021, 10, 1822.
- Peña-Ramos, E.A.; Xiong, Y.L. Antioxidative activity of whey protein hydrolysates in a liposomal system. J. Dairy Sci. 2001, 84, 2577–2583.
- Aluko, R.E. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Elsevier Inc.: Cambridge, UK, 2015; pp. 105–140.
- Shi, Y.; Mandal, R.; Singh, A.; Pratap Singh, A. Legume lipoxygenase: Strategies for application in food industry. Legume Sci. 2020, 2, e44.
- Lončarić, M.; Strelec, I.; Moslavac, T.; Šubarić, D.; Pavić, V.; Molnar, M. Lipoxygenase inhibition by plant extracts. Biomolecules 2021, 11, 152.
- Heinrich, L.; Booijink, R.; Khurana, A.; Weiskirchen, R.; Bansal, R. Lipoxygenases in chronic liver diseases: Current insights and future perspectives. Trends Pharmacol. Sci. 2022, 43, 188–205.
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310.
- Ciganović, P.; Jakimiuk, K.; Tomczyk, M.; Zovko Končić, M. Glycerolic licorice extracts as active cosmeceutical ingredients: Extraction optimization, chemical characterization, and biological activity. Antioxidants 2019, 8, 445.
- Jakupović, L.; Bačić, I.; Jablan, J.; Marguí, E.; Marijan, M.; Inić, S.; Nižić Nodilo, L.; Hafner, A.; Zovko Končić, M. Hydroxypropyl-β-cyclodextrin-based Helichrysum italicum extracts: Antioxidant and cosmeceutical activity and biocompatibility. Antioxidants 2023, 12, 855.
- Krieg, P.; Fürstenberger, G. The role of lipoxygenases in epidermis. Biochim. Biophys. Acta-Mol. Cell. Biol. Lipids 2014, 1841, 390–400.
- Jakubczyk, A.; Szymanowska, U.; Karaś, M.; Złotek, U.; Kowalczyk, D. Potential anti-inflammatory and lipase inhibitory peptides generated by in vitro gastrointestinal hydrolysis of heat treated millet grains. CyTA-J. Food 2019, 17, 324–333.
- Grancieri, M.; Martino, H.S.D.; Gonzalez de Mejia, E. Digested total protein and protein fractions from chia seed (Salvia hispanica L.) had high scavenging capacity and inhibited 5-LOX, COX-1-2, and iNOS enzymes. Food Chem. 2019, 289, 204–214.
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 970.
- Jakubczyk, A.; Karaś, M.; Baraniak, B.; Pietrzak, M. The impact of fermentation and in vitro digestion on formation angiotensin converting enzyme (ACE) inhibitory peptides from pea proteins. Food Chem. 2013, 141, 3774–3780.
- Sheng, Z.; Turchini, G.M.; Xu, J.; Fang, Z.; Chen, N.; Xie, R.; Zhang, H.; Li, S. Functional properties of protein hydrolysates on growth, digestive enzyme activities, protein metabolism, and intestinal health of larval largemouth bass (Micropterus salmoides). Front. Immunol. 2022, 13, 913024.
- Kshetri, P.; Roy, S.S.; Sharma, S.K.; Singh, T.S.; Ansari, M.A.; Sailo, B.; Singh, S.; Prakash, N. Feather degrading, phytostimulating, and biocontrol potential of native actinobacteria from North Eastern Indian Himalayan Region. J. Basic Microbiol. 2018, 58, 730–738.
- Kshetri, P.; Roy, S.S.; Chanu, S.B.; Singh, T.S.; Tamreihao, K.; Sharma, S.K.; Ansari, M.A.; Prakash, N. Valorization of chicken feather waste into bioactive keratin hydrolysate by a newly purified keratinase from Bacillus sp. RCM-SSR-102. J. Environ. Manag. 2020, 273, 111195.
- Rival, S.G.; Fornaroli, S.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 1. Lipoxygenase inhibitory properties. J. Agric. Food Chem. 2001, 49, 287–294.
- Gan, T.J. Diclofenac: An update on its mechanism of action and safety profile. Curr. Med. Res. Opin. 2010, 26, 1715–1731.
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001, 49, 295–302.




