Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bingchen He | -- | 2936 | 2023-07-12 05:39:41 | | | |
| 2 | Wendy Huang | Meta information modification | 2936 | 2023-07-12 06:30:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
He, B.; Chen, S.; Su, Z. Crystal Structural Characterization Techniques in Degradation of Perovskite. Encyclopedia. Available online: https://encyclopedia.pub/entry/46666 (accessed on 07 February 2026).
He B, Chen S, Su Z. Crystal Structural Characterization Techniques in Degradation of Perovskite. Encyclopedia. Available at: https://encyclopedia.pub/entry/46666. Accessed February 07, 2026.
He, Bingchen, Shi Chen, Zhenghuang Su. "Crystal Structural Characterization Techniques in Degradation of Perovskite" Encyclopedia, https://encyclopedia.pub/entry/46666 (accessed February 07, 2026).
He, B., Chen, S., & Su, Z. (2023, July 12). Crystal Structural Characterization Techniques in Degradation of Perovskite. In Encyclopedia. https://encyclopedia.pub/entry/46666
He, Bingchen, et al. "Crystal Structural Characterization Techniques in Degradation of Perovskite." Encyclopedia. Web. 12 July, 2023.
Copy Citation
The general chemical formula of halide perovskite materials is ABX3, where the A site can be occupied by organic cations, such as methyl ammonium (MA), formamidinium (FA), or inorganic cation cesium. The B site can be divalent cations, such as lead(Pb) or tin(Sn), while the X site consists of halide anions, such as chlorine(Cl), bromine(Br) or iodine(I). Due to the great variety of its chemical composition, halide perovskite forms a big material family with tunable optoelectronic properties.
perovskite materials
in situ/operando
characterization techniques
crystal structure
degradation
X-ray diffraction
1. Introduction
Currently, the major hurdle in halide perovskite research and applications is its intrinsic stability problem [1]. It is well known that halide perovskite is vulnerable to various environmental stresses, including water, heat, light, oxygen, and electric fields [2][3][4][5]. In early studies, this vulnerability was easily identified by a quick color change of the film and short device lifetime [6]. Later on, multiple characterization techniques, such as X-ray diffraction (XRD), scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM), and so on, were used to study the degradation process in detail [7][8][9][10]. At first glance, the degradation of perovskite seems simple, but systematic studies found that the degradation is a complex process, which is highly dependent on the type of perovskite and the details of the exposure conditions. Therefore, it is important to control the exposure conditions and to monitor the degradation in real time. Keeping this complexity in mind, we could understand why studies with in situ and operando approaches reveals more degradation details than those studies performed in an ex situ manner. With the help of in situ and operando approaches, the degradation mechanism of 3D perovskite (MAPbI3 and FAPbI3) has been thoroughly studied [3][7][8]. It has been found that the degradation of 3D perovskite can be divided into three different categories: morphological change, ion migration, and decomposition. Morphological changes in perovskite materials are primarily due to recrystallization, which usually involves water. A morphological change can happen much earlier than decomposition [11]. However, it may damage the device integrity and be responsible for premature device failure. Ion migration is mainly driven by internal or external electrical fields during the operation of devices [12]. Ion migration threatens the long-term stability and cannot be alleviated by protection methods like encapsulation. The decomposition of perovskite is due to irreversible loss of certain components (mainly organic parts), causing permanent changes in perovskite materials. In general, all three degradation pathways can be accelerated when more than one stresses are present. In a real device, degradation is usually caused by the combination of all three categories, depending on the types and magnitude of degradation stresses it is facing.
In situ and operando are two approaches used in characterization techniques to study the perovskite degradation process. The concept of “in situ” is widely used in surface-related research aiming to study surface changes in an ultrahigh vacuum (UHV) condition to avoid ambient exposure. A precisely controlled environment helps to reveal the surface change that are specific to certain factors. In the study of perovskite degradation, an in situ approach is capable of revealing the degradation clearly due to a specific stress (such as water or heat). However, due to the constraints of a UHV condition, the “strength” of the stress is significantly limited (for water, an in situ study can only reach ~10−5 mbar). On the other hand, the concept of “operando” was first introduced by chemists in 2002 to study catalytical reactions in real time and in realistic conditions [13][14][15]. It a much wider reaction conditions than an in situ approach. In perovskite studies, an operando approach helps to investigate degradation under realistic conditions or beyond. For example, in water-induced degradation studies, an operando approach can reach 90% relative humidity (RH) at room temperature, which is equivalent to ~27 mbar, too high for any ultrahigh vacuum equipment [16]. The two approaches are complementary to each other because an in situ approach pinpoints the detailed mechanism under one of multiple degradation stresses while an operando approach reveals the real degradation dynamics in an environment much closer to a real environment.
Crystallinity is a fundamental property of materials. In fact, the name of perovskite materials is from their crystal structure. Perovskite materials have black and yellow phases, where the black phase includes α, β, and γ structures, corresponding to cubic, tetragonal, and orthorhombic structures, respectively. The yellow phase refers to the δ structure (orthorhombic structure) [17][18][19]. It is well known that the crystal structure of perovskite depends on its composition and temperature [20][21]. For example, MAPbI3 has three crystal structures: cubic (>330 K), tetragonal (160 K~330 K), and orthorhombic (<160 K). Since the degradation of perovskite materials is commonly associated with structural change, many structural characterization techniques have been used in degradation study, such as X-ray diffraction (XRD), grazing incidence wide-angle X-ray scattering (GIWAXS), and grazing incidence neutron scattering (GISANS). These techniques are based on diffraction laws and are sensitive to the structural changes that occur during degradation.
2. Perovskite Degradation Studies by In Situ/Operando X-ray Scattering Techniques
X-ray diffraction is the most widely used technique in crystal structure determination. It uses Bragg’s diffraction law to probe the long-range crystalline order in samples. In iodide perovskite studies, ex situ XRD typically uses the signal of PbI2 at 12.7° to infer the status of perovskite degradation or the quality of pristine perovskite [22][23]. In FAPbI3, XRD also provides its phase information because the photoactive α phase has a different diffraction peak (13.9°) from the photo inactive δ phase (11.9°) [24]. In addition, ex situ XRD can also confirm the existence of a metastable dihydrate (CH3NH3)4PbI6·2H2O if the perovskite were kept in the dark [22][23]. By using the ex situ XRD technique, perovskite degradation under different stresses, such as water, light, and heat, can be easily confirmed [4][6].
Because the hard X-ray used in XRD has a high penetration depth, there is no limit on pressure or other factors in the XRD measurement. Therefore, for this technique, in situ and operando approaches can be easily realized simultaneously. To perform in situ/operando XRD, the sample is usually kept in a commercially available flat airtight chamber with an X-ray window. The window material can be Kapton films. Other accessories, such as for gas regulation and temperature control, may be added to this chamber during in situ studies. For example, in water-induced degradation, a pump and gas inlet are connected to adjust the humidity level [25][26]. A humidity sensor is connected either inside the chamber or along the gas line to monitor the actual humidity level.
The most notable discovery made from operando XRD is the revealing of the monohydrate and dihydrate phases in the water-induced degradation of MAPbI3. Their characteristic peaks are at 8.7° and 11.6°, respectively [27][28][29]. There are two influencing conditions for the formation of hydrate: humidity level and exposure time. Hydrates need higher-than-threshold humidity conditions (~70% RH) to form. It was also observed that the formation of hydrates can be shortened when the relative humidity is even higher. The monohydrate forms first, ~30 min at 80% RH, and the dihydrate phase forms later ~120 min at 80% RH condition. The monohydrate has a molecular formula of MAPbI3·H2O, and it can turn back to MAPbI3 under dry conditions. The formation of monohydrate changes the perovskite structure from 3D to 1D. It incorporates one-dimensional, isolated [PbI3]− double chains, forming a two-octahedra-wide “ribbon” that is similar to δ structures. As the exposure time to water conditions increases, the monohydrate turns into dihydrate, (CH3NH3)4PbI6·2H2O, in which each primary cell of perovskite includes two water molecules. This process is partially reversible due to PbI2 formation. The perovskite structure changes from 1D to 0D. The intermediate phases were found in devices when exposed to water [30]. The formation condition varies depending on the details of the device structure, which may be due to interfacial influences or local water aggregation, resulting in hydrate formation at low humidity. Chen et al. investigated the degradation process of FTO/TiO2/MAPbI3/spiro-OMeTAD/Au devices under ~65% RH humidity using operando XRD [30]. This degradation process can be divided into three stages. In the first stage, the perovskite material first forms hydrate CH3NH3PbI3·H2O, which leads to the loss of its fill factor. In the second stage, the hydrate is transformed into PbI2, which reduces the JSC and VOC values of the device. In the third stage, PbI2 decomposes to form PbIOH (~20.5°) due to the combined effect of light and moisture. operando XRD also observed structural changes due to water-induced ion migration. At 30% RH, Pb0 (31.2°) is formed due to a redox reaction in perovskite with Al electrodes. In devices, perovskite films could form hydrates at RH as low as 30%, which is different for the degradation perovskite thin films (~70% RH) [27]. These results reveal that water molecules in devices could induce a redox reaction between electrode and perovskite, reducing the activation energy of ion migration and facilitating the diffusion of ions.
In heat-induced degradation, operando XRD could observe the composition change more closely. It was observed that MA-perovskite is less heat-stable than FA-perovskite. MA-perovskite decomposed at ~130 °C, while FA-perovskite decomposed at 160 °C. The mixed perovskite material (FA0.83Cs0.17PbI2Br) shows better thermal stability than the MA- and FA-perovskite. Its thermal decomposition process can be divided into three phases [31]. Below 160 °C, the crystal structure is Pm3m, and no phase change or significant decomposition occurs. Though the peak at 14.24° is slightly lower angle, it is thought that the change is caused by the expansion of the structure due to the loss of Br elements. When the temperature is higher than 160 °C, the perovskite decomposes into PbI2 due to the volatilization of FA ions and halogen elements. When the temperature rises to 220 °C, new substances, such as CsPbI3−zBrz (z << 1) with Pnma structures, are formed. Temperature-induced decomposition is highly sensitive to the types of ions in perovskite. Tan et al. compared the thermal degradation processes of FA0.83Cs0.17Pb(I0.83Br0.17)3 and Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3, by in situ XRD [32]. The experimental results show that the triple-cation perovskite material is less thermally stable than the dual-cation perovskite material because of the MA component. Prepared perovskite films containing MACl show better thermal stability than their counterpart without Cl−. In comparison to MAPbI3, perovskite materials with containing Cl− can maintain the ABX3 structure at higher temperature (~300 °C) because the addition of Cl− can suppress PbI2 formation [33].
Light-induced degradation is also studied by in situ XRD in vacuum. The results show that FACs-perovskite shows better light stability than MA-perovskite [34]. Under light conditions with an Xe lamp at 400 W/m2 and after 30 min, the (100) peak of MA-perovskite showed an increased peak width followed by peak splitting at 14.3°, while FACs-perovskite shows no obvious change. For longer light exposure, MA-perovskite further decomposed into metallic lead (Pb0) and lead iodide (PbI2), as shown by diffraction peaks at 12.7°. Factors other than light could greatly affect the degradation pathway of FACs-perovskite. In vacuum, photodegradation mainly creates iodine vacancies. But in air, the lead salts are formed due to removal of organic parts under the presence of oxygen and water. The reason for this is that FACs-perovskites would form I-element domains under light conditions and thus accumulate strain, which can have a stabilizing effect on the perovskite.
Synchrotron-based grazing incidence X-ray diffraction (GIXRD) and grazing incidence wide-angle X-ray scattering (GIWAXS) has been widely used in the structural determination of perovskite solar cells with better surface sensitivity [35][36]. It studies the crystallinity of the top surface by using an X-ray with a very shallow angle of incident. The advantages of GIXRD and GIWAXS are (1) the large detection area due to elongated incident X-ray spot from a very shallow angle; (2) the adjustable sensitivity in the z-direction by changing the grazing incidence angle from very shallow ~0.02° (ultrahigh surface sensitivity) to less shallow ~1° (relative bulk sensitive); and (3) the high sensitivity from high X-ray flux and quick angular information in combination with a two-dimensional surface detector (MarCCD). Ex situ GIXRD and GIWAXS have been widely used as a complementary technique to confirm the structural information of perovskite surfaces [37][38][39].
To perform in situ/operando GIXRD measurements, the test chamber must adapt to the grazing incidence of the X-ray. A flat top window is not suitable for a very shallow incidence angle (~0.02°). Therefore, the setup must use side widows to perform GIXRD measurements. The typical window materials to seal the chamber is Kapton films, which have good mechanical strength and low X-ray absorption coefficient. Alternatively, a complete glovebox as the test chamber can be used to conduct more complicated studies with stable environmental control, one of which is located in the Shanghai synchrotron radiation facility (SSRF). [40][41][42]. Although this setup is mainly to study the formation of perovskite [43], it can also be used to study the degradation process, as shown in Figure 1.
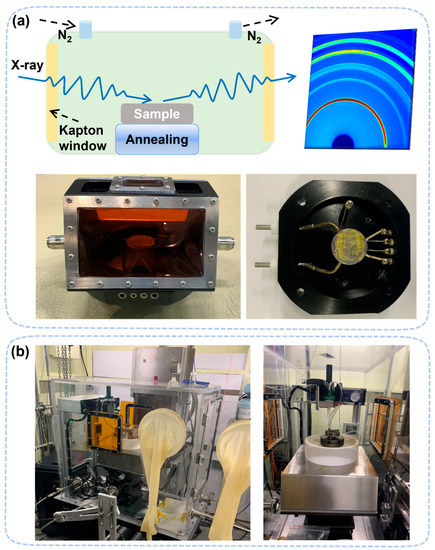
Figure 1. (a) In situ heating device with Kapton film windows on both sides. The operating temperature can range from −180 °C (≈liquid nitrogen temperature) to 200 °C. (b) In situ glove box, which can be used for in situ spin-coating experiments and in situ degradation experiments, including thermal, humidity, and light decomposition.
In situ GIXRD studies have not only confirmed the formation of hydrate, but it also observed surface-related degradation under various stresses. It confirmed the formation of hydrate and PbI2 under a similar relative humidity [25]. Using in situ GIWAXS, Fransishyn et al. showed [26] that the introduction of moisture could increase ion mobility, leading to the screening of the built-in potential and resulting in electrode corrosion. In situ GIWAXS measurements have found that the heat-induced decomposition of the surface of MAPbI3 actually starts as low as 80 °C, which is evidenced by the appearance of a PbI2-related diffraction ring at ≈0.9 Å−1. When the temperature increased from 80 °C to 100 °C, the decomposition progresses faster, showing a degradation time decrease from 1 h to 20 min. The decomposition process, unlike the tetragonal-to-cubic phase transformation, is irreversible. The angle-dependent GIWAXS also revealed that decomposition starts from the surface and progressively advances into the bulk.
3. Perovskite Degradation Studies by In Situ/Operando Neutron Scattering Techniques
The structural information from degradation can also be studied by neutron scattering. It is a less reported method, mainly due to very few experimental facilities capable of performing it. Neutron scattering can provide considerable and unique structural information, which cannot be obtained using XRD. For example, (i) neutron scattering is capable of studying amorphous phases using a radial distribution function, (ii) a neutron scattering cross-section is less dependent on the atomic number, which is useful to study hydrogen atoms in the crystal structure of perovskite, and (iii) neutrons have a higher penetration depth than lab-based X-ray photons, which enables the study of the structure of large crystals. Right now, there are only two papers reported using an in situ GISANS method [29][44]. To perform such study, an air-tight chamber, like the one used for in situ XRD, is used to study the humidity-induced degradation of MAPbI3. Instead of glass, Al was used as the window material because of its low absorption coefficient to neutrons.
GISANS was used to study water distribution in perovskite. Chlipf et al. determined the formation conditions of hydrates in MAPbI3 [29]. The RH required for monohydrate is greater than ~70% while the dihydrate requires RH greater than 90%, which are consistent with the in situ XRD studies [27][28][29]. In addition, GISANS can find the RH-dependence of the water content in perovskite as it absorbs on the surface or into the bulk of perovskite depending on the humidity level. Surprisingly, at 41% RH, there is already 10 vol% of water in perovskite. The water content can be as high as 50 vol% at 96% RH. The results also show that water can enter the perovskite without forming a new phase or domain expansion.
GISANS has confirmed that quasi-two-dimensional (3D/2D) perovskite has better moisture stability at a high humidity level [44]. GISANS also revealed that 3D/2D perovskite degrades mainly by phase separation at high humidity levels (~90% RH). The two-dimensional phase with n = 5 (PEA)2(MA)4PbI16 transformed into a more stable n = 2 and n = 3 phase. The presence of the two-dimensional phase may reduce the migration of ions to the crystal’s surface, such as MA+ and I−, thus inhibiting the formation of PbI2. For the degradation process of 3D/2D perovskite films, the inhibition of ion migration in 2D perovskite may be more important than the hydrophobicity of organic ligands. Although only water-induced degradation was reported in the GISANS study, other types of degradation can also be studied by GISANS because all hybrid perovskites contain hydrogen.
All structural characterization techniques have long probing distance (>1 μm). The operando approach can be easily achieved in a commercial or home-made airtight cell/chamber. However, these setups are not specially design for the study of perovskite and can be further improved by the integration of a heater and/or electrical fields in the cell/chamber to study the structural changes under multiple stresses. For example, a cell with 85 °C and 85% RH condition will be helpful to study degradation under accelerated aging. Table 1 lists most relevant in situ/operando papers on the perovskite film degradation process using crystal structural characterization techniques.
Table 1. Summary of in situ/operando techniques applied in crystal structure studies.
| Perovskite Films | Exposure Condition | Techniques | Ref. |
|---|---|---|---|
| MAPbI3 | Water (27~90% RH) | XRD | [16] |
| MAPbI3 | Water (80% RH) | XRD | [27] |
| MAPbI3 | Water (80% RH) | XRD | [28] |
| MAPbI3 | Water (65% RH) | XRD | [30] |
| CsFAPb(IBr)3 | Heat (80~320 °C) | XRD | [31] |
| MAPbI3−xClx | Heat (28~400 °C) | XRD | [33] |
| FACsPb(IBr)3, MAPb(IBr)3 | Light | XRD | [34] |
| 3D/2D | Water (90% RH) | XRD | [44] |
| MAPbI3 | Liquid water | XRD | [45] |
| MAPbX3(X = I, Br, Cl) | Heat (30~320 °C) | XRD | [46] |
| MAPbI3 | Vacuum/light | XRD | [47] |
| (CsFAMA)Pb(IBr)3 | Light and bias | XRD | [48] |
| MAPb(IBr)3 | Light | XRD | [49] |
| MAPbI3 | Water (80% RH) | GIXRD | [25] |
| MAPbI3 | Water (85% RH) | GIWAXS | [26] |
| MAPbI3 | Heat (25~130 °C) | GIWAXS | [50] |
| MAPbI3 | D2O (73% and 93% RH) | GISANS | [29] |
| 3D/2D | Water (90% RH) | GISANS | [44] |
References
- Singh, A.N.; Kajal, S.; Kim, J.; Jana, A.; Kim, J.Y.; Kim, K.S. Interface Engineering Driven Stabilization of Halide Perovskites against Moisture, Heat, and Light for Optoelectronic Applications. Adv. Energy Mater. 2020, 10, 2000768.
- Béchu, S.; Ralaiarisoa, M.; Etcheberry, A.; Schulz, P. Photoemission Spectroscopy Characterization of Halide Perovskites. Adv. Energy Mater. 2020, 10, 1904007.
- Kosasih, F.U.; Ducati, C. Characterising degradation of perovskite solar cells through in-situ and operando electron microscopy. Nano Energy 2018, 47, 243–256.
- Wei, J.; Wang, Q.; Huo, J.; Gao, F.; Gan, Z.; Zhao, Q.; Li, H. Mechanisms and Suppression of Photoinduced Degradation in Perovskite Solar Cells. Adv. Energy Mater. 2020, 11, 2002326.
- Hidalgo, J.; Castro-Méndez, A.F.; Correa-Baena, J.P. Imaging and Mapping Characterization Tools for Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900444.
- Boyd, C.C.; Cheacharoen, R.; Leijtens, T.; McGehee, M.D. Understanding Degradation Mechanisms and Improving Stability of Perovskite Photovoltaics. Chem. Rev. 2019, 119, 3418–3451.
- Kundu, S.; Kelly, T.L. In situ studies of the degradation mechanisms of perovskite solar cells. EcoMat 2020, 2, e12025.
- Zhou, Y.; Sternlicht, H.; Padture, N.P. Transmission Electron Microscopy of Halide Perovskite Materials and Devices. Joule 2019, 3, 641–661.
- Szostak, R.; Goncalves, A.d.S.; Freitas, J.N.d.; Marchezi, P.E.; Araujo, F.L.d.; Tolentino, H.C.N.; Toney, M.F.; Marques, F.d.C.; Nogueira, A.F. In Situ and Operando Characterizations of Metal Halide Perovskite and Solar Cells: Insights from Lab-Sized Devices to Upscaling Processes. Chem. Rev. 2023, 123, 3160–3236.
- Meng, X.; Tian, X.; Zhang, S.; Zhou, J.; Zhang, Y.; Liu, Z.; Chen, W. In Situ Characterization for Understanding the Degradation in Perovskite Solar Cells. Sol. RRL 2022, 6, 2200280.
- Dunfield, S.P.; Bliss, L.; Zhang, F.; Luther, J.M.; Zhu, K.; Hest, M.F.A.M.; Reese, M.O.; Berry, J.J. From Defects to Degradation: A Mechanistic Understanding of Degradation in Perovskite Solar Cell Devices and Modules. Adv. Energy Mater. 2020, 10, 1904054.
- Zhang, S.; Liu, Z.; Zhang, W.; Jiang, Z.; Chen, W.; Chen, R.; Huang, Y.; Yang, Z.; Zhang, Y.; Han, L.; et al. Barrier Designs in Perovskite Solar Cells for Long-Term Stability. Adv. Energy Mater. 2020, 10, 2001610.
- Nguyen, L.; Tao, F.F.; Tang, Y.; Dou, J.; Bao, X.-J. Understanding Catalyst Surfaces during Catalysis through Near Ambient Pressure X-ray Photoelectron Spectroscopy. Chem. Rev. 2019, 119, 6822–6905.
- Chakrabarti, A.; Ford, M.E.; Gregory, D.; Hu, R.; Keturakis, C.J.; Lwin, S.; Tang, Y.; Yang, Z.; Zhu, M.; Bañares, M.A.; et al. A decade+ of operando spectroscopy studies. Catal. Today 2017, 283, 27–53.
- Bañares, M.A.; Guerrero-Pérez, M.O.; Fierro, J.L.G.; Cortez, G.G. Raman spectroscopy during catalytic operations with on-line activity measurement (operando spectroscopy): A method for understanding the active centres of cations supported on porous materials. J. Mater. Chem. 2002, 12, 3337–3342.
- Zhao, L.; Kerner, R.A.; Xiao, Z.; Lin, Y.L.; Lee, K.M.; Schwartz, J.; Rand, B.P. Redox Chemistry Dominates the Degradation and Decomposition of Metal Halide Perovskite Optoelectronic Devices. ACS Energy Lett. 2016, 1, 595–602.
- Yin, J.; Teobaldi, G.; Liu, L.M. The Role of Thermal Fluctuations and Vibrational Entropy: A Theoretical Insight into the delta-to-alpha Transition of FAPbI(3). J. Phys. Chem. Lett. 2022, 13, 3089–3095.
- Niu, T.; Chao, L.; Dong, X.; Fu, L.; Chen, Y. Phase-Pure α-FAPbI3 for Perovskite Solar Cells. J. Phys. Chem. Lett. 2022, 13, 1845–1854.
- Masi, S.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Stabilization of Black Perovskite Phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020, 5, 1974–1985.
- Su, Z.; Wang, C.; Zheng, G.; Gao, X. Impacts of MAPbBr3 Additive on Crystallization Kinetics of FAPbI3 Perovskite for High Performance Solar Cells. Coatings 2021, 11, 545.
- Li, G.; Su, Z.; Canil, L.; Hughes, D.; Aldamasy, M.H.; Dagar, J.; Trofimov, S.; Wang, L.; Zuo, W.; Jerónimo-Rendon, J.J.; et al. Highly efficient p-i-n perovskite solar cells that endure temperature variations. Science 2023, 379, 399–403.
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3 films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710.
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the excited state and photovoltaic efficiency of CH3NH3PbI3 perovskite upon controlled exposure to humidified air. J. Am. Chem. Soc. 2015, 137, 1530–1538.
- Zhang, M.; Zhang, F.; Wang, Y.; Zhu, L.; Hu, Y.; Lou, Z.; Hou, Y.; Teng, F. High-Performance Photodiode-Type Photodetectors Based on Polycrystalline Formamidinium Lead Iodide Perovskite Thin Films. Sci. Rep. 2018, 8, 11157.
- Yang, J.; Siempelkamp, B.D.; Liu, D.; Kelly, T.L. Investigation of CH3NH3PbI3 degradation rates and mechanisms in controlled humidity environments using in situ techniques. ACS Nano 2015, 9, 1955–1963.
- Fransishyn, K.M.; Kundu, S.; Kelly, T.L. Elucidating the Failure Mechanisms of Perovskite Solar Cells in Humid Environments Using In Situ Grazing-Incidence Wide-Angle X-ray Scattering. ACS Energy Lett. 2018, 3, 2127–2133.
- Leguy, A.M.A.; Hu, Y.; Campoy-Quiles, M.; Alonso, M.I.; Weber, O.J.; Azarhoosh, P.; van Schilfgaarde, M.; Weller, M.T.; Bein, T.; Nelson, J.; et al. Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals, and Solar Cells. Chem. Mater. 2015, 27, 3397–3407.
- Li, D.; Bretschneider, S.A.; Bergmann, V.W.; Hermes, I.M.; Mars, J.; Klasen, A.; Lu, H.; Tremel, W.; Mezger, M.; Butt, H.-J.; et al. Humidity-Induced Grain Boundaries in MAPbI3 Perovskite Films. J. Phys. Chem. C 2016, 120, 6363–6368.
- Schlipf, J.; Biessmann, L.; Oesinghaus, L.; Berger, E.; Metwalli, E.; Lercher, J.A.; Porcar, L.; Muller-Buschbaum, P. In Situ Monitoring the Uptake of Moisture into Hybrid Perovskite Thin Films. J. Phys. Chem. Lett. 2018, 9, 2015–2021.
- Chen, B.-A.; Lin, J.-T.; Suen, N.-T.; Tsao, C.-W.; Chu, T.-C.; Hsu, Y.-Y.; Chan, T.-S.; Chan, Y.-T.; Yang, J.-S.; Chiu, C.-W.; et al. In Situ Identification of Photo- and Moisture-Dependent Phase Evolution of Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 342–348.
- Long, M.; Zhang, T.; Liu, M.; Chen, Z.; Wang, C.; Xie, W.; Xie, F.; Chen, J.; Li, G.; Xu, J. Abnormal Synergetic Effect of Organic and Halide Ions on the Stability and Optoelectronic Properties of a Mixed Perovskite via In Situ Characterizations. Adv. Mater. 2018, 30, 1801562.
- Tan, W.; Bowring, A.R.; Meng, A.C.; McGehee, M.D.; McIntyre, P.C. Thermal Stability of Mixed Cation Metal Halide Perovskites in Air. ACS Appl. Mater. Interfaces 2018, 10, 5485–5491.
- Nenon, D.P.; Christians, J.A.; Wheeler, L.M.; Blackburn, J.L.; Sanehira, E.M.; Dou, B.; Olsen, M.L.; Zhu, K.; Berry, J.J.; Luther, J.M. Structural and chemical evolution of methylammonium lead halide perovskites during thermal processing from solution. Energy Environ. Sci. 2016, 9, 2072–2082.
- Sutter-Fella, C.M.; Ngo, Q.P.; Cefarin, N.; Gardner, K.L.; Tamura, N.; Stan, C.V.; Drisdell, W.S.; Javey, A.; Toma, F.M.; Sharp, I.D. Cation-Dependent Light-Induced Halide Demixing in Hybrid Organic-Inorganic Perovskites. Nano Lett. 2018, 18, 3473–3480.
- Li, M.; Wang, Z.-K.; Yang, Y.-G.; Hu, Y.; Feng, S.-L.; Wang, J.-M.; Gao, X.-Y.; Liao, L.-S. Copper Salts Doped Spiro-OMeTAD for High-Performance Perovskite Solar Cells. Adv. Energy Mater. 2016, 6, 1601156.
- Miyadera, T.; Shibata, Y.; Koganezawa, T.; Murakami, T.N.; Sugita, T.; Tanigaki, N.; Chikamatsu, M. Crystallization Dynamics of Organolead Halide Perovskite by Real-Time X-ray Diffraction. Nano Lett. 2015, 15, 5630–5634.
- Shen, Y.; Shen, K.C.; Li, Y.Q.; Guo, M.; Wang, J.; Ye, Y.; Xie, F.M.; Ren, H.; Gao, X.; Song, F.; et al. Interfacial Potassium-Guided Grain Growth for Efficient Deep-Blue Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2020, 31, 2006736–2006738.
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for alpha-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385.
- Wang, Y.; Dar, M.I.; Ono, L.K.; Zhang, T.; Kan, M.; Li, Y.; Zhang, L.; Wang, X.; Yang, Y.; Gao, X.; et al. Thermodynamically stabilized β-CsPbI3-based perovskite solar cells with efficiencies. Science 2019, 365, 591–595.
- Qin, M.; Tse, K.; Lau, T.K.; Li, Y.; Su, C.J.; Yang, G.; Chen, J.; Zhu, J.; Jeng, U.S.; Li, G.; et al. Manipulating the Mixed-Perovskite Crystallization Pathway Unveiled by In Situ GIWAXS. Adv. Mater. 2019, 31, e1901284.
- Lu, L.; Shen, K.C.; Wang, J.; Su, Z.; Li, Y.; Chen, L.; Luo, Y.; Song, F.; Gao, X.; Tang, J.X. Interaction of the Cation and Vacancy in Hybrid Perovskites Induced by Light Illumination. ACS Appl. Mater. Interfaces 2020, 12, 42369–42377.
- Song, J.; Zhou, G.; Chen, W.; Zhang, Q.; Ali, J.; Hu, Q.; Wang, J.; Wang, C.; Feng, W.; Djurisic, A.B.; et al. Unraveling the Crystallization Kinetics of 2D Perovskites with Sandwich-Type Structure for High-Performance Photovoltaics. Adv. Mater. 2020, 32, e2002784.
- Qin, M.; Chan, P.F.; Lu, X. A Systematic Review of Metal Halide Perovskite Crystallization and Film Formation Mechanism Unveiled by In Situ GIWAXS. Adv. Mater. 2021, 33, e2105290.
- Schlipf, J.; Hu, Y.; Pratap, S.; Bießmann, L.; Hohn, N.; Porcar, L.; Bein, T.; Docampo, P.; Müller-Buschbaum, P. Shedding Light on the Moisture Stability of 3D/2D Hybrid Perovskite Heterojunction Thin Films. ACS Appl. Energy Mater. 2019, 2, 1011–1018.
- Hada, M.; Hasegawa, Y.; Nagaoka, R.; Miyake, T.; Abdullaev, U.; Ota, H.; Nishikawa, T.; Yamashita, Y.; Hayashi, Y. In-situ X-ray diffraction reveals the degradation of crystalline CH3NH3PbI3 by water-molecule collisions at room temperature. Jpn. J. Appl. Phys. 2018, 57, 028001.
- Pistor, P.; Burwig, T.; Brzuska, C.; Weber, B.; Fränzel, W. Thermal stability and miscibility of co-evaporated methyl ammonium lead halide (MAPbX3, X = I, Br, Cl) thin films analysed by in situ X-ray diffraction. J. Mater. Chem. A 2018, 6, 11496–11506.
- Tang, X.; Brandl, M.; May, B.; Levchuk, I.; Hou, Y.; Richter, M.; Chen, H.; Chen, S.; Kahmann, S.; Osvet, A.; et al. Photoinduced degradation of methylammonium lead triiodide perovskite semiconductors. J. Mater. Chem. A 2016, 4, 15896–15903.
- Ruf, F.; Rietz, P.; Aygüler, M.F.; Kelz, I.; Docampo, P.; Kalt, H.; Hetterich, M. The Bandgap as a Moving Target: Reversible Bandgap Instabilities in Multiple-Cation Mixed-Halide Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 2995–3001.
- Barker, A.J.; Sadhanala, A.; Deschler, F.; Gandini, M.; Senanayak, S.P.; Pearce, P.M.; Mosconi, E.; Pearson, A.J.; Wu, Y.; Srimath Kandada, A.R.; et al. Defect-Assisted Photoinduced Halide Segregation in Mixed-Halide Perovskite Thin Films. ACS Energy Lett. 2017, 2, 1416–1424.
- Kim, N.K.; Min, Y.H.; Noh, S.; Cho, E.; Jeong, G.; Joo, M.; Ahn, S.W.; Lee, J.S.; Kim, S.; Ihm, K.; et al. Investigation of Thermally Induced Degradation in CH3NH3PbI3 Perovskite Solar Cells using In-situ Synchrotron Radiation Analysis. Sci. Rep. 2017, 7, 4645.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
699
Revisions:
2 times
(View History)
Update Date:
12 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




