Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roger jones | -- | 3408 | 2023-07-07 05:43:53 | | | |
| 2 | Wendy Huang | Meta information modification | 3408 | 2023-07-07 05:58:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jones, R.A.C. Bean Yellow Mosaic Virus in Grain Lupins. Encyclopedia. Available online: https://encyclopedia.pub/entry/46552 (accessed on 07 February 2026).
Jones RAC. Bean Yellow Mosaic Virus in Grain Lupins. Encyclopedia. Available at: https://encyclopedia.pub/entry/46552. Accessed February 07, 2026.
Jones, Roger A. C.. "Bean Yellow Mosaic Virus in Grain Lupins" Encyclopedia, https://encyclopedia.pub/entry/46552 (accessed February 07, 2026).
Jones, R.A.C. (2023, July 07). Bean Yellow Mosaic Virus in Grain Lupins. In Encyclopedia. https://encyclopedia.pub/entry/46552
Jones, Roger A. C.. "Bean Yellow Mosaic Virus in Grain Lupins." Encyclopedia. Web. 07 July, 2023.
Copy Citation
Lupins (Lupinus spp.) are grown as annual cool-season grain legume (pulse) crops in all continents apart from Antarctica. The main production areas include countries surrounding the Mediterranean Sea (European, North African, and Middle Eastern), northeast Europe, the Andean region of South America, southern Australia, southern Africa, and southeast USA. The most important, widespread, and damaging viral pathogen of grain lupins is bean yellow mosaic virus (BYMV).
lupins
virus
BYMV
disease
yield losses
breeding
1. Introduction
Lupin grain is used to feed humans not only directly but also indirectly by providing feed for domestic animals and fishmeal in aquaculture. Depending upon the world region, they are grown as summer crops where the climate is temperate, as winter crops where it is Mediterranean or subtropical, and as wet season, high altitude crops where it is tropical at lower altitudes [1][2][3][4][5][6][7][8]. From 2014 to 2021, the 10 countries that produced the most lupin seed, which are located in Australasia, Europe, South America, and Africa, produced a combined 1.1–1.6 million tonnes of seed annually [9]. The main species cultivated for their grain are narrow-leafed lupin (L. angustifolius), white lupin (L. albus), yellow lupin (L. luteus), and pearl lupin (L. mutabilis). Both pearl and white lupins were first domesticated to produce land races in their centres of origin in the Andean region of South America and the Mediterranean region, respectively. The centre of origin of both yellow and narrow-leafed lupin was also in the Mediterranean region. Of these four species, pearl and white lupin were first domesticated >2,500 years ago, whereas narrow-leafed and yellow lupin were domesticated recently in northern Europe (Baltic countries and Germany) [3][5][6][7][10][11]. Lupin land races show evidence of early farmer selection of beneficial traits, such as drought avoidance, reduced vegetative growth, permeable seeds, and greater seed size. However, full domestication of crop lupins requires crossing programs designed to increase other key domestication traits, such as vernalisation insensitivity, low alkaloid levels, early flowering, non-shattering pods, yield stability, and pest and disease resistance, so this did not commence until the early 20th century. This was in Germany in 1928, when the first alkaloid-free lupin plants were isolated [1][2][5][6][7][12]. Although the rough-seeded lupin species sandplain lupin (L. cosentinii) was domesticated in the 1970s in Australia, it currently only persists in pastures as feed for domestic animals [1][2][3][5][6][12][13]. Additional lupin species that are recently domesticated, under domestication, or potentially suitable for domestication include three other rough-seeded lupin species, L. atlanticus, L. pilosus, and L. digitatus [5][6][7][14][15], and L. hispanicus, which resembles yellow lupin [7]. Lupin species grown as ornamental plants include yellow and pearl lupins, L. pilosus, L. hartwegii, L. polyphyllus, and the interspecies cross L. polyphyllus × L. arboreus [5][7].
Lupins not only tolerate growing in poor, nitrogen-deficient soils but also contribute nitrogen to the soil, making them ideal for sowing in rotation with crops unable to fix nitrogen. However, they also suffer from diverse abiotic and biotic constraints that limit their productivity [2][3][5][6][12][16][17]. Amongst these constraints, disease is a major contributor, as lupins become infected by a wide range of fungal and viral pathogens that diminish both the yield and the quality of their seeds [18][19][20][21][22]. The magnitude of the disease-induced losses in seed yield and quality that develops varies between different cultivated lupin species, pathogen species and types, climatic differences, and world region [18][19][20][21][22].
2. Bean Yellow Mosaic Virus
The most important, widespread, and damaging viral pathogen of grain lupins is bean yellow mosaic virus (BYMV) [18]. Although BYMV causes a mild disease in pearl lupin, a damaging disease develops in the other four cultivated lupin species (Table 1). Its principal foliage symptoms vary between lupin species: pearl lupin—mild mosaic and slight plant stunting; yellow lupin—narrowing of leaflets, vein mosaic, bunchy growth, and plant dwarfing; and both white and sandplain lupin—severe mosaic, necrotic spotting and deformation of leaves, and plant stunting (Figure 1A–C) [18]. In narrow-leafed lupin, BYMV symptom development depends upon the virus strain present (necrotic or non-necrotic) and the growth stage when infection occurs. Early infection with the necrotic strain causes bending over of the shoot tip, necrotic stem streaking, and plant death (Figure 1D,E), whereas late infection of mature plants remains restricted to one or some branches, which develop black pod syndrome (BPS) and/or systemic necrosis (Figure 1F) [18][23]. In contrast, the necrotic phenotype is lacking when plants become infected by the non-necrotic strain, which causes mosaic and stunting symptoms (Figure 1G) [24][25]. The earliest reports of virus symptoms resembling those caused by BYMV were in yellow lupin in Germany in 1929, in Argentina in white lupin in 1932, and in narrow-leafed lupin in New Zealand in 1934. During the period from 1938 to 1960, typical BYMV symptoms were reported under different names in plants of these three lupin species in Europe, Australasia, North America, and Southern Africa. They were also reported in pearl lupin in Australia, New Zealand, and South Africa, and in sandplain lupin in Australia [18]. Because it occurs worldwide [26][27][28], BYMV infection poses a serious threat to the lupin crop wherever it is grown in the world. It infects many species of flowering plants (both monocots and dicots) and causes damaging diseases in legume species [26][28][29][30]. It is vectored non-persistently by >50 aphid species, including Myzus persicae, Aphis craccivora, A. fabae, Acyrthosiphon kondoi, Acyrthosiphon pisum, and Macrosiphum euphorbiae [18][29][30][31]. It is readily seed-borne in yellow and white lupin, and sowing their infected seed stocks creates primary infection foci from which aphid vectors spread the virus within the crop. In contrast, seed transmission has never been found in narrow-leafed, pearl, or sandplain lupin. Therefore, with them, lupin crop infection depends solely on aphid vectors bringing in BYMV from infected alternative hosts growing nearby, such as legume weeds, pasture plants, and crops [18][32]. Weather conditions that promote aphid build-up both before and during the growing season (especially rainfall and warm temperatures) favor its spread within lupin crops [32][33].

Figure 1. Plants of different lupin (Lupinus) species with foliage symptoms caused by infection with bean yellow mosaic virus (BYMV) (A–G), or being screened for BYMV resistance in the field (H). (A), Plants of yellow lupin (L. luteus) with typical narrow-leaflet symptoms and reduction in leaf size (South Perth 1995). (B), Plants of white lupin (L. albus) with typical leaf symptoms of mosaic and deformation (front) and unaffected plants with larger dark green leaves (behind) (South Perth 1997). (C), Plant of sandplain lupin (L. costentinii) with typical leaf symptoms of severe mosaic and deformation, and reduction in size (front) with unaffected plant (behind) (South Perth 1989). (D), Plant of narrow-leafed lupin (L. angustifolius) with typical initial early necrotic strain symptom consisting of shoot tip bending over (‘shepherds crook’) (South Perth 1989). (E), Three plants of narrow-leafed lupin killed by early necrotic strain infection (right), and healthy plant (left) (South Perth 1992). (F), Plant of narrow-leafed lupin with black pod syndrome caused by late necrotic strain infection (centre), and healthy plant with normal-looking pods (top left) (South Perth 1995). (G), Plant of narrow-leafed lupin with typical chlorosis and downcurling of leaflets in apical leaves caused by recent infection with the non-necrotic strain (Avondale 1995). (H), Single row plots of cultivars, breeding lines, and germplasm accessions of different lupin species undergoing BYMV resistance screening (South Perth 1993). Note the BYMV-infected clover transplants positioned at both ends of each row to provide a uniform infection source for naturally occurring aphid vectors to spread the virus.
Table 1. Viral pathogens causing diseases in grain lupin species.
| Pathogen | Virus Genus | Mode of Vector Transmission | Main Disease Symptoms | World Regions Where Lupin Infection Reported | Narrow-Leafed Lupin | White Lupin | Yellow Lupin | Pearl Lupin | Sandplain Lupin |
|---|---|---|---|---|---|---|---|---|---|
| Main pathogens | |||||||||
| Bean yellow mosaic virus * | Potyvirus | Aphid | Leaf mosaic, chlorosis, narrowing, deformation, plant stunting, or strain-specific systemic necrosis or black pod syndrome | Australasia, Europe, North and South America, Southern Africa | +++++ | +++++ | +++++ | ++++ | +++++ |
| Cucumber mosaic virus * | Cucumovirus | Aphid | Leaf mosaic, chlorosis, downcurling, plant stunting | Australasia, Europe, South America, Southern Africa | +++++ | - | +++++ | ++++ | - |
| Minor pathogens | |||||||||
| Alfalfa mosaic virus * | Alfamovirus | Aphid | Mild leaf mosaic, downcurling, plant stunting | Australasia, Europe | +++ | (+) | ++ | + | - |
| Bean common mosaic virus | Potyvirus | Aphid | Mild leaf mosaic, deformation, stunting | Europe | (+) | - | + | - | - |
| Bidens mottle virus | Potyvirus | Aphid | Leaf narrowing, rugosity | North America | + | - | - | - | - |
| Broad bean wilt virus | Fabavirus | Aphid | Mosaic, leaf deformation, shoot apical necrosis, necrotic stem streaking, plant stunting, death | Europe | - | - | + | - | - |
| Clover yellow vein virus | Potyvirus | Aphid | Leaf chlorosis, necrotic spotting, shoot apical necrosis, stem necrosis, plant stunting | Australasia, Europe | ++ | + | + | - | - |
| Pea early browning virus | Tobravirus | Nematode | Necrotic stem streaking, shoot apical necrosis | Europe | - | (+) | + | - | - |
| Pea enation mosaic virus | Enamovirus | Aphid | Leaf deformation, axillary shoot proliferation | Europe | - | - | + | - | - |
| Peanut stunt virus | Cucumovirus | Aphid | Severe leaf and flower deformation, plant stunting | Europe | + | + | + | - | - |
| Lettuce necrotic yellows virus | Cytorhabdovirus | Aphid | Leaf chlorosis, plant stunting | Australasia | + | + | - | - | - |
| Tobacco streak virus | Ilarvirus | Thrips | Not reported | North America | (+) | (+) | - | - | - |
| Tomato black ring virus | Nepovirus | Nematode | Leaf mosaic, deformation, necrotic spotting, plant stunting | Europe | (+) | (+) | + | (+) | - |
| Tomato spotted wilt virus | Orthotospovirus | Thrips | Leaf ringspots (chlorotic or necrotic), deformation, and necrosis (stem streaking or dieback) | Australasia, Europe, North America | + | + | - | + | + |
| Soybean dwarf virus | Luteovirus | Aphid | Leaf chlorosis, reddening, and cupping/rolling | Australasia, East Asia | + | + | + | - | + |
* = host resistance studies; +++++ = very important, ++++ = important, +++ = moderately important, ++ = minor importance, + unimportant; (+) = from glasshouse inoculations; - = no record found. The principal sources of the information in this table are the following published reviews and scientific papers: [7][11][18][19][20][21][22][29][30][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60]; however, several other documents cited in the reference list also contributed data.
Phytosanitary (sow healthy seeds, isolate from external virus sources, and sow perimeter non-host barrier crop), cultural (sow early maturing cultivars, deter aphid landings with stubble groundcover, and promote early canopy closure), and chemical (apply insecticide to suppress aphid vectors in adjacent legume pasture) control measures are available for managing BYMV in lupin crops [18][19][32][34][61]. However, host resistance offers an alternative approach towards BYMV management [18]. In the 1950s in southeast USA, selections of yellow lupin and 31 other lupin species were screened for BYMV resistance [35][36][37]. None was found in any cultivated lupin species, but six perennial lupin species had extreme BYMV resistance (immunity). Unfortunately, all attempts to transfer this resistance to yellow, white, and narrow-leafed lupin were unsuccessful. Similarly, from 1970 to 1980 in Europe, screening for BYMV resistance found none in yellow lupin breeding selections in Poland [38] or in yellow or white lupin cultivars in Hungary [39]. In Byelorussia, however, screening of 21 lupin species for resistance to BYMV found some ‘resistant’ lines (= no symptoms developed, so some might have been tolerant) amongst several wild lupin species and one ‘resistant’ pearl lupin line (K2153) [40]. In Russian studies, partial resistance to BYMV infection by aphids was reported in several yellow and white lupin breeding lines [41]. In Ukraine, similar partial resistance was found in yellow lupin cv. Motiv [42]. In addition, when resistance to BYMV seed transmission was studied in Russia, yellow lupin breeding lines with ‘intrinsic’ BYMV seed transmission rates as low as 3% were identified despite 30% being the typical seed transmission rate [42]. Moreover, when 102 breeding lines and other populations of yellow lupin were tested for possible BYMV resistance in Germany, 21 of them had quantitatively inherited partial resistance, which was linked to reduced seed transmission [43]. Similar resistance to BYMV seed transmission was reported in yellow lupin in Poland [44]. Within Eastern European yellow and white lupin breeding programs, therefore, large-scale BYMV resistance screening focused on partial resistance to infection by aphids and resistance to seed transmission [42][45][46]. This was achieved by field exposure in the presence of ‘spreader rows’ sown with BYMV-infected lupin seed or spray gun inoculation [42].
In Australia, annual routine field screening for resistance to the necrotic BYMV strain commenced for the Australian national lupin breeding program in 1989 [47][48]. Single row plots of lupin test lines were exposed to uniform BYMV inoculum pressure by placing BYMV-infected subterranean clover transplants at each of their ends and allowing naturally occurring aphid vectors to spread the virus along the rows (Figure 1H). Over the years, this annual BYMV resistance screening included not only very large numbers of narrow-leafed lupin germplasm accessions, breeding lines, and cultivars, but also smaller numbers of yellow, white, pearl, and rough-seeded lupins (Figure 2A). Although no extreme BYMV resistance was ever found in any lupin species, two different types of BYMV resistance were detected in narrow-leafed lupin: systemic hypersensitive resistance (SHR) and the partial resistance to BYMV transmission by aphids found previously in yellow and white lupin in Europe (see previous paragraph) [47][48]. SHR (i.e., the typical systemic necrosis and plant death syndrome that results from early BYMV infection) was exhibited by all of the numerous narrow-leafed lupin lines evaluated, apart from accession P26697 and lupin breeding line 90L423-07-13, both of which always developed a ‘non-necrotic’ phenotype (Figure 2B–D) [25][32][48]. SHR is called a resistance reaction because it is controlled by single resistance genes and limits virus spread in the field [62][63]. When a diverse range of necrotic and non-necrotic strain isolates were aphid-inoculated to plants of narrow-leafed lupin cultivars Danja and/or Merrit and of 90L423-07-13 and/or P26697, all of the necrotic strain isolates (but none of the non-necrotic strain isolates) elicited SHR phenotypes when inoculated to Danja or Merrit (Figure 2E) [25]. In contrast, only two of the necrotic strain isolates (neither of which came from lupin) and none of the non-necrotic strain isolates elicited SHR phenotypes when inoculated to 90L423-07-13 or P26697. This suggested the presence of two putative strain-specific, independently inherited SHR genes and four BYMV strain groups (= pathotypes). Strain group 1 contained the two isolates that elicited necrotic phenotypes with both putative SHR genes. Strain group 2 contained the isolates that elicited the putative gene in the two cultivars but not the putative gene in 90L423-07-13 and P26697. Strain group 3 is made up of hypothetical isolates that only elicit the putative gene in 90L423-07-13 and P26697. Strain group 4 contained isolates that elicited neither putative gene, and therefore always caused non-necrotic phenotypes (= the non-necrotic strain [25].
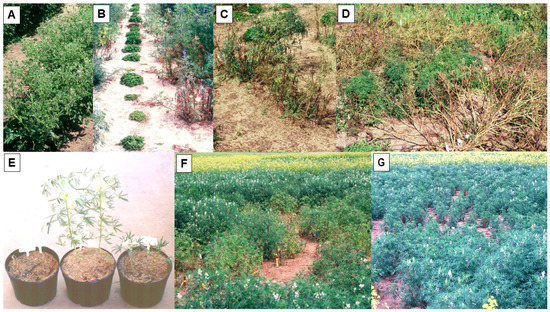
Figure 2. Plants of the rough-seeded lupin species sandplain lupin (Lupinus costentinii) or narrow-leafed lupin (L. angustifolius) being screened for resistance to the necrotic strain of bean yellow mosaic virus in the field (A–D), and plants of narrow-leafed lupin infected with its necrotic or non-necrotic strains being evaluated for symptom expression in the glasshouse (E) or their patterns of spread in the field (F,G). (A), Single row plot of sandplain lupin with all plants infected showing symptoms of severe mosaic and leaf deformation, reduction in leaf size, and stunting (South Perth 1995). (B), Plants of narrow-leafed lupin closest to infected subterranean clover transplants showing systemic necrotic symptoms after being the first ones to become infected (South Perth 1992). (C), Row of infected narrow-leafed lupin plants showing systemic necrotic symptoms (South Perth 1992). (D), Row of narrow-leafed lupin germplasm accession P26697 in which plants show systemic mosaic and leaf deformation symptoms without necrosis (centre), and rows of other accessions killed by infection (front and on top left behind) (South Perth 1995). (E), Plants of narrow-leafed lupin cv. Danja (2/pot) left uninoculated (centre), and aphid-inoculated with the necrotic strain (left) or the non-necrotic strain (right). Infected plants both killed (necrotic strain) or severely stunted without any necrosis (non-necrotic strain) (South Perth 1994). (F,G), Plots of narrow-leafed lupin cv. Gungurru within which necrotic or non-necrotic strains were being spread from centrally placed infection foci (infected clover transplants) by naturally occurring aphids (Avondale 1999). (F), Slow, localized spread of the necrotic strain killing plants in the plot central region. (G), Faster spread of the non-necrotic strain causing more widespread infection of plants that became stunted without developing any necrosis. Stakes mark individual infected plants.
Proof that the putative SHR gene present in Danja and Merrit exists was obtained following inoculation of a strain group 2 isolate to F2 progeny plants of six different crosses [62]. A 3:1 ratio for necrotic:non-necrotic phenotypes was obtained with the crosses 90L423-07-13 × Danja, 90L423-07-13 × Merrit, P26697 × Danja, and P26697 × Merrit, but entirely non-necrotic or 99% necrotic phenotypes were obtained with 90L423-07-13 × P26697 or Danja × Merrit, respectively. This single, independently inherited, dominant SHR gene was named Nbm-1 [62]. Moreover, evidence was obtained that independently segregating modifier genes present in the genetic background altered necrotic phenotype expression elicited by Nbm-1. This was because in F2 progeny plants derived from crosses between parents with and without Nbm-1, the delay between inoculation and the plant being killed varied markedly from plant to plant [62]. This delay was most evident when P26697 was a parent. Proving that the second putative SHR gene exists would require inoculation of a subgroup 3 isolate to progeny plants of similar crosses. Because all narrow-leafed lupin genotypes apart from 90L423-07-13 and P26697 developed SHR when infected with necrotic BYMV strain isolates from lupins, there is no need for active Nbm-1 gene incorporation into new narrow-leafed cultivars. However, the inadvertent selection of new cultivars that behave like 90L423-07-13 and P26697 should be avoided when advanced narrow-leafed lupin breeding lines are screened for BYMV resistance in the field. Furthermore, a search for resistance to the non-necrotic BYMV strain would be worthwhile as, by spreading faster, it causes greater yield losses [63]. Both Nbm-1 and the second putative SHR gene were absent from other cultivated lupin species as, during routine BYMV resistance screening, the rapid necrosis followed by death syndrome never developed in any of them [25]. The suspected quantitatively inherited partial resistance trait in narrow-leafed lupin was characterised by the need for inoculation by many more viruliferous aphids to establish necrotic phenotype infection successfully and was unrelated to aphid susceptibility, flowering date, or alkaloid content [48]. Breeding line 84A086-5-20-31 had outstanding partial resistance of this type both under routine BYMV field screening conditions and in larger-scale field evaluations. Therefore, it seems likely to be a suitable parent for crosses focused on breeding narrow-leafed lupin cultivars destined for BYMV-prone regions [32][48][64].
The question arises as to how the presence of the SHR gene Nbm-1 would be beneficial to narrow-leafed lupin crops growing in the field despite the rapid killing of plants infected early by the necrotic strain, which then produce no seeds. The answer is that instead of intervening to prevent virus spread at the level of individual plants, SHR does this at the plant population level. Thus, the killing of plants infected early by the necrotic BYMV strain prevents them from becoming a virus source for further spread by aphid vectors (Figure 2F) [65][66][67][68]. In contrast, because the non-necrotic strain breaks this resistance by overcoming Nbm-1, lupin plants infected with it remain alive throughout the life of the crop, acting as sources for further virus acquisition and spread by naturally occurring aphid vectors, which results in many more infected plants (Figure 2G) [66][67]. The greater yield losses caused when the non-necrotic strain infected more plants was demonstrated clearly in large-scale field experiments in which both strains were introduced into narrow-leafed lupin plots and allowed to spread by naturally occurring aphids [63]. In contrast, when both strains infected subterranean clover plants, the necrotic strain outcompeted the non-necrotic strain. This explains why there are always more primary infection foci of the former than the latter when BYMV spreads from BYMV-infected subterranean clover pastures into narrow-leafed lupin crops [24][25].
When late infection with the necrotic BYMV strain occurred in narrow-leafed plants in the field, cv. Mandellup was ranked as more ‘BPS-susceptible’ than cv. Jenabillup [69]. However, sap inoculation of the necrotic BYMV strain to plants at different growth stages failed to confirm this because, although its development was slower in Jenabillup than in Mandellup, the BPS symptoms that formed later were as severe as those in Mandellup [23]. Despite this finding, the slower BPS development in Jenabillup might still be a trait of interest for future breeding for BPS resistance in narrow-leafed lupin. Therefore, further studies on BPS are warranted, including obtaining an understanding of its genetic basis (likely polygenic) and the mechanism responsible for it (e.g., mature plant resistance or partial resistance to systemic infection via the phloem) [23].
The possibility of using genetic engineering to introduce BYMV resistance to narrow-leafed and yellow lupins was also investigated [70][71]. Different protease (NIa) gene constructs derived from BYMV were introduced to both lupin species. However, when later generation transgenic progeny plants were inoculated with BYMV, only partial resistance (slow systemic movement) was found. This was restricted to some yellow lupin plants but was absent from any narrow-leafed lupin plants [72][73]. In addition, a synthetic ‘hairpin’ construct derived from the replicase (NIb) gene of BYMV was introduced to plants of narrow-leafed lupin cv. Wonga [74]. When the progeny plants of forty-five lines with this construct were inoculated with BYMV, seven of them had extreme resistance. However, when later generation progeny plants were tested, the resistance derived from the NIb gene construct had become silenced [49].
References
- Gladstones, J.S. Lupin cultivation and breeding. J. Aust. Inst. Agric. Sci. 1960, 26, 19–25.
- Gladstones, J.S. Lupins as crop plants. Field Crop Abstr. 1970, 23, 123–148.
- Gladstones, J.S. Distribution, origin, taxonomy, history and importance. In Lupins as Crop Plants: Biology: Production and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: London, UK, 1998; pp. 1–39.
- Hondelmann, W. The lupin—Ancient and modern crop plant. Theor. Appl. Genet. 1984, 68, 1–9.
- Cowling, W.A.; Buirchell, B.J.; Tapia, M.E. Lupin. Lupinus spp. In Promoting the Conservation and Use of Underutilized and Neglected Crops; Monograph No. 23; Institute of Plant Genetics and Crop Plant Research: Gatersleben, Germany; International Plant Genetic Resources Institute: Rome, Italy, 1998; p. 105.
- Cowling, W.A.; Huyghe, C.; Swiecicki, W. Lupin breeding. In Lupins as Crop Plants: Biology, Production and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: London, UK, 1998; pp. 93–120.
- Wolko, B.; Clements, J.C.; Naganowska, B.; Nelson, M.N.; Yang, H.A. Lupinus. In Wild Crop Relatives: Genomic and Breeding Resources, Legume Crops and Forages, Chapter 9; Kole, C., Ed.; Springer: Berlin, Germany, 2011; p. 105.
- Szczepański, A.; Adamek-Urbańska, D.; Kasprzak, R.; Szudrowicz, H.; Śliwiński, J.; Kamaszewski, M. Lupin: A Promising Alternative Protein Source for Aquaculture Feeds? Aquac. Rep. 2022, 26, 101281.
- TRIDGE. Lupin. Production Trends Overview of Top 10 Countries. 2021. Available online: https://www.tridge.com/intelligences/lupin-bean/production (accessed on 1 April 2023).
- Kurlovich, B.S. (Ed.) Lupins: Geography, Classification, Genetic Resources and Breeding; Intan Publishing House: St. Petersberg, Russia, 2002; p. 480.
- Clements, J.C.; Buirchell, B.J.; Yang, H.; Smith, P.M.C.; Sweetingham, M.W.; Smith, C.G. Chapter 9: Lupin. In Genetic Resources, Chromosome Engineering, and Crop Improvement, Series-II Grain Legumes; Singh, R.J., Jauhar, P.P., Eds.; CRC Press: Boca Raton, Florida, 2005; pp. 231–323.
- Gladstones, J.S. Recent developments in the understanding, improvement, and use of Lupinus. In Advances in Legume Science; Summerfield, R.J., Bunting, A.H., Eds.; University of Reading: Reading, UK, 1980; pp. 603–661.
- Cowling, W.A.; Gladstones, J.S. Lupin breeding in Australia. In Linking Research and Marketing Opportunities for Pulses in the 21st Century: Proceedings of the Third International Food Legumes Research Conference; Springer: Dordrecht, The Netherlands, 2000; pp. 541–547.
- Roy, N.N.; Gladstones, J.S. Prospects for interspecific hybridization of Lupinus atlanticus Gladst. with L. cosentinii Guss. Theor. Appl. Genetics. 1985, 71, 238–241.
- Buirchell, B.J.; Cowling, W.A. Domestication of rough seeded lupins. West. Aust. J. Agric. Fourth Ser. 2008, 33, 131–137.
- Small, E. Lupins–Benefit and Harm Potentials. Biodiversity 2012, 13, 54–64. Available online: https://www.tandfonline.com/doi/pdf/10.1080/14888386.2012.658327 (accessed on 12 April 2023).
- Seymour, M.; Kirkegaard, J.A.; Peoples, M.B.; White, P.F.; French, R.J. Break-crop benefits to wheat in Western Australia–insights from over three decades of research. Crop Pasture Sci. 2012, 63, 1–6.
- Jones, R.A.C.; McLean, G.D. Virus diseases of lupins. Ann. Appl. Biol. 1989, 114, 609–637.
- Sweetingham, M.W.; Jones, R.A.C.; Brown, A.G.P. Diseases and pests. Chapter. In Lupins as Crop Plants: Biology, Production and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Wallingford, UK, 1998; pp. 263–289.
- Salam, M.U.; Davidson, J.A.; Thomas, G.J.; Ford, R.; Jones, R.A.C.; Lindbeck, K.D.; MacLeod, W.J.; Kimber, R.B.; Galloway, J.; Mantri, N.; et al. Advances in winter pulse pathology research in Australia. Australas. Plant Pathol. 2011, 40, 549–567.
- Rubiales, D.; Fondevilla, S.; Chen, W.; Gentzbittel, L.; Higgins, T.J.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and challenges in legume breeding for pest and disease resistance. Crit. Rev. Plant Sci. 2015, 34, 195–236.
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.; Jóhannsson, M.H.; Mateos, C.; et al. State and progress of Andean lupin cultivation in Europe: A review. Agronomy 2020, 10, 1038.
- Kehoe, M.A.; Buirchell, B.J.; Coutts, B.A.; Jones, R.A.C. Black pod syndrome of Lupinus angustifolius is caused by late infection with bean yellow mosaic virus. Plant Dis. 2014, 98, 739–745.
- Cheng, Y.; Jones, R.A.C. Distribution and incidence of necrotic and non-necrotic strains of bean yellow mosaic virus in wild and crop lupins. Aust. J. Agric. Res. 1999, 50, 589–600.
- Cheng, Y.; Jones, R.A.C. Biological properties of necrotic and non-necrotic strains of bean yellow mosaic virus in cool season grain legumes. Ann. Appl. Biol. 2000, 136, 215–227.
- Bos, L. Bean Yellow Mosaic Virus. Descriptions of Plant Viruses; No. 40; Associations of Applied Biologists: Warwick, UK, 1970; Available online: https://www.dpvweb.net/dpv/showdpv/?dpvno=40 (accessed on 5 April 2023).
- Bos, L. Legume virology. In Encyclopedia of Virology, 3rd ed.; Mahy, W.J., Van Regenmortel, M.H.N., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 212–220.
- Boswell, K.F.; Gibbs, A.J. Viruses of Legumes 1983: Descriptions and Keys from VIDE; Research School of Biological Science, Australian National University: Canberra, Australia, 1983; p. 139.
- Edwardson, J.R.; Christie, R.G. Viruses Infecting Forage Legumes; Volumes I-III.; Monograph No. 14; Agricultural Experiment Stations, Institute of Food and Agricultural Sciences, University of Florida: Gainsville, Florida, 1986; p. 742.
- Edwardson, J.R.; Christie, R.G. CRC Handbook of Viruses Infecting Legumes; CRC Press: Boca Raton, Florida, 1991; p. 293.
- Berlandier, F.A.; Thackray, D.J.; Jones, R.A.C.; Latham, L.J.; Cartwright, L. Determining the relative roles of different aphid species as vectors of cucumber mosaic and bean yellow mosaic viruses in lupins. Ann. Appl. Biol. 1997, 131, 297–314.
- Jones, R.A.C. Developing integrated disease management strategies against non-persistently aphid-borne viruses: A model programme. Integr. Pest Man. Rev. 2001, 6, 15–46.
- Maling, T.; Diggle, A.J.; Thackray, D.J.; Siddique, K.H.M.; Jones, R.A.C. An epidemiological model for externally sourced vector-borne viruses applied to bean yellow mosaic virus in lupin crops in a Mediterranean-type environment. Phytopathology 2008, 98, 1280–1290.
- Edwardson, J.R.; Corbett, M.K. Lupines for forage production. Proc. Fla. Agric. Exp. Stn. J. 1959, 19, 119–132.
- Corbett, M.K. Virus diseases of lupines in Florida. Proc. Soil Crop Sci. Soc. Fla. 1955, 15, 35–39.
- Corbett, M.K. A virus disease of lupines caused by bean yellow mosaic virus. Phytopathology 1958, 48, 86–91.
- Corbett, M.K.; Edwardson, J.R. Virus diseases of yellow lupine: Preliminary investigation on control by the use of a protecting border. Proc. Soil Crop Sci. Soc. Fla. 1957, 17, 294–301.
- Frencel, I.M.; Pospieszny, H.; Swiecicki, W. Viruses in natural infections of yellow lupin (Lupinus luteus L.) in Poland. II. Susceptibility of varieties of yellow lupin to bean yellow mosaic virus. Acta Phytopathol. Acad. Sci. Hung. 1978, 13, 45–49.
- Beczner, L.; Horvath, J.; Borbely, F. Susceptibility of Pisum sativum and Lupinus species and cultivars to some economically important viruses. Tag. Akad. Landwwirtschaftwissenshaften Berlin 1983, 216, 313–322.
- Luchina, N.N.; Sapun, V.N.; Sergeenko, M.I. Study of the resistance to lupin species of the American group to Fusarium wilt and virus diseases. Sb. Nauchnykl Tr.-Belorasskii Nauchno-Isseledovated-Ski. Insitut Zemled. 1981, 25, 149–155. (In Russian)
- Larionova, L.I.; Polivanova, T.A.; Ovchinnikova, A.M. Developing Lupin Varieties with Resistance to Fusarium and Viruses. Nauchno-Tekhnicheskii Byulleten’ Vsesoyuznogo Ordena Lenina Ordena Druz. Nar. Nauchno-Issledovatel’skogo Inst. Rastenievod. Im. N. I. Vavilova 1986, 139, 70–72. Available online: https://eurekamag.com/research/001/333/001333841.php (accessed on 20 April 2023). (In Russian).
- Gladstones, J.S.; Cowling, W.A.; Sweetingham, M.W. Report on Australian Mission to USSR on Collaborative Research in Lupin Breeding and Lupin Diseases; Department of Primary Industries: Canberra, Australia, 1987; p. 30.
- Schmidt, H.E.; Meyer, U.; Misko, I.; Jagoda, G.K.; Hellmund, J.; Jakuševa, A.; Haack, I. Quantitative resistance of yellow lupin (Lupinus luteus L.) to bean yellow mosaic virus and correlations of its traits. Arch. Für Züchtungsforschung 1990, 20, 81–91.
- Pospieszny, H.; Wojtowicz, A. The influence of yellow lupin (Lupinus luteus) cultivars on seed-transmission of the bean yellow mosaic virus. Int. Lupin Assoc. Lupin Newsl. 1989, 13, 32–35.
- Yakusheva, A.S. Virus diseases on lupin. Zashchita Rasteniĭ 1991, 11, 26. Available online: https://www.cabdirect.org/cabdirect/abstract/19922316265 (accessed on 10 April 2023).
- Yakovenko, G.L.; Lukashevisn, M.I.; Ageeva, P.A.; Novik, N.V.; Zakharova, M.V. Status and Prospects of Breeding of Cultivated Species of Lupin in Russia. Earth Environ. Sci. 2021, 663, 012014. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/663/1/012014/pdf (accessed on 20 April 2023).
- McKirdy, S.J.; Jones, R.A.C. Bean yellow mosaic potyvirus infection of alternative hosts associated with subterranean clover (Trifolium subterraneum) and narrow-leafed lupins (Lupinus angustifolius): Field screening procedure, relative susceptibility/resistance rankings, seed transmission and persistence between growing seasons. Aust. J. Agric. Res. 1995, 46, 135–152.
- Jones, R.A.C.; Coutts, B.A. Screening for resistance to bean yellow mosaic virus in lupins using a disease nursery. In Highlights of Lupin Research and Development in Western Australia; Shea, G., Ed.; Agriculture Western Australia: Perth, Western Australia, 1998; pp. 29–30.
- Jones, R.A.C.; Coutts, B.A.; Buirchell, G.M.; Wylie, S.J. Bean yellow mosaic virus in lupins: Strains, losses, epidemiology and control. In Lupins for Health and Wealth. Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14–18 September 2008; International Lupin Association: Canterbury, New Zealand, 2008; pp. 420–424.
- Jones, R.A.C.; Latham, L.J. Natural resistance to cucumber mosaic virus in lupin species. Ann. Appl. Biol. 1996, 130, 187–206.
- Frencel, I.M.; Pospieszny, H. Viruses in natural infections of yellow lupin (Lupinus luteus L.) in Poland. V. Cucumber mosaic virus. Acta Phytopathol. Acad. Sci. Hung. 1985, 20, 87–90.
- Jones, R.A.C.; Cowling, W.A. Resistance to seed transmission of cucumber mosaic virus in narrow-leafed lupins (Lupinus angustifolius). Aust. J. Agric. Res. 1995, 46, 1339–1352.
- Eppler, A.; Hinz, U.; Romer, P. Virus-diseases of Lupinus mutabilis Sweet in Germany. Meded. Fac. Landbou Wet. Rijksuniv. Gent. 1986, 51, 817–826.
- Buchen-Osmond, C.; Crabtree, K.; Gibbs, A.J.; McLean, G.D. Viruses of Plants in Australia: Descriptions and Lists from the VIDE Database; Australian National University: Canberra, Australia, 1988.
- Jones, R.A.C.; Latham, L.J. Natural resistance to cucumber mosaic virus in broad-leafed lupins. In Proceedings of the First Australian Lupin Technical Symposium, Dracup, M., Palta, J., Eds. Department of Agriculture: Perth, Australia, 1994; p. 278.
- Jones, R.A.C.; Buirchell, B.J. Resistance to cucumber mosaic virus in Lupinus mutabilis (pearl lupin). Australas. Plant Pathol. 2004, 33, 591–593.
- Jones, R.A.C.; Coutts, B.A.; Reeve, N.; Cowling, W.A.; Buirchell, B.J. Screening for resistance to cucumber mosaic virus in lupins. In Highlights of Lupin Research and Development in Western Australia; Shea, G., Ed.; Agriculture Western Australia: Perth, Australia, 1999; pp. 27–28.
- Jones, R.A.C.; Pearce, R.M.; Prince, R.T.; Coutts, B.A. Natural resistance to alfalfa mosaic virus in different lupin species. Australas. Plant Pathol. 2008, 37, 112–116.
- Frencel, I.M.; Pospieszny, H. Viruses in natural infections of yellow lupin (Lupinus luteus L.) in Poland. III. Alfalfa mosaic virus (AMV). Acta Phytopathol. Acad. Sci. Hung. 1979, 14, 269–278.
- Frencel, I.M. Infectious diseases of lupin in Poland, with special reference to yellow lupin (Lupinus luteus L.). Lupin Newsl. 1988, 11, 13–19.
- Jones, R.A.C. Using epidemiological information to develop effective integrated virus disease management strategies. Virus Res. 2004, 100, 5–30.
- Jones, R.A.C.; Smith, L.J. Inheritance of hypersensitive resistance to bean yellow mosaic virus in narrow-leafed lupin (Lupinus angustifolius). Ann. Appl. Biol. 2005, 146, 539–543.
- Jones, R.A.C.; Coutts, B.A.; Cheng, Y. Yield limiting potential of necrotic and non-necrotic strains of bean yellow mosaic virus in narrow-leafed lupin (Lupinus angustifolius). Aust. J. Agric. Res. 2003, 54, 849–859.
- Gladstones, J.S. Natural resistance to escape from BYMV in L. angustifolius. In Highlights of Lupin Research and Development in Western Australia; Shea, G., Ed.; Agriculture Western Australia: Perth, Western Australia, 1998; pp. 31–32.
- Jones, R.A.C. Effects of cereal borders, admixture with cereals and plant density on the spread of bean yellow mosaic potyvirus into narrow-leafed lupins (Lupinus angustifolius). Ann. Appl. Biol. 1993, 122, 501–518.
- Cheng, Y.; Jones, R.A.C.; Thackray, D.J. Deploying strain specific hypersensitive resistance to diminish temporal virus spread. Ann. Appl. Biol. 2002, 140, 69–79.
- Thackray, D.J.; Smith, L.J.; Cheng, Y.; Perry, J.N.; Jones, R.A.C. Effect of strain-specific hypersensitive resistance on spatial patterns of virus spread. Ann. Appl. Biol. 2002, 141, 45–59.
- Jones, R.A.C. Patterns of spread of two non-persistently aphid-borne viruses in lupin stands under four different infection scenarios. Ann. Appl. Biol. 2005, 146, 337–350.
- Buirchell, B.J. Narrow-leafed lupin breeding in Australia–where to from here. In Lupins for Health and Wealth—Proceedings of the 12th International Lupin Conference, Fremantle, Western Australia, 14-18 September 2008; International Lupin Association: Canterbury, New Zealand, 2008; pp. 14–18.
- Li, H.; Wylie, S.J.; Jones, M.G.K. Transgenic yellow lupin (Lupinus luteus). Plant Cell Rep. 2000, 19, 634–637.
- Jones, M.G.K.; Wylie, S.J.; Berryman, D.; Selladurai, S.; Brien, S.J.; Li, D.; Yang, R.; Ryan, K.; Li, H.; Li, L. Biotechnological tools in lupin breeding and disease diagnosis. In Lupin, an Ancient Crop for the New Millennium, Proceedings of the 9th International Lupin Conference, Klink/Muritz, Germany, 20–24 June 1999; International Lupin Association: Canterbury, New Zealand, 1999; pp. 111–114.
- Li, D.A.; Wylie, S.J.; Jones, M.G.K. Preliminary assessment for resistance to bean yellow mosaic virus in transgenic lupins. In Proceedings of the 15th International Working Group on Legume Viruses, Fremantle, Australia, 15–17 August 1999; Centre for Legumes in Mediterranean Agriculture: Perth, Western, Australia, 1999; p. 66.
- Jones, R.A.C.; Smith, L.J. Evaluation of transgenic lupin plants containing pathogen-derived constructs for bean yellow mosaic virus. In Proceedings of the 15th International Working Group on Legume Viruses, Fremantle, Australia, 15–17 August 1999; Centre for Legumes in Mediterranean Agriculture: Perth, Western, Australia, 1999; p. 67.
- Wylie, S.J.; Welsh, B.; Hodgson, L.; Chapple, S.; Fletcher, N.; Barker, S.; Smith, P.; Jones, M.G.K. Transgenic Lupin Cultivar Immune to Bean Yellow Mosaic Virus. In Research Repository; Murdoch University: Perth, Western Australia, 2004; Available online: https://researchrepository.murdoch.edu.au/id/eprint/19710/1/WYLIE-20040624162812.pdf (accessed on 10 May 2023).
More
Information
Subjects:
Agronomy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
771
Revisions:
2 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




