Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Payal Agarwal | -- | 2239 | 2023-07-06 17:28:12 | | | |
| 2 | Rita Xu | Meta information modification | 2239 | 2023-07-07 04:05:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs in Cancer Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/46531 (accessed on 07 February 2026).
Chakrabortty A, Patton DJ, Smith BF, Agarwal P. miRNAs in Cancer Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/46531. Accessed February 07, 2026.
Chakrabortty, Atonu, Daniel J. Patton, Bruce F. Smith, Payal Agarwal. "miRNAs in Cancer Treatment" Encyclopedia, https://encyclopedia.pub/entry/46531 (accessed February 07, 2026).
Chakrabortty, A., Patton, D.J., Smith, B.F., & Agarwal, P. (2023, July 06). miRNAs in Cancer Treatment. In Encyclopedia. https://encyclopedia.pub/entry/46531
Chakrabortty, Atonu, et al. "miRNAs in Cancer Treatment." Encyclopedia. Web. 06 July, 2023.
Copy Citation
MicroRNAs (miRNAs) are single-stranded, non-coding RNA molecules that regulate gene expression post-transcriptionally by binding to messenger RNAs. miRNAs are important regulators of gene expression, and their dysregulation is implicated in many human and canine diseases. Most cancers tested to date have been shown to express altered miRNA levels, which indicates their potential importance in the oncogenic process.
miRNAs
cancer
oncomiRs
tumor-suppressor miRNAs
1. Introduction
miRNAs are small non-coding RNA sequences with an average length of 18–22 bps. To date, 2654 mature miRNAs have been reported in humans [1]. miRNAs play an essential role in biological processes by regulating gene expression at the post-transcription level. miRNAs bind to messenger RNA (mRNA) in the cytoplasm, resulting in mRNA degradation or temporary inhibition of translation until needed [2]. Downregulation of a specific miRNA leads to upregulation of the corresponding proteins’ expression and vice-versa. Conversely, upregulation of miRNA leads to decreased target protein(s) expression. miRNAs bind at the 3′ and 5′ untranslated regions (UTRs) and coding regions of mRNA to induce translation repression. miRNAs are also involved in inducing gene transcription by binding within the promoter regions of a gene [3]. miRNAs are typically found inside cells; however, a portion of them are shed into circulation in lipid-coated particles known as exosomes [4]. Circulatory exosomal miRNAs have been identified as possible disease biomarkers as they are stable in blood and are protected from endogenous RNAse activity [5].
miRNAs play an important role in cancer cell transformation. miRNAs can function as tumor-suppressor genes or oncogenes by targeting genes involved in tumor development and progression or cell-cycle inhibition, respectively. Since the discovery of microRNAs, they have held great promise for cancer diagnosis, prognosis, and therapy. Different miRNA profiles can be identified for different tumor types, which could then serve as phenotypic signatures for exploitation in cancer diagnosis, prognosis, and treatment. If miRNA profiles can accurately predict malignancies, this technology could be used as a tool to overcome many diagnostic challenges [6].
2. Role of miRNAs in Cancer
2.1. Humans
2.1.1. OncomiRs
OncomiRs are defined as miRNAs that are overexpressed in tumors, repress tumor-suppressor mRNAs, and stimulate tumor cell proliferation and metastasis (Figure 1) [7]. There are many different oncomiRNAs with different roles in cancer growth that have been identified so far.
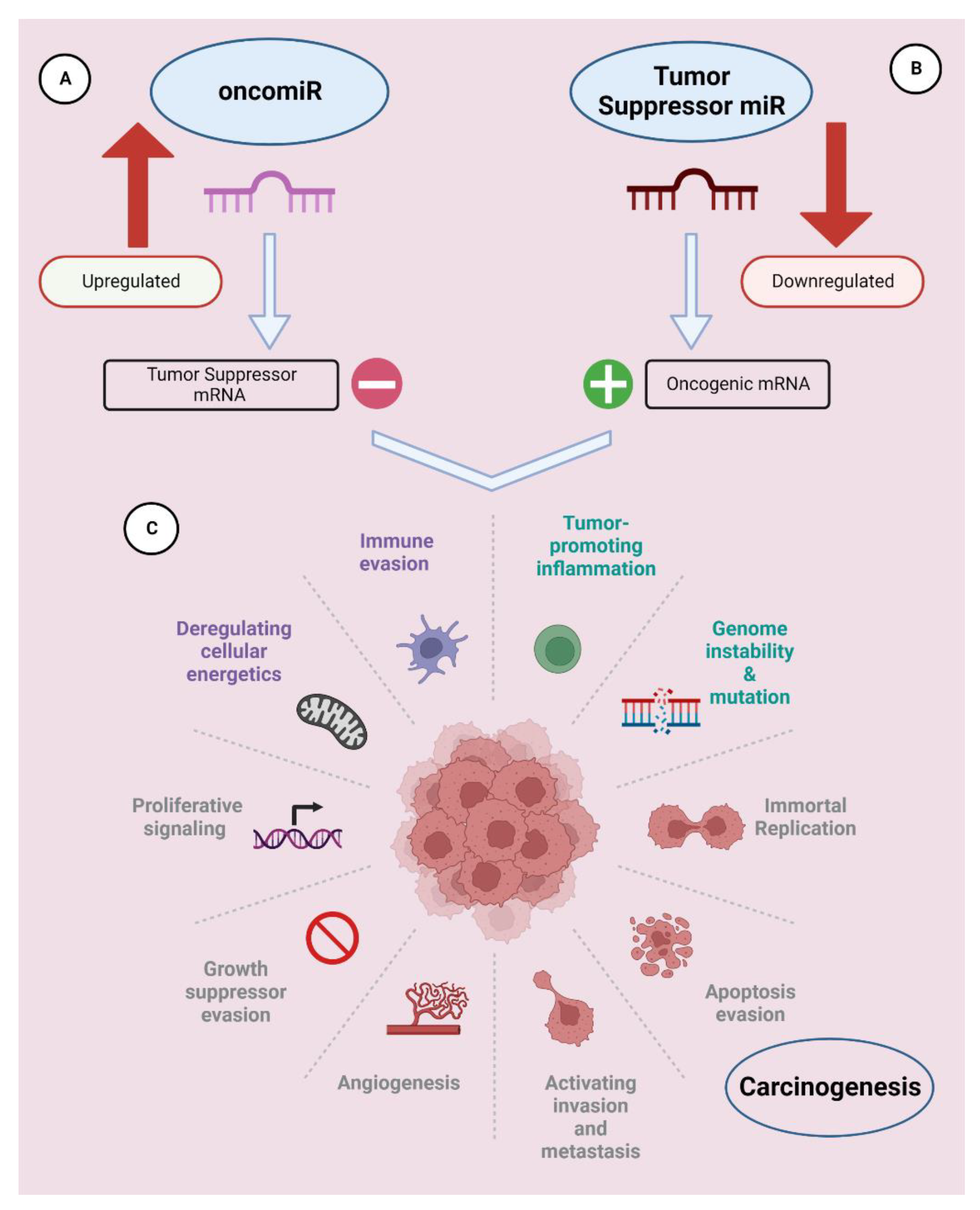
Figure 1. miRNAs can be classified as oncomiRs and tumor suppressors. (A) OncomiRs suppress tumor-suppressor gene translation and promote tumor cell growth through constitutive overexpression. (B) Tumor-suppressor miRNAs inhibit tumorigenesis and subsequent cancer development by suppressing the translation of mRNAs that encode for oncogenes. (C) Hallmarks of carcinogenesis. This figure was created using Biorender.
The miR-17-92 cluster (miRs-17, -18a, -19a, -20a, -19b, and -92a) downregulates PTEN (phosphate and tensin homolog), E2F, the transforming growth factor-β (TGF-β) signaling pathway, B cell lymphoma/leukemia 2-like protein 11 (BCL2L11), and thrombospondin-1 (TSP-1) [8]. Functionally, it favors tumor growth and is reported to be overexpressed in small-cell lung cancer, colon cancer, hepatocellular carcinoma, thyroid cancer, colorectal adenoma organoids, and renal cell carcinoma [9][10][11][12][13][14].
miR-21 is associated with phosphatase and tensin homolog (PTEN), Tropomyosin 1 (TPM1), and programmed cell death 4 (PDCD4) downregulation. miR21 overexpression is reported in a variety of cancers, such as breast, ovarian, colon, etc. [15][16][17]. Elevated levels of miR-21 were also reported in the serum, plasma, and tumor tissues in breast, lung, ovarian, colon, prostate, pancreatic, and gastric cancer patients [18][19][20][21][22][23][24][25][26][27]. Downregulation of miR-21 reduces cancer proliferation and reverses drug resistance in pancreatic, ovarian, and breast cancers [25][26][27].
miR-181 is an oncomiR that is also upregulated in various cancer types [28]. miR-181a-5p promotes breast tumor progression through N-Myc downstream-regulated gene 2 (NDRG2)-induced activation of the PTEN/AKT signaling pathway and inhibition of sprouty RTK signaling antagonist 4 (SPRY4), PH domain, leucine-rich repeat protein phosphatase 2 (PHLPP2), and inositol polyphosphate 4-phosphatase type II (INPP4B) [29][30][31]. miR-181 facilitates prostate cancer cell proliferation by targeting dosage-sensitive sex reversal, adrenal hypoplasia critical region on chromosome X, gene 1 (DAX-1) [32]. Similarly, miR-181 upregulation is associated with poor prognosis and survival in oral squamous cell carcinoma and drug resistance in melanoma [33]. miR-146a is significantly higher in plasma samples from breast cancer patients [34]. miR-221/222 is overexpressed in liver tumorigenesis and breast, colon, and pancreatic tumors [22][35][36][37].
2.1.2. Tumor-Suppressor miRNAs (TS-miRNAs)
Ts-miRNSa are defined as miRNAs that downregulate cancer progression (Figure 1). The downregulation of tumor-suppressor miRNAs plays a crucial role in cancer development and proliferation [38]. TS-miRNAs are more susceptible to mutations due to their location in cancer-associated genomic regions or fragile sites. Downregulation of TS-miRNAs may occur due to dysfunctional proteins involved in their biogenesis or due to any genetic alteration [39]. Inhibition of the expression of important miRNA biogenesis machineries, such as Drosha, DiGeorge Critical Region 8 (DCGR8), and Dicer, substantially decreases miRNA production and promotes a more transformed cell phenotype [40][41][42][43][44][45].
Loss of TS-miRNA miR-16 is correlated with the progression and expansion of chronic lymphocytic leukemia, gastric, prostate, and pancreatic tumors [46][47][48][49][50]. Let-7 family miRNAs are tumor suppressors that target the Rasa and Myc oncogenes [51]. Ectopic expression of the Let-7 miRNA family induces cell death in lung cancer cells [52]. The Let-7 miRNA family is also reported to target other oncogenes, such as high-mobility group A2 (HMGA2) and MYCN [53]. The Let-7 miRNA family also acts as a tumor suppressor in breast cancer by inhibiting ERα-mediated cellular malignant growth [54].
miR-29 and miR-34 are tumor-suppressor miRNAs whose downregulated expression is associated with the progression and invasion of breast cancer, lung cancer, neuroblastoma and glioblastoma, colon cancer, stomach cancer, osteoblastoma, ovarian cancer, bladder cancer, cervical cancer, cholangiocarcinoma, melanoma, and prostate cancer [55][56][57][58][59]. miR-29 downregulation is also associated with cisplatin resistance in ovarian cancer and elevated cell proliferation in osteosarcoma [60][61][62]. Downregulation of miR-34 is associated with proliferation in pancreatic cancer, lung squamous cell carcinoma, head and neck cancer, colorectal cancer, gastric cancer, and epithelial ovarian cancer [63][64][65][66][67][68]. Elevated expression of miR-362-3p interrupts the cell cycle and inhibits tumor growth, resulting in an improved prognosis in colorectal carcinoma patients [69].
Upregulated miR-193b expression results in reduced fatty acid synthase (FASN), which in turn makes triple-negative breast cancer cells more sensitive to the effects of metformin [70]. The expression of miRNA-193b acts as a tumor suppressor in pancreatic cancer and is markedly reduced in tissues with advanced neoplasia. Cell lines transfected with miRNA-193b exhibited significantly decreased proliferation, migration, and invasiveness [71].
The impact of miRNA polymorphisms and their associated impact on cancer risk have been studied [72][73]. Single-nucleotide polymorphisms (SNPs) rs3746444 in miR-499 and rs4919510 in miR-608 are significantly associated with an increased risk of lung cancer [74]. An SNP in miRNA-499 increases the risk of prostate cancer [72]. X-inactivation-specific transcript (XIST) is a carcinogenic long coding RNA involved in ovarian tumor progression by regulating miR-355/BCL2L2 [75].
2.2. Dogs
Dogs have high similarity to humans in gene sequence and gene function. Dogs share the same environmental exposures and risks as humans. Almost 50% of dogs, 10 years old or older, are diagnosed with cancer at some point in their lives [76]. Due to these similarities, dogs are excellent translational models for complex human diseases, such as cancer. As in humans, miRNAs play an important role in canine cancer.
Upregulation of miRNA-19a, -19b, -17, -5p were reported in T and B cell lymphomas in dogs [77]. Additionally, miRNA-203, -181a, and -218 were reported to be underexpressed in canine lymphoma cell lines and tissues [77]. miRNA-9 enhances mast cell tumor progression [78]. miRNA-145, -203, and -205 are downregulated in canine melanoma [79][80]. miRNA-123b is significantly overexpressed in B cell chronic lymphocytic leukemia (CLL). miRNA-155 is preferentially overexpressed in T lymphocytes and some B cell CLLs, and miRNA-150 is overexpressed in T cell CLL in comparison to B cell CLL [81]. miRNA-214 promotes apoptosis in hemangiosarcoma, and miRNA dysregulation is also involved in canine splenic hemangiosarcoma [82][83]. miRNA expression profiles differ in canine splenic hemangiosarcoma, nodular hyperplasia, and normal spleens. A total of 22 miRNAs were differentially expressed in canine hemangiosarcoma samples compared to normal spleen and nodular hyperplasia [82].
In canine mammary tumors (CMTs), expression of mi-RNA-141 showed post-transcriptional downregulation of the tumor-suppressor gene family INK4A/CDKN2A [84]. miRNA-21 and -29b were reported to be upregulated in mammary gland tumor tissues, and miR-141 was reported to be overexpressed in canine mammary tumor cell lines, whereas miRNA-31, -34a, and -143/145 were reported to be downregulated in canine mammary tissues [84]. Similar miRNA expression is reported in human and dog mammary tumor patients. miR-15a and miR-16 are downregulated in canine ductal carcinomas, while miR-181b, miR-21, miR-29b, and miRlet-7f are upregulated in tubular papillary carcinomas [85]. miR-29b, miR-101, miR-143, and miR-145 expression levels were downregulated and miR-125a expression levels are upregulated in canine mammary tumors compared to normal mammary cells [86].
3. Role of miRNAs in Cancer Treatment
3.1. Humans
As stated above, each cancer possesses a specific combination of miRNAs, either overexpressed oncomiRNAs targeting tumor-suppressor genes or downregulated tumor-suppressor miRNAs targeting oncogenes [87]. This profile of expressed miRNAs may be used to establish a “fingerprint” that could potentially identify specific tumor types and even subtypes with a given tumor. Since miRNAs are involved in cancer cell gene regulation, these may provide excellent opportunities to design personalized therapeutics for cancer patients. miRNA-based anti-cancer therapies have recently generated interest either as monotherapies or in combination with other cancer therapies. Targeting oncomiRNAs induces the expression of tumor-suppressor genes, which in turn enhance tumor cell killing and promote tumor regression [88]. However, physiological and cellular barriers hamper the in vivo efficacy of anti-miRNA technologies.
As previously mentioned, one of the first miRNAs detected in the human genome, miR-21, is overexpressed in glioblastoma [89] and could be used as a therapeutic target in this type of cancer. In glioblastoma cells, the additive interaction of antisense oligonucleotide inhibitors to both miRNA-21 and miRNA-10b may constitute an effective therapeutic strategy to control glioblastoma growth by inhibiting oncogene expression and inducing tumor-suppressor gene expression. miRNA-21 inhibitors also interrupt the activity of the EGFR pathway, thereby increasing the expression of PDCD4 and Tropomyosin 1 (TPM1) and reducing the activities of matrix metalloproteinases (MMPs) [89]. Inhibition of NADPH oxidase (NOX) dramatically lowered the invasive potential of lung cancer in vitro by decreasing miRNA-21-expression [90].
When miRNA inhibitors are co-administered with an anti-cancer agent, they can induce synergistic effects (e.g., in glioblastoma) [91]. A concern in miRNA modulation strategies is the proper identification, in silico, of miRNA inhibitors or analogs that can effectively inhibit or mimic the function of specific miRNAs to achieve miRNA loss or gain of function, respectively. Another challenge to miRNA-directed therapies’ efficiency is the long-term release of these miRNA inhibitors or mimics at their specific target sites. A new form of miRNA inhibitor delivery has been developed to answer these concerns, specifically targeting miRNA-155 in an acidic tumor microenvironment in murine lymphoma. To achieve this, peptide nucleic acid anti-miRs were attached to a peptide with a low pH-induced transmembrane structure (pHLIP). This construct could target the tumor microenvironment and transport anti-miRs under acidic conditions across the plasma membrane. This approach evades the hepatic barrier (removal of foreign proteins from circulation by the hepatic reticuloendothelial system) and facilitates miRNA targeting through a non-specific endocytic pathway [92]. An alternative miRNA inhibitor delivery strategy using R3V6 peptide was evaluated as a transporter of antisense oligodeoxynucleotides [93]. Serum stability assays showed that R3V6 protected miRNA inhibitors from nucleases more efficiently than polyethyleneimine (PEI; 25 kDa, PEI25k). In an in vitro transfection assay, R3V6 transported antisense oligodeoxynucleotide anti-miRNA-21 into cells more efficiently than PEI (25 kDa, PEI25k) and lipofectamine [93].
microRNA can also serve as a candidate for developing oncolytic virotherapy. A new miRNA-modified Coxsackievirus B3 (CVB3) was developed by inserting miR-145/143, miR-1, and miR-216 target sequences into the 5′ untranslated region (5′ UTR) of the CVB3 genome. miR-145/143 is downregulated in tumors, miR-1is muscle-specific, and miR-216 is pancreas-selective [94]. The virus is downregulated in any cell expressing any of the three miRNAs but is replication-competent in cells, such as a tumor, that do not express any of the three. This novel miRNA-modified oncolytic virus inhibited triple-negative breast cancer growth in immunocompromised mouse models [94].
Chemotherapy and miRNA therapy combinations have shown synergistically increased antineoplastic activities. The combination of a miRNA-21 inhibitor and Taxol is an effective therapeutic strategy to control the growth of glioblastoma multiforme (GBM) by inhibiting the expression and phosphorylation of STAT3 in vitro [95].
3.2. Dogs
Canine hemangiosarcoma has an extremely poor prognosis. Upregulation of miR-214 induces apoptosis in hemangiosarcoma cell lines. Intraperitoneal administration of synthetic miR-214 (miR-214/5AE) exhibits anti-tumor effects in a murine model of canine hemangiosarcoma. It induces apoptosis and prohibits cell proliferation [96]. Similarly, intratumoral administration of synthetic miRNA-205 (miR-205BP/S3) can be used to treat canine malignant melanoma. Administration of miR-205BP/S3 in eleven dogs led to five complete remissions, three dogs with stable diseases, and three cases of progressive disease [97].
Novel miRNA vectors are being explored to induce oncolysis and disease remission in solid tumors leading to a new wave of cancer treatments [98]. Canine osteosarcoma patients with metastatic disease have poor prognosis. miR-34a suppresses the oncogene Eag-1, and the downregulation of miR-34a has been correlated with the progression of canine osteosarcoma. In vitro and in vivo models showed that administration of miR-34a inhibited osteosarcoma progression and decreased Eag-1 production [99]. A bioengineered miRNA prodrug (tRNA/miR-34a) was successfully processed into mature miR-34a in canine osteosarcoma cells. The administration of tRNA/miR-34a in murine models with canine OSA xenografts caused delayed tumor growth, increased necrosis and apoptosis, and reduced cellular proliferation [98]. tRNA/miR-34a treatment showed 32% less tumor growth and more prolonged survival versus the control groups [98]. The emergence of new research on the effects of miRNA on tumor progression allows for novel treatment development.
References
- www.miRBase.org. Available online: https://www.mirbase.org/cgi-bin/browse.pl (accessed on 8 November 2022).
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379.
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333.
- Schwarzenbach, H. The clinical relevance of circulating, exosomal miRNAs as biomarkers for cancer. Expert Rev. Mol. Diagn. 2015, 15, 1159–1169.
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659.
- Ahmad, N.; Haider, S.; Jagannathan, S.; Anaissie, E.; Driscoll, J.J. MicroRNA theragnostics for the clinical management of multiple myeloma. Leukemia 2014, 28, 732–738.
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670.
- Mogilyansky, E.; Rigoutsos, I. The miR-17/92 cluster: A comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ. 2013, 20, 1603–1614.
- Zhu, H.; Han, C.; Wu, T. MiR-17-92 cluster promotes hepatocarcinogenesis. Carcinogenesis 2015, 36, 1213–1222.
- Tsuchida, A.; Ohno, S.; Wu, W.; Borjigin, N.; Fujita, K.; Aoki, T.; Ueda, S.; Takanashi, M.; Kuroda, M. miR-92 is a key oncogenic component of the miR-17-92 cluster in colon cancer. Cancer Sci. 2011, 102, 2264–2271.
- Takakura, S.; Mitsutake, N.; Nakashima, M.; Namba, H.; Saenko, V.A.; Rogounovitch, T.I.; Nakazawa, Y.; Hayashi, T.; Ohtsuru, A.; Yamashita, S. Oncogenic role of miR-17-92 cluster in anaplastic thyroid cancer cells. Cancer Sci. 2008, 99, 1147–1154.
- Hayashita, Y.; Osada, H.; Tatematsu, Y.; Yamada, H.; Yanagisawa, K.; Tomida, S.; Yatabe, Y.; Kawahara, K.; Sekido, Y.; Takahashi, T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005, 65, 9628–9632.
- Martens-de Kemp, S.R.; Komor, M.A.; Hegi, R.; Bolijn, A.S.; Tijssen, M.; de Groen, F.L.M.; Depla, A.; van Leerdam, M.; Meijer, G.A.; Fijneman, R.J.A.; et al. Overexpression of the miR-17-92 cluster in colorectal adenoma organoids causes a carcinoma-like gene expression signature. Neoplasia 2022, 32, 100820.
- Lu, F.; Zhao, X.; Zhang, Z.; Xiong, M.; Wang, Y.; Sun, Y.; He, B.; Zhu, J. The diagnostic and prognostic value of the miR-17-92 cluster in hepatocellular carcinoma: A meta-analysis. Front. Genet. 2022, 13, 927079.
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. miRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502.
- Ozgun, A.; Karagoz, B.; Bilgi, O.; Tuncel, T.; Baloglu, H.; Kandemir, E.G. MicroRNA-21 as an indicator of aggressive phenotype in breast cancer. Onkologie 2013, 36, 115–118.
- Echevarria-Vargas, I.M.; Valiyeva, F.; Vivas-Mejia, P.E. Upregulation of miR-21 in cisplatin resistant ovarian cancer via JNK-1/c-Jun pathway. PLoS ONE 2014, 9, e97094.
- Zhang, X.; Wang, C.; Shan, S.; Liu, X.; Jiang, Z.; Ren, T. TLR4/ROS/miRNA-21 pathway underlies lipopolysaccharide instructed primary tumor outgrowth in lung cancer patients. Oncotarget 2016, 7, 42172–42182.
- Si, H.; Sun, X.; Chen, Y.; Cao, Y.; Chen, S.; Wang, H.; Hu, C. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 223–229.
- Baez-Vega, P.M.; Echevarria Vargas, I.M.; Valiyeva, F.; Encarnacion-Rosado, J.; Roman, A.; Flores, J.; Marcos-Martinez, M.J.; Vivas-Mejia, P.E. Targeting miR-21-3p inhibits proliferation and invasion of ovarian cancer cells. Oncotarget 2016, 7, 36321–36337.
- Zhang, Z.; Li, Z.; Gao, C.; Chen, P.; Chen, J.; Liu, W.; Xiao, S.; Lu, H. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab. Investig. 2008, 88, 1358–1366.
- Yaman Agaoglu, F.; Kovancilar, M.; Dizdar, Y.; Darendeliler, E.; Holdenrieder, S.; Dalay, N.; Gezer, U. Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumor Biol. 2011, 32, 583–588.
- Toiyama, Y.; Takahashi, M.; Hur, K.; Nagasaka, T.; Tanaka, K.; Inoue, Y.; Kusunoki, M.; Boland, C.R.; Goel, A. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J. Natl. Cancer Inst. 2013, 105, 849–859.
- Simonian, M.; Mosallayi, M.; Mirzaei, H. Circulating miR-21 as novel biomarker in gastric cancer: Diagnostic and prognostic biomarker. J. Cancer Res. Ther. 2018, 14, 475.
- Sicard, F.; Gayral, M.; Lulka, H.; Buscail, L.; Cordelier, P. Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. 2013, 21, 986–994.
- Si, M.L.; Zhu, S.; Wu, H.; Lu, Z.; Wu, F.; Mo, Y.Y. miR-21-mediated tumor growth. Oncogene 2007, 26, 2799–2803.
- Chan, J.K.; Blansit, K.; Kiet, T.; Sherman, A.; Wong, G.; Earle, C.; Bourguignon, L.Y. The inhibition of miR-21 promotes apoptosis and chemosensitivity in ovarian cancer. Gynecol. Oncol. 2014, 132, 739–744.
- Tian, W.; Pang, X.; Luan, F. Diagnosis value of miR-181, miR-652, and CA72-4 for gastric cancer. J. Clin. Lab. Anal. 2022, 36, e24411.
- Tian, J.; Shen, R.; Yan, Y.; Deng, L. miR-186 promotes tumor growth in cutaneous squamous cell carcinoma by inhibiting apoptotic protease activating factor-1. Exp. Ther. Med. 2018, 16, 4010–4018.
- Zhai, Z.; Mu, T.; Zhao, L.; Li, Y.; Zhu, D.; Pan, Y. MiR-181a-5p facilitates proliferation, invasion, and glycolysis of breast cancer through NDRG2-mediated activation of PTEN/AKT pathway. Bioengineered 2022, 13, 83–95.
- Strotbek, M.; Schmid, S.; Sanchez-Gonzalez, I.; Boerries, M.; Busch, H.; Olayioye, M.A. miR-181 elevates Akt signaling by co-targeting PHLPP2 and INPP4B phosphatases in luminal breast cancer. Int. J. Cancer 2017, 140, 2310–2320.
- Tong, S.J.; Liu, J.; Wang, X.; Qu, L.X. microRNA-181 promotes prostate cancer cell proliferation by regulating DAX-1 expression. Exp. Ther. Med. 2014, 8, 1296–1300.
- Barbato, A.; Iuliano, A.; Volpe, M.; D’Alterio, R.; Brillante, S.; Massa, F.; De Cegli, R.; Carrella, S.; Salati, M.; Russo, A.; et al. Integrated Genomics Identifies miR-181/TFAM Pathway as a Critical Driver of Drug Resistance in Melanoma. Int. J. Mol. Sci. 2021, 22, 1801.
- Abrahamsson, A.; Dabrosin, C. Tissue specific expression of extracellular microRNA in human breast cancers and normal human breast tissue in vivo. Oncotarget 2015, 6, 22959–22969.
- Tao, K.; Yang, J.; Guo, Z.; Hu, Y.; Sheng, H.; Gao, H.; Yu, H. Prognostic value of miR-221-3p, miR-342-3p and miR-491-5p expression in colon cancer. Am. J. Transl. Res. 2014, 6, 391–401.
- Pineau, P.; Volinia, S.; McJunkin, K.; Marchio, A.; Battiston, C.; Terris, B.; Mazzaferro, V.; Lowe, S.W.; Croce, C.M.; Dejean, A. miR-221 overexpression contributes to liver tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 264–269.
- Chen, W.X.; Hu, Q.; Qiu, M.T.; Zhong, S.L.; Xu, J.J.; Tang, J.H.; Zhao, J.H. miR-221/222: Promising biomarkers for breast cancer. Tumor Biol. 2013, 34, 1361–1370.
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004.
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561.
- Yang, W.; Lee, D.Y.; Ben-David, Y. The roles of microRNAs in tumorigenesis and angiogenesis. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 140–155.
- Qu, H.; Zheng, L.; Song, H.; Jiao, W.; Li, D.; Fang, E.; Wang, X.; Mei, H.; Pu, J.; Huang, K.; et al. microRNA-558 facilitates the expression of hypoxia-inducible factor 2 α through binding to 5’-untranslated region in neuroblastoma. Oncotarget 2016, 7, 40657–40673.
- Baradaran, B.; Shahbazi, R.; Khordadmehr, M. Dysregulation of key microRNAs in pancreatic cancer development. Biomed. Pharmacother. 2019, 109, 1008–1015.
- Wen, J.; Fu, J.; Zhang, W.; Guo, M. Genetic and epigenetic changes in lung carcinoma and their clinical implications. Mod. Pathol. 2011, 24, 932–943.
- Zhao, L.; Duan, Y.T.; Lu, P.; Zhang, Z.J.; Zheng, X.K.; Wang, J.L.; Feng, W.S. Epigenetic Targets and their Inhibitors in Cancer Therapy. Curr. Top. Med. Chem. 2018, 18, 2395–2419.
- Link, S.; Grund, S.E.; Diederichs, S. Alternative splicing affects the subcellular localization of Drosha. Nucleic Acids Res. 2016, 44, 5330–5343.
- Xia, L.; Zhang, D.; Du, R.; Pan, Y.; Zhao, L.; Sun, S.; Hong, L.; Liu, J.; Fan, D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer 2008, 123, 372–379.
- Shen, J.; Wan, R.; Hu, G.; Yang, L.; Xiong, J.; Wang, F.; Shen, J.; He, S.; Guo, X.; Ni, J.; et al. miR-15b and miR-16 induce the apoptosis of rat activated pancreatic stellate cells by targeting Bcl-2 in vitro. Pancreatology 2012, 12, 91–99.
- Musumeci, M.; Coppola, V.; Addario, A.; Patrizii, M.; Maugeri-Sacca, M.; Memeo, L.; Colarossi, C.; Francescangeli, F.; Biffoni, M.; Collura, D.; et al. Control of tumor and microenvironment cross-talk by miR-15a and miR-16 in prostate cancer. Oncogene 2011, 30, 4231–4242.
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949.
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529.
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS is regulated by the let-7 microRNA family. Cell 2005, 120, 635–647.
- Kumar, M.S.; Erkeland, S.J.; Pester, R.E.; Chen, C.Y.; Ebert, M.S.; Sharp, P.A.; Jacks, T. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc. Natl. Acad. Sci. USA 2008, 105, 3903–3908.
- Buechner, J.; Tomte, E.; Haug, B.H.; Henriksen, J.R.; Lokke, C.; Flaegstad, T.; Einvik, C. Tumour-suppressor microRNAs let-7 and mir-101 target the proto-oncogene MYCN and inhibit cell proliferation in MYCN-amplified neuroblastoma. Br. J. Cancer 2011, 105, 296–303.
- Sun, X.; Qin, S.; Fan, C.; Xu, C.; Du, N.; Ren, H. Let-7: A regulator of the ERalpha signaling pathway in human breast tumors and breast cancer stem cells. Oncol. Rep. 2013, 29, 2079–2087.
- Slusarz, A.; Pulakat, L. The two faces of miR-29. J. Cardiovasc. Med. 2015, 16, 480–490.
- Zhang, W.; Wu, Q.; Liu, Y.; Wang, X.; Ma, C.; Zhu, W. LncRNA HOTAIR Promotes Chemoresistance by Facilitating Epithelial to Mesenchymal Transition through miR-29b/PTEN/PI3K Signaling in Cervical Cancer. Cells Tissues Organs 2022, 211, 16–29.
- Vera, O.; Bok, I.; Jasani, N.; Nakamura, K.; Xu, X.; Mecozzi, N.; Angarita, A.; Wang, K.; Tsai, K.Y.; Karreth, F.A. A MAPK/miR-29 Axis Suppresses Melanoma by Targeting MAFG and MYBL2. Cancers 2021, 13, 1408.
- Hozaka, Y.; Seki, N.; Tanaka, T.; Asai, S.; Moriya, S.; Idichi, T.; Wada, M.; Tanoue, K.; Kawasaki, Y.; Mataki, Y.; et al. Molecular Pathogenesis and Regulation of the miR-29-3p-Family: Involvement of ITGA6 and ITGB1 in Intra-Hepatic Cholangiocarcinoma. Cancers 2021, 13, 2804.
- Grassilli, S.; Bertagnolo, V.; Brugnoli, F. Mir-29b in Breast Cancer: A Promising Target for Therapeutic Approaches. Diagnostics 2022, 12, 2139.
- Zhang, W.; Qian, J.X.; Yi, H.L.; Yang, Z.D.; Wang, C.F.; Chen, J.Y.; Wei, X.Z.; Fu, Q.; Ma, H. The microRNA-29 plays a central role in osteosarcoma pathogenesis and progression. Mol. Biol. 2012, 46, 622–627.
- Yu, P.N.; Yan, M.D.; Lai, H.C.; Huang, R.L.; Chou, Y.C.; Lin, W.C.; Yeh, L.T.; Lin, Y.W. Downregulation of miR-29 contributes to cisplatin resistance of ovarian cancer cells. Int. J. Cancer 2014, 134, 542–551.
- Li, M.H.; Wu, Z.Y.; Wang, Y.; Chen, F.Z.; Liu, Y. Expression of miR-29 and STAT3 in osteosarcoma and its effect on proliferation regulation of osteosarcoma cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7275–7282.
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; Desano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 2009, 4, e6816.
- Corney, D.C.; Hwang, C.I.; Matoso, A.; Vogt, M.; Flesken-Nikitin, A.; Godwin, A.K.; Kamat, A.A.; Sood, A.K.; Ellenson, L.H.; Hermeking, H.; et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin. Cancer Res. 2010, 16, 1119–1128.
- Wu, X.; Cheng, Y.L.; Matthen, M.; Yoon, A.; Schwartz, G.K.; Bala, S.; Taylor, A.M.; Momen-Heravi, F. Down-regulation of the tumor suppressor miR-34a contributes to head and neck cancer by up-regulating the MET oncogene and modulating tumor immune evasion. J. Exp. Clin. Cancer Res. 2021, 40, 70.
- Sun, D.; Wu, Y.; Zhang, S.; Han, Y.; Shen, J.; Zheng, W.; Wei, L.; Liu, Y.; Ren, L.; Gu, Z.; et al. Distinct roles of miR-34 family members on suppression of lung squamous cell carcinoma. Biomed. Pharmacother. 2021, 142, 111967.
- Shi, L.; Fan, B.; Chen, D.; Guo, C.; Xiang, H.; Nie, Y.; Zhong, D.; Shi, X. Human cytomegalovirus protein UL136 activates the IL-6/STAT3 signal through MiR-138 and MiR-34c in gastric cancer cells. Int. J. Clin. Oncol. 2020, 25, 1936–1944.
- Dehghan, R.; Najafi, R.; Azizi Jalilian, F.; Saidijam, M.; Radaei, Z.; Zamani, A.; Ezati, R.; Asna-Ashari, F.; Amini, R. A promising effect of zerumbone with improved anti-tumor-promoting inflammation activity of miR-34a in colorectal cancer cell lines. Mol. Biol. Rep. 2021, 48, 203–218.
- Christensen, L.L.; Tobiasen, H.; Holm, A.; Schepeler, T.; Ostenfeld, M.S.; Thorsen, K.; Rasmussen, M.H.; Birkenkamp-Demtroeder, K.; Sieber, O.M.; Gibbs, P.; et al. MiRNA-362-3p induces cell cycle arrest through targeting of E2F1, USF2 and PTPN1 and is associated with recurrence of colorectal cancer. Int. J. Cancer 2013, 133, 67–78.
- Wahdan-Alaswad, R.S.; Cochrane, D.R.; Spoelstra, N.S.; Howe, E.N.; Edgerton, S.M.; Anderson, S.M.; Thor, A.D.; Richer, J.K. Metformin-Induced Killing of Triple-Negative Breast Cancer Cells Is Mediated by Reduction in Fatty Acid Synthase via miRNA-193b. Horm. Cancer 2014, 5, 374–389.
- Li, J.; Kong, F.; Wu, K.; Song, K.; He, J.; Sun, W. miR-193b directly targets STMN1 and uPA genes and suppresses tumor growth and metastasis in pancreatic cancer. Mol. Med. Rep. 2014, 10, 2613–2620.
- Hashemi, M.; Moradi, N.; Ziaee, S.A.; Narouie, B.; Soltani, M.H.; Rezaei, M.; Shahkar, G.; Taheri, M. Association between single nucleotide polymorphism in miR-499, miR-196a2, miR-146a and miR-149 and prostate cancer risk in a sample of Iranian population. J. Adv. Res. 2016, 7, 491–498.
- Ren, Y.G.; Zhou, X.M.; Cui, Z.G.; Hou, G. Effects of common polymorphisms in miR-146a and miR-196a2 on lung cancer susceptibility: A meta-analysis. J. Thorac. Dis. 2016, 8, 1297–1305.
- Li, D.; Zhu, G.; Di, H.; Li, H.; Liu, X.; Zhao, M.; Zhang, Z.; Yang, Y. Associations between genetic variants located in mature microRNAs and risk of lung cancer. Oncotarget 2016, 7, 41715–41724.
- Meng, Q.; Wang, N.; Duan, G. Long non-coding RNA XIST regulates ovarian cancer progression via modulating miR-335/BCL2L2 axis. World J. Surg. Oncol. 2021, 19, 165.
- Eckstein, S. Dogs and Cancer: Get the Facts. Available online: https://pets.webmd.com/dogs/guide/dogs-and-cancer-get-the-facts#1 (accessed on 20 May 2023).
- Uhl, E.; Krimer, P.; Schliekelman, P.; Tompkins, S.M.; Suter, S. Identification of altered MicroRNA expression in canine lymphoid cell lines and cases of B- and T-Cell lymphomas. Genes Chromosomes Cancer 2011, 50, 950–967.
- Fenger, J.M.; Bear, M.D.; Volinia, S.; Lin, T.Y.; Harrington, B.K.; London, C.A.; Kisseberth, W.C. Overexpression of miR-9 in mast cells is associated with invasive behavior and spontaneous metastasis. BMC Cancer 2014, 14, 84.
- Noguchi, S.; Mori, T.; Hoshino, Y.; Yamada, N.; Maruo, K.; Akao, Y. MicroRNAs as tumour suppressors in canine and human melanoma cells and as a prognostic factor in canine melanomas. Vet. Comp. Oncol. 2013, 11, 113–123.
- Noguchi, S.; Mori, T.; Hoshino, Y.; Yamada, N.; Nakagawa, T.; Sasaki, N.; Akao, Y.; Maruo, K. Comparative study of anti-oncogenic microRNA-145 in canine and human malignant melanoma. J. Vet. Med. Sci. 2012, 74, 1–8.
- Gioia, G.; Mortarino, M.; Gelain, M.E.; Albonico, F.; Ciusani, E.; Forno, I.; Marconato, L.; Martini, V.; Comazzi, S. Immunophenotype-related microRNA expression in canine chronic lymphocytic leukemia. Vet. Immunol. Immunopathol. 2011, 142, 228–235.
- Grimes, J.A.; Prasad, N.; Levy, S.; Cattley, R.; Lindley, S.; Boothe, H.W.; Henderson, R.A.; Smith, B.F. A comparison of microRNA expression profiles from splenic hemangiosarcoma, splenic nodular hyperplasia, and normal spleens of dogs. BMC Vet. Res. 2016, 12, 272.
- Heishima, K.; Mori, T.; Sakai, H.; Sugito, N.; Murakami, M.; Yamada, N.; Akao, Y.; Maruo, K. MicroRNA-214 Promotes Apoptosis in Canine Hemangiosarcoma by Targeting the COP1-p53 Axis. PLoS ONE 2015, 10, e0137361.
- Lutful Kabir, F.M.; DeInnocentes, P.; Bird, R.C. Altered microRNA Expression Profiles and Regulation of INK4A/CDKN2A Tumor Suppressor Genes in Canine Breast Cancer Models. J. Cell. Biochem. 2015, 116, 2956–2969.
- Boggs, R.M.; Wright, Z.M.; Stickney, M.J.; Porter, W.W.; Murphy, K.E. MicroRNA expression in canine mammary cancer. Mamm. Genome 2008, 19, 561–569.
- von Deetzen, M.C.; Schmeck, B.T.; Gruber, A.D.; Klopfleisch, R. Malignancy Associated MicroRNA Expression Changes in Canine Mammary Cancer of Different Malignancies. ISRN Vet. Sci. 2014, 2014, 148597.
- Sethi, S.; Ali, S.; Sethi, S.; Sarkar, F.H. MicroRNAs in personalized cancer therapy. Clin. Genet. 2014, 86, 68–73.
- Costa, P.M.; Pedroso de Lima, M.C. MicroRNAs as Molecular Targets for Cancer Therapy: On the Modulation of MicroRNA Expression. Pharmaceuticals 2013, 6, 1195–1220.
- Liu, Y.; Zheng, M.; Jiao, M.; Yan, C.; Xu, S.; Du, Q.; Morsch, M.; Yin, J.; Shi, B. Polymeric nanoparticle mediated inhibition of miR-21 with enhanced miR-124 expression for combinatorial glioblastoma therapy. Biomaterials 2021, 276, 121036.
- Yan, S.; Liu, G.; Pei, C.; Chen, W.; Li, P.; Wang, Q.; Jin, X.; Zhu, J.; Wang, M.; Liu, X. Inhibition of NADPH oxidase protects against metastasis of human lung cancer by decreasing microRNA-21. Anticancer Drugs 2015, 26, 388–398.
- Dong, C.G.; Wu, W.K.; Feng, S.Y.; Wang, X.J.; Shao, J.F.; Qiao, J. Co-inhibition of microRNA-10b and microRNA-21 exerts synergistic inhibition on the proliferation and invasion of human glioma cells. Int. J. Oncol. 2012, 41, 1005–1012.
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110.
- Song, H.; Oh, B.; Choi, M.; Oh, J.; Lee, M. Delivery of anti-microRNA-21 antisense-oligodeoxynucleotide using amphiphilic peptides for glioblastoma gene therapy. J. Drug Target. 2015, 23, 360–370.
- Bahreyni, A.; Liu, H.; Mohamud, Y.; Xue, Y.C.; Zhang, J.; Luo, H. A new miRNA-Modified coxsackievirus B3 inhibits triple negative breast cancer growth with improved safety profile in immunocompetent mice. Cancer Lett. 2022, 548, 215849.
- Ren, Y.; Zhou, X.; Mei, M.; Yuan, X.B.; Han, L.; Wang, G.X.; Jia, Z.F.; Xu, P.; Pu, P.Y.; Kang, C.S. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer 2010, 10, 27.
- Yoshikawa, R.; Maeda, A.; Ueno, Y.; Sakai, H.; Kimura, S.; Sawadaishi, T.; Kohgo, S.; Yamada, K.; Mori, T. Intraperitoneal administration of synthetic microRNA-214 elicits tumor suppression in an intraperitoneal dissemination mouse model of canine hemangiosarcoma. Vet. Res. Commun. 2022, 46, 447–457.
- Yoshikawa, R.; Mori, T.; Noguchi, S.; Akao, Y.; Maruo, K.; Kitade, Y. Synthetic microRNA-205 exhibited tumour suppression in spontaneous canine malignant melanoma by intratumoral injection. Vet. Comp. Oncol. 2019, 17, 407–412.
- Alegre, F.; Ormonde, A.R.; Snider, K.M.; Woolard, K.; Yu, A.M.; Wittenburg, L.A. A genetically engineered microRNA-34a prodrug demonstrates anti-tumor activity in a canine model of osteosarcoma. PLoS ONE 2018, 13, e0209941.
- Xu, X.; Zhu, Y.; Liang, Z.; Li, S.; Xu, X.; Wang, X.; Wu, J.; Hu, Z.; Meng, S.; Liu, B.; et al. c-Met and CREB1 are involved in miR-433-mediated inhibition of the epithelial-mesenchymal transition in bladder cancer by regulating Akt/GSK-3beta/Snail signaling. Cell Death Dis. 2016, 7, e2088.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
705
Revisions:
2 times
(View History)
Update Date:
07 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




