
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tânia Nascimento | -- | 7600 | 2023-07-05 11:29:35 | | | |
| 2 | Peter Tang | Meta information modification | 7600 | 2023-07-06 07:32:52 | | |
Video Upload Options
Tea tree oil is an essential oil extracted from Melaleuca alternifolia (Maiden & Betch) Cheel with known antibacterial, anti-inflammatory, and antioxidant properties and widely used in cosmetic products to treat acne vulgaris.
1. Components of Tea Tree Oil (TTO) and Their Variability
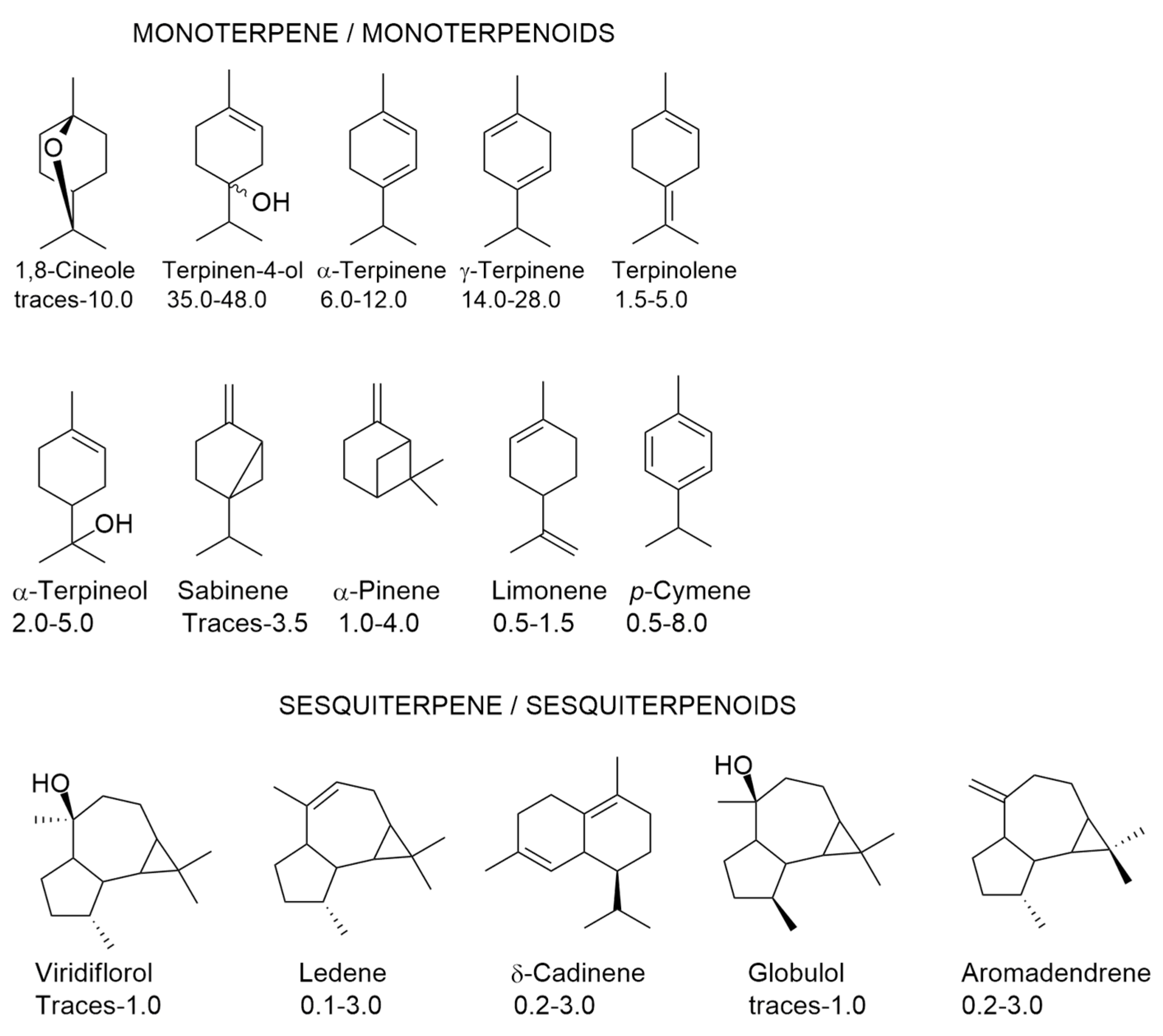
2. Biological Properties of TTO
2.1. Antimicrobial Activity of TTO
|
Main Components, Percentage (>5%) |
Microorganism |
Diameters of Inhibition Zones (mm) |
Minimal Bactericidal Concentration (MBC) TTO |
Minimal Inhibitory Concentration (MIC) TTO |
Biofilm Reduction (R) or Inhibition (I) (Concentration) |
Reference |
|---|---|---|---|---|---|---|
|
Terpinen-4-ol, 42 Terpinen-4-ol standard |
Legionella pneumophila |
- |
0.25–0.5% (v/v) |
0.06–0.125% (v/v) |
- |
[32] |
|
β-Pinene, 9 β-Terpineol, 6 Terpinen-4-ol, 10 α-Terpineol, 20 |
Planktonic Enterococcus faecalis E. faecalis biofilm inhibition |
- |
0.5% |
0.25% |
≥0.25% (I) |
[33] |
|
α-Terpinene, 9 γ-Terpinene, 20 Terpinen-4-ol, 43 |
Escherichia coli 22BT E. coli 45DT Enterococcus faecium A29 E. faecalis VAN3 Staphylococcus aureus C3 S. aureus O |
- |
- |
128 μg/mL 1 μg/mL 1 μg/mL 64 μg/mL 8 μg/mL 8 μg/mL |
128 μg/mL (R) 1 μg/mL (R) 1 μg/mL (R) 64 μg/mL (R) 8 μg/mL (R) 8 μg/mL (R) |
[34] |
|
α-Terpinene, 11 γ-Terpinene, 19 Terpinen-4-ol, 33 |
14 Clinical and 2 references S. aureus strains |
Liquid 8–30 Volatile 0–15 |
- |
Liquid 0.1–0.8% (v/v) Liquid (biofilm) |
Liquid (biofilm) 0.8–6.3% (v/v) (minimal biofilm eradication) |
[35] |
|
Area by standard GC-MS α-Pinene, 12 1,8-Cineole, 15 γ-Terpinene, 10 o-Cymene, 6 Terpinen-4-ol, 35 Area by Head Space GC-MS α-Pinene, 23 1,8-Cineole, 17 γ-Terpinene, 11 o-Cymene, 9 Terpinen-4-ol, 29 |
Methicillin-susceptibility Staphylococcus aureus Methicillin-resistant Staphylococcus aureus Escherichia coli Extended Spectrum Beta-Lactamases Carbapenem-Susceptible Kp Extended Spectrum Beta-Lactamases Carbapenem-Resistant Acinetobacter baumannii Pseudomonas aeruginosa Methicillin-susceptibility Staphylococcus aureus + oxacillin Methicillin-resistant Staphylococcus aureus + oxacillin |
2% (v/v) 2% (v/v) 0.25 0.50 0.25 0.25 1 |
1% (v/v) 0.50% (v/v) 0.25% (v/v) 0.50% (v/v) 0.25% (v/v) 0.25% (v/v) 1% (v/v) Fractional inhibitory concentration index 0.32 (synergism) 0.32 (synergism) |
[36] |
||
|
Terpinen-4-ol, 40 γ-Terpinene, 12 1,8-Cineole, 7 p-Cymene, 6 |
Bacillus subtilis Enterococcus faecalis Micrococcus luteus Staphylococcus aureus Pseudomonas aeruginosa Yersinia enterocolitica Salmonella enterica Serratia marcescens Pseudomonas fluorescens (biofilm) Salmonella enterica (biofilm) Candida albicans C. glabrata C. krusei C. tropicalis |
9.33 10.67 7.67 7.33 6.00 6.00 7.33 6.67 6.00 6.00 10.67 7.67 6.33 8.33 |
MIC 90 (μL/mL) 18.36 18.45 18.68 14.26 12.32 15.46 16.36 16.24 28.59 25.43 26.76 29.85 26.32 27.46 |
[37] |
||
|
Terpinen-4-ol, 36 |
Staphylococcus aureus Coliform bacilli Proteus spp. Klebsiella spp. Escherichia coli Citrobacter spp. Enterobacter spp. E. coli (NCTC 11560) Fecal streptococci Fecal streptococci β-Hemolytic streptococci GP.2 Enterococcus faecalis (ATC29212) β-Hemolytic streptococci Streptococcus pyogenes Coagulase-negative staphylococci MRSA Staphylococcus aureus (NCTC 6571) Candida spp. P. aeruginosa P. aeruginosa (NCTC10662) |
1 2 4 2 2 2 4 4 4 >8 >8 >8 >8 1–4 4 4 2 1 1–5 >8 |
0.5 1–2 2 1 1 1 2 2 2 >8 >8 8 >8 0.5–2 2 2–4 2 2 0.5 2–6 8 |
[38] |
||
|
Without a chemical profile, only with the following information: TTO complied with the ISO 4730 and European Pharmacopoeia standards |
Twenty-seven clinical isolates of S. aureus and the reference strain S. aureus NCTC 8325-4 |
0.25–1% (v/v) 0.5% (v/v) |
0.125–0.5% (v/v) 0.5% (v/v) |
2% (v/v) 1% (v/v) |
[39] |
|
|
TTO (enterprise 1) TTO (enterprise 2) Terpinen-4-ol (racemic) L-Terpinen-4-ol TTO (enterprise 1) TTO (enterprise 2) Terpinen-4-ol (racemic) L-Terpinen-4-ol |
Thirty MRSA isolates Twenty-eight CoNS isolates |
1–8% (v/v) 1->8% (v/v) 0.125–1% (v/v) 0.125–1% (v/v) 0.5–2% (v/v) 0.5–2% (v/v) 0.25–0.5% (v/v) 0.25–0.5% (v/v) |
0.125–1% (v/v) 0.125–1% (v/v) 0.0625–0.5 (v/v) 0.0625–0.5 (v/v) 0.25–0.5% (v/v) 0.125–0.5% (v/v) 0.0625–0.25% (v/v) 0.0625–0.25% (v/v) |
[40] |
||
|
Terpinen-4-ol, 40 α-Terpinene, 9 γ-Terpinene, 21 |
Staphylococcus aureus
Escherichia coli
Candida albicans |
1 mg/mL (16.57) 0.5 mg/mL (15.54) 0.01 mg/mL (11.08) 1 mg/mL (16.75) 0.5 mg/mL (15.13) 0.01 mg/mL (9.87) 0.01 mg/mL (12.21) |
[41] |
|||
|
Terpinen-4-ol, 44 γ-Terpinene, 22 α-Terpinene, 7 α-Terpineol, 6 |
C. albicans Trichophyton mentagrophytes S. aureus S. epidermidis Streptococcus pyogenes MRSA Klebsiella pneumoniae P. aeruginosa
C. albicans Trichophyton mentagrophytes S. aureus S. epidermidis Streptococcus pyogenes MRSA Klebsiella pneumoniae P. aeruginosa |
TTO 20.3 21.1 19.2 21.7 19.2 19.5 18.1 13.2 AgNO3 17.7 19.2 18.2 19.2 22.4 17.6 24.2 15.3 |
[42] |
|||
|
Not determined |
Bacteroides Prevotella Fusobacterium Peptostreptococcus anaerobius Other gram-positive anaerobic cocci |
0.03–0.5% (v/v) 0.03–0.25% (v/v) 0.06–0.55% (v/v) 0.06–0.25% (v/v) 0.03–0.25% (v/v) |
[43] |
|||
|
Three batches Terpinen-4-ol, 41–44 α-Terpinene, 10–11 γ-Terpinene, 21–23 |
Cutibacterium acnes |
0.25% (v/v) |
[44] |
|||
|
Terpinen-4-ol, 40 1,8-Cineole, 5 |
Trichophyton rubrum T. mentagrophytes Microsporum canis Candida albicans Candida sp. Trichosporon cutaneum Malassezia furfur isolated from patients with Dandruff Seborrheic dermatitis Pityriasis versicolor |
0.11–0.22% (m/v) 0.11–0.44% (m/v) 0.11% (m/v) 0.44% (m/v) 0.22–0.44% (m/v) 0.22% (m/v) 0.05–0.44% (m/v) 0.11–0.22% (m/v) 0.05–0.22% (m/v) |
[45] |
|||
|
α-Terpinene, 9 γ-Terpinene, 19 Terpinen-4-ol, 46 |
Chromobacterium violaceum CV026 |
At MIC 0.25 mg/mL: 14.3 |
2 mg/mL |
[46] |
||
|
α-Terpinene, 10 p-Cymene, 24 Terpinen-4-ol, 25 β-Fenchyl alcohol, 9 Oregano + TTO TTO + Cinamom TTO + Lavender TTO + Laurel Oregano + TTO TTO + Cinamom TTO + Lavender TTO + Laurel Oregano + TTO TTO + Cinamom TTO + Lavender TTO + Laurel |
Streptococcus pyogenes ATCC 19625 Staphylococcus aureus ATCC 25923 Streptococcus agalactiae ATCC 12386
Streptococcus pyogenes ATCC 19625 S. aureus ATCC 25923 Streptococcus agalactiae ATCC 12386 |
15.00 29.50 26.50 No interaction Additive effect Synergic effect No interaction Synergic effect No interaction No interaction Additive effect Synergic effect No interaction Synergic effect No interaction |
2.00 0.25 Growth |
1.00 0.125 1.00 |
[47] |
|
|
Cupressus sempervirens + TTO Myrtus communis + TTO Origanum marjorana + TTO Origanum vulgare + TTO |
Streptococcus agalactiae ATCC 55618 S. pneumoniae ATCC 49619 S. pyogenes ATCC 12344 Mycobacterium smegmatis ATCC 19420 Moraxella catarrhalis ATCC 23246 Cryptococcus neoformans ATCC 14116 Staphylococcus aureus ATCC 25924 Streptococcus agalactiae ATCC 55618 S. pneumoniae ATCC 49619 S. pyogenes ATCC 12344 Mycobacterium smegmatis ATCC 19420 Klebsiella pneumoniae ATCC 13883 Moraxella catarrhalis ATCC 23246 Cryptococcus neoformans ATCC 14116 Staphylococcus aureus ATCC 25924 Streptococcus agalactiae ATCC 55618 S. pneumoniae ATCC 49619 Mycobacterium smegmatis ATCC 19420 Klebsiella pneumoniae ATCC 13883 Moraxella catarrhalis ATCC 23246 Cryptococcus neoformans ATCC 14116 Streptococcus agalactiae ATCC 55618 S. pneumoniae ATCC 49619 Mycobacterium smegmatis ATCC 19420 Klebsiella pneumoniae ATCC 13883 Cryptococcus neoformans ATCC 14116 |
Additive effect Additive effect Additive effect Synergic effect Additive effect Synergic effect Synergic effect Additive effect Synergic effect Synergic effect Synergic effect Synergic effect Synergic effect Synergic effect Additive effect Additive effect Additive effect Synergic effect Synergic effect Synergic effect Additive effect Synergic effect Synergic effect Synergic effect Additive effect |
1.00 mg/mL 2.00 mg/mL 1.50 mg/mL 1.00 mg/mL 2.00 mg/mL 0.09 mg/mL 2.00 mg/mL 1.00 mg/mL 1.00 mg/mL 2.00 mg/mL 1.00 mg/mL 1.00 mg/mL 0.25 mg/mL 2.00 mg/mL 1.00 mg/mL 1.50 mg/mL 3.00 mg/mL 1.00 mg/mL 1.00 mg/mL 0.25 mg/mL 1.00 mg/mL 1.00 mg/mL 2.00 mg/mL 1.00 mg/mL 0.50 mg/mL |
[48] |
||
|
γ-Terpinene, 17 4-Terpinenyl acetate, 67 |
Cutibacterium acnes Staphylococcus epidermidis |
0.053 g/mL 0.053 g/mL |
0.053 g/mL 0.053 g/mL |
R: No effect I: 0.107 g/mL |
[49] |
|
|
The quantification of the components were not provided |
Staphylococcus aureus strain EG-AE1 Staphylococcus epidermidis strain EG-AE2 Cutibacterium acnes Strain EG-AE1 |
15.5 21.02 20.85 |
78 mg/mL 78 mg/mL 39 mg/mL |
78 mg/mL 78 mg/mL 39 mg/mL |
[50] |
2.1.1. Tea Tree Oil Formulations with Antimicrobial Activity
3. Efficacy in acne vulgaris
Various studies have demonstrated the efficacy of TTO in several human pathologies, such as dentistry, infectious diseases, ophthalmology, and dermatology, amongst others [78]. In dermatology, and specifically, in acne vulgaris, several essential oils have shown beneficial results. Tea tree oil is one of the oils described in tests in vivo as having biological activity on acne, thanks to its antimicrobial, anti-inflammatory, and antioxidant properties [79].
Several human studies have been found evaluating the efficacy of TTO in topical preparations for acne vulgaris [80][81][82][83][84][85][86][87][88][89]. All of them have shown efficacy in the treatment of this pathology, namely in inflammatory lesions. In addition to this demonstrated efficacy, the low incidence of adverse effects described is also an advantage for the use of this oil in topical products for the treatment of acne vulgaris.
In 1990, a single-blind random clinical trial (RCT) was carried out on subjects with mild-moderate acne, for three months, comparing the use of a TTO (5%) water-based gel and benzoyl peroxide (BP) 5% water-based lotion. Although both groups showed a reduction in the inflammatory lesions, the group that applied BP showed a significantly greater improvement than the group that applied TTO (p < 0.001). Although the results point towards greater efficacy of the BP in reducing acne lesions, the results regarding safety point to better results with the TTO gel. In this group, the adverse events reported were less (44%) than in the BP group (79%), with a statistically significant difference (p < 0.001) [80].
Compared with the placebo (carbomer gel), the TTO 5% gel demonstrated good anti-inflammatory and antibacterial capacities, leading to a significant reduction in inflammatory lesions (papules 46.06%; pustules 47.45%) in a double-blind RCT developed by Enshaieh et al. [81], on subjects with mild-moderate acne, for 45 days. This RCT also showed a significant decrease in the number of comedones (40.24%), the total number of lesions (43.64%), and the acne severity index (ASI) (40.49%) [81].
The use of TTO in cleansing and moisturizing products for acne was evaluated in a dual-center, open-label, phase II pilot study, which assessed the efficacy and safety of two products containing TTO, a Face Wash (7 mg/g) and a Gel (200 mg/g), on mild-moderate acne vulgaris, for 12 weeks. The results showed a significant 54% decrease in the total number of lesions (p < 0.001), as well as in the investigator's global assessment score (p < 0.05). Furthermore, skin oiliness decreased significantly (p < 0.01). Even with a twice-daily application, no serious adverse reactions were reported [83].
Compared with Lactobacillus-fermented Chamaecyparis obtusa (LFCO), TTO demonstrated less efficacy in reducing the number of inflammatory and non-inflammatory lesions [82]. However, the anti-inflammatory properties of TTO were enhanced in this research with a significant decrease in inflammation-related proteins, namely IL-8 and TLR-2 mRNA. Adverse reactions such as dryness, erythema, and desquamation were once again described as the most prevalent in the use of topical TTO [82].
The efficacy of the association of adapalene and TTO vs. adapalene was evaluated in a triple-blind RCT in subjects with mild-moderate acne vulgaris for 12 weeks, The efficacy was evaluated demonstrating good results of the association of TTO with adapalene in reducing the number of total lesions, inflammatory and non-inflammatory lesions. The association between adapalene and TTO makes it possible to obtain a topical formulation that covers the four main factors in the development of acne: hyperseborrhoea, hyperkeratinization, inflammation, and bacterial colonization. Despite the good results obtained, further studies should be carried out to obtain more consistent results that corroborate those already obtained in the work developed by Najafi-Taher et al. [85].
The anti-inflammatory and antibacterial properties of TTO have been described and are mainly due to terpinen-4-ol [90], being widely used and studied in the treatment of acne. However, this compound does not have excellent antiseborrheic and keratolytic properties, and it may therefore be necessary to associate it with other compounds that not only reinforce but also complement its activity in the treatment of acne, as described above. Other studies have associated TTO with various plant extracts with known activity on acne, also showing good results in reducing acne lesions [84][86][87][88][89].
In general, the studies found in the literature demonstrate the good properties of TTO in treating acne, both in isolation and associated with other ingredients with complementary activities.
References
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca Alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50.
- Zhang, X.; Chen, S.; Zhang, Y.; Xiao, Y.; Qin, Y.; Li, Q.; Liu, L.; Liu, B.; Chai, L.; Yang, H.; et al. Draft Genome of the Medicinal Tea Tree Melaleuca alternifolia. Mol. Biol. Rep. 2023, 50, 1545–1552.
- Caldefie-Chézet, F.; Fusillier, C.; Jarde, T.; Laroye, H.; Damez, M.; Vasson, M.P.; Guillot, J. Potential Anti-Inflammatory Effects of Melaleuca alternifolia Essential Oil on Human Peripheral Blood Leukocytes. Phytother. Res. 2006, 20, 364–370.
- American Botanical Council. Botanical Adulterants Program Publishes Bulletin on Tea Tree Oil Adulteration. Available online: https://www.herbalgram.org/news/press-releases/2017/botanical-adulterants-program-publishes-bulletin-on-tea-tree-oil-adulteration/ (accessed on 20 February 2023).
- Voelker, J.; Mauleon, R.; Shepherd, M. The Terpene Synthase Genes of Melaleuca alternifolia (Tea Tree) and Comparative Gene Family Analysis Among Myrtaceae Essential Oil Crops. Plant Syst. Evol. 2023, 309, 13.
- Homer, L.E.; Leach, D.N.; Lea, D.; Slade Lee, L.; Henry, R.J.; Baverstock, P.R. Natural Variation in the Essential Oil Content of Melaleuca alternifolia Cheel (Myrtaceae). Biochem. Syst. Ecol. 2000, 28, 367–382.
- ISO 4730:2017; Essential Oil of Melaleuca, Terpinen-4-Ol Type (Tea Tree Oil). Technical Committee ISO/TC 54: Geneva, Switzerland, 2017.
- Gloerfelt-Tarp, F.; Micog, J.C.; Bigland, M.; Wheeler, S.; Palmer, W.M.; Kretzschmar, T. Predicting Tea Tree Oil Distillate Composition Using Portable Spectrometric Technology. J. Raman Spectrosc. 2022, 53, 771–784.
- Shelton, D.; Aitken, K.; Doimo, L.; Leach, D.; Baverstock, P.; Henry, R. Genetic Control of Monoterpene Composition in the Essential Oil of Melaleuca alternifolia (Cheel). Theor. Appl. Genet. 2002, 105, 377–383.
- Southwell, I.; Dowell, A.; Morrow, S.; Allen, G.; Savins, D.; Shepherd, M. Monoterpene Chiral Ratios: Chemotype Diversity and Interspecific Commonality in Melaleuca alternifolia and M. linariifolia. Ind. Crops Prod. 2017, 109, 850–856.
- Butcher, P.A.; Doran, J.C.; Slee, M.U. Intraspecific Variation in Leaf Oils of Melaleuca alternifolia (Myrtaceae). Biochem. Syst. Ecol. 1994, 22, 419–430.
- Butcher, P.A.; Matheson, A.C.; Slee, M.U. Potential for Genetic Improvement of Oil Production in Melaleuca alternifolia and M. linariifolia. New For. 1996, 11, 31–51.
- Zabaras, D.; Spooner-hart, R.N.; Wyllie, S.G. Effects of Mechanical Wounding on Concentration and Composition of Essential Oil from Melaleuca alternifolia Leaves. Biochem. Syst. Ecol. 2002, 30, 399–412.
- Figueiredo, M. Chemical Composition and oil Concentration of Tea Tree Leaf Oil Grown in South Africa During a One-Year Vegetative Cycle. J. Essent. Oil Res. 2006, 18, 52–53.
- Silva, C.J.; Barbosa, L.C.A.; Maltha, C.R.A.; Pinheiro, A.L.; Ismail, F.M.D. Comparative Study of the Essential Oils of Seven Melaleuca (Myrtaceae) Species Grown in Brazil. Flavour Fragr. J. 2007, 22, 474–478.
- Baker, G.R.; Lowe, R.F.; Southwell, I.A. Comparison of Oil Recovered from Tea Tree Leaf by Ethanol Extraction and Steam Distillation. J. Agric. Food Chem. 2000, 48, 4041–4043.
- Wong, V.; Wyllie, S.G.; Cornwell, C.P.; Tronson, D. Supercritical Fluid Extraction (SFE) of Monoterpenes from the Leaves of Melaleuca alternifolia (Tea Tree). Molecules 2001, 6, 92–103.
- ISO 9235: 2021; Aromatic Natural Raw Materials—Vocabulary. Technical Committee ISO: Geneva, Switzerland, 2021.
- Carson, C.F.; Riley, T.V. Antimicrobial Activity of the Essential Oil of Melaleuca alternifolia. Lett. Appl. Microbiol. 1993, 16, 49–55.
- Bezabh, S.A.; Tesfaye, W.; Christenson, J.K.; Carson, C.F.; Thomas, J. Antiparasitic Activity of Tea Tree Oil (TTO) and Its Components against Medically Important Ectoparasites: A Systematic Review. Pharmaceutics 2022, 14, 1587.
- Lam, N.S.K.; Long, X.X.; Griffin, R.C.; Chen, M.K.; Doery, J.C.G. Can the Tea Tree Oil (Australian Native Plant: Melaleuca alternifolia Cheel) Be an Alternative Treatment for Human Demodicosis on Skin? Parasitology 2018, 145, 1510–1520.
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Iriti, M. Plants of the Melaleuca Genus as Antimicrobial Agents: From Farm to Pharmacy. Phytother. Res. 2017, 31, 1475–1494.
- Johnson, J.B.; Thani, P.R.; Mani, J.S.; Cozzolino, D.; Naiker, M. Mid-Infrared Spectroscopy for the Rapid Quantification of Eucalyptus Oil Adulteration in Australian Tea Tree Oil (Melaleuca alternifolia). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 283, 121766.
- May, J.; Chan, C.H.; King, A.; Williams, L.; French, G.L. Time-Kill Studies of Tea Tree Oils on Clinical Isolates. J. Antimicrob. Chemother. 2000, 45, 639–643.
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. Determining the Antimicrobial Actions of Tea Tree Oil. Molecules 2001, 6, 87–91.
- Brun, P.; Bernabè, G.; Filippini, R.; Piovan, A. In Vitro Antimicrobial Activities of Commercially Available Tea Tree (Melaleuca alternifolia) Essential Oils. Curr. Microbiol. 2019, 76, 108–116.
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The Mode of Antimicrobial Action of the Essential Oil of Melaleuca alternifolia (Tea Tree Oil). J. Appl. Microbiol. 2000, 88, 170–175.
- Cox, S.D.; Gustafson, J.E.; Mann, C.M.; Markham, J.L.; Liew, Y.C.; Hartland, R.P.; Bell, H.C.; Warmington, J.R.; Wyllie, S.G. Tea Tree Oil Causes K+ Leakage and Inhibits Respiration in Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 355–358.
- Gustafson, J.E.; Liew, Y.C.; Chew, S.; Markham, J.; Bell, H.C.; Wyllie, S.G.; Warmington, J.R. Effects of Tea Tree Oil on Escherichia coli. Lett. Appl. Microbiol. 1998, 26, 194–198.
- Li, W.R.; Li, H.L.; Shi, Q.S.; Sun, T.L.; Xie, X.B.; Song, B.; Huang, X.M. The Dynamics and Mechanism of the Antimicrobial Activity of Tea Tree Oil against Bacteria and Fungi. Appl. Microbiol. Biotechnol. 2016, 100, 8865–8875.
- Cuaron, J.A.; Dulal, S.; Song, Y.; Singh, A.K.; Montelongo, C.E.; Yu, W.; Nagarajan, V.; Jayaswal, R.K.; Wilkinson, B.J.; Gustafson, J.E. Tea Tree Oil-Induced Transcriptional Alterations in Staphylococcus aureus. Phytother. Res. 2013, 27, 390–396.
- Mondello, F.; Fontana, S.; Scaturro, M.; Girolamo, A.; Colone, M.; Stringaro, A.; Di Vito, M.; Ricci, M.L. Terpinen-4-Ol, the Main Bioactive Component of Tea Tree Oil, as an Innovative Antimicrobial Agent against Legionella pneumophila. Pathogens 2022, 11, 682.
- Qi, J.; Gong, M.; Zhang, R.; Song, Y.; Liu, Q.; Zhou, H.; Wang, J.; Mei, Y. Evaluation of the Antibacterial Effect of Tea Tree Oil on Enterococcus faecalis and Biofilm in Vitro. J. Ethnopharmacol. 2021, 281, 114566.
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E.; Messi, P. Effects of Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. Essential Oils on Antibiotic-Resistant Bacterial Biofilms. Molecules 2023, 28, 1671.
- Brożyna, M.; Paleczny, J.; Kozłowska, W.; Chodaczek, G.; Dudek-Wicher, R.; Felińczak, A.; Gołębiewska, J.; Górniak, A.; Junka, A. The Antimicrobial and Antibiofilm in Vitro Activity of Liquid and Vapour Phases of Selected Essential Oils against Staphylococcus aureus. Pathogens 2021, 10, 1207.
- Oliva, A.; Costantini, S.; de Angelis, M.; Garzoli, S.; Božović, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High Potency of Melaleuca alternifolia Essential Oil Against Multi-Drug Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Molecules 2018, 23, 2584.
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Tvrdá, E.; Kačániová, M. Chemical and Biological Characterization of Melaleuca alternifolia Essential Oil. Plants 2022, 11, 558.
- Banes-Marshall, L.; Cawley, P.; Phillips, C.A. In Vitro Activity of Melaleuca alternifolia (Tea Tree) Oil against Bacterial and Candida spp. Isolates from Clinical Specimens. Br. J. Biomed. Sci. 2001, 58, 139–145.
- Kwieciński, J.; Eick, S.; Wójcik, K. Effects of Tea Tree (Melaleuca alternifolia) Oil on Staphylococcus aureus in Biofilms and Stationary Growth Phase. Int. J. Antimicrob. Agents 2009, 33, 343–347.
- Loughlin, R.; Gilmore, B.F.; McCarron, P.A.; Tunney, M.M. Comparison of the Cidal Activity of Tea Tree Oil and Terpinen-4-Ol against Clinical Bacterial Skin Isolates and Human Fibroblast Cells. Lett. Appl. Microbiol. 2008, 46, 428–433.
- Blejan, E.I.; Popa, D.E.; Costea, T.; Cioacă, A.; Olariu, L.; Ghica, M.; Georgescu, M.; Stancov, G.; Arsene, A.L. The in Vitro Antimicrobial Activity of Some Essential Oils from Aromatic Plants. Farmacia 2021, 69, 290–298.
- Ramadan, M.A.; Shawkey, A.E.; Rabeh, M.A.; Abdellatif, A.O. Promising Antimicrobial Activities of Oil and Silver Nanoparticles Obtained from Melaleuca alternifolia Leaves against Selected Skin-Infecting Pathogens. J. Herb. Med. 2020, 2020, 100289.
- Hammer, K.A.; Carson, C.F.; Riley, T.V. In Vitro Susceptibilities of Lactobacilli and Organisms Associated with Bacterial Vaginosis to Melaleuca alternifolia (Tea Tree) Oil. Antimicrob. Agents Chemother. 1999, 43, 196.
- Ossa-Tabares, J.C.; Llanos, C.J.; García, A.M. Evaluation of Tea Tree Oil Physicochemical Features and Its Antimicrobial Activity against Cutibacterium acnes (Propionibacterium acnes) ATCC 6919. Biomedica 2020, 40, 693–694.
- Nenoff, P.; Haustein, U.F.; Brandt, W. Antifungal Activity of the Essential Oil of Melaleuca alternifolia (Tea Tree Oil) against Pathogenic Fungi in vitro. Ski. Pharmacol. 1996, 9, 388–394.
- Ngenge, A.T.; Kucukaydin, S.; Ceylan, O.; Duru, M.E. Evaluation of Enzyme Inhibition and Anti-Quorum Sensing Potentials of Melaleuca alternifolia and Citrus sinensis Essential Oils. Nat. Prod. Commun. 2021, 16, 1934578X211044565.
- Altun, M.; Yapici, B.M. Determination of Chemical Compositions and Antibacterial Effects of Selected Essential Oils against Human Pathogenic Strains. An. Acad. Bras. Cienc. 2022, 94, e20210074.
- Leigh-de Rapper, S.; Viljoen, A.; van Vuuren, S. Essential Oil Blends: The Potential of Combined Use for Respiratory Tract Infections. Antibiotics 2021, 10, 1517.
- Abdelhamed, F.M.; Abdeltawab, N.F.; ElRakaiby, M.T.; Shamma, R.N.; Moneib, N.A. Antibacterial and Anti-Inflammatory Activities of Thymus vulgaris Essential Oil Nanoemulsion on Acne Vulgaris. Microorganisms 2022, 10, 1874.
- Esmael, A.; Hassan, M.G.; Amer, M.M.; Abdelrahman, S.; Hamed, A.M.; Abd-raboh, H.A.; Foda, M.F. Antimicrobial Activity of Certain Natural-Based Plant Oils against the Antibiotic-Resistant Acne Bacteria. Saudi J. Biol. Sci. 2020, 27, 448–455.
- Homeyer, D.C.; Sanchez, C.J.; Mende, K.; Beckius, M.L.; Murray, C.K.; Wenke, J.C.; Akers, K.S. In Vitro Activity of Melaleuca alternifolia (Tea Tree) Oil on Filamentous Fungi and Toxicity to Human Cells. Med. Mycol. 2015, 53, 285–294.
- Villar Rodríguez, J.; Pérez-Pico, A.M.; Mingorance-Álvarez, E.; Mayordomo Acevedo, R. Meta-Analysis of the Antifungal Activities of Three Essential Oils as Alternative Therapies in Dermatophytosis Infections. J. Appl. Microbiol. 2022, 133, 241–253.
- Pormohammad, A.; Hansen, D.; Turner, R.J. Antibacterial, Antibiofilm, and Antioxidant Activity of 15 Different Plant-Based Natural Compounds in Comparison with Ciprofloxacin and Gentamicin. Antibiotics 2022, 11, 1099.
- Carson, C.F.; Riley, T.V.; Cookson, B.D. Efficacy and Safety of Tea Tree Oil as a Topical Antimicrobial Agent. J. Hosp. Infect. 1998, 40, 175–178.
- Minghetti, P.; Casiraghi, A.; Cilurzo, F.; Gambaro, V.; Montanari, L. Formulation Study of Tea Tree Oil Patches. Nat. Prod. Commun. 2009, 4, 133–137.
- Muta, T.; Parikh, A.; Kathawala, K.; Haidari, H.; Song, Y.; Thomas, J.; Garg, S. Quality-by-Design Approach for the Development of Nano-Sized Tea Tree Oil Formulation-Impregnated Biocompatible Gel with Antimicrobial Properties. Pharmaceutics 2020, 12, 1091.
- Low, W.L.; Kenward, K.; Cairul, M.; Amin, I.M.; Martin, C.; Nahar, L.; Basar, N.; Sarker, S.D.; Adams, J.D. Ionically Crosslinked Chitosan Hydrogels for the Controlled Release of Antimicrobial Essential Oils and Metal Ions for Wound Management Applications. Medicines 2016, 3, 8.
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérz-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023.
- Ghosh, B.; Bhattacharya, D.; Mukhopadhyay, M. A Hydrogel Sheet Mask with Tea Tree Essential Oil Entrapment and Targeted Dose Delivery Capability. Mater. Today Proc. 2022, 57, 77–83.
- Catanzano, O.; Straccia, M.C.; Miro, A.; Ungaro, F.; Romano, I.; Mazzarella, G.; Santagata, G.; Quaglia, F.; Laurienzo, P.; Malinconico, M. Spray-by-Spray in Situ Cross-Linking Alginate Hydrogels Delivering a Tea Tree Oil Microemulsion. Eur. J. Pharm. Sci. 2015, 66, 20–28.
- Sinha, P.; Srivastava, S.; Mishra, N.; Singh, D.K.; Luqman, S.; Chanda, D.; Yadav, N.P. Development, Optimization, and Characterization of a Novel Tea Tree Oil Nanogel Using Response Surface Methodology. Drug Dev. Ind. Pharm. 2016, 42, 1434–1445.
- de Assis, K.M.A.; da Silva Leite, J.M.; de Melo, D.F.; Borges, J.C.; Santana, L.M.B.; dos Reis, M.M.L.; Moreira, V.M.; da Rocha, W.R.V.; Catão, R.M.R.; dos Santos, S.G.; et al. Bicontinuous Microemulsions Containing Melaleuca alternifolia Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. Drug Deliv. Transl. Res. 2020, 10, 1748–1763.
- Bao, X.; Wu, J.; Ma, G. Sprayed Pickering Emulsion with High Antibacterial Activity for Wound Healing. Prog. Nat. Sci. Mater. Int. 2020, 30, 669–676.
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372.
- Low, W.L.; Martin, C.; Hill, D.J.; Kenward, M.A. Antimicrobial Efficacy of Liposome-Encapsulated Silver Ions and Tea Tree Oil against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans. Lett. Appl. Microbiol. 2013, 57, 33–39.
- Aguilar-Pérez, K.M.; Medina, D.I.; Parra-Saldívar, R.; Iqbal, H.M.N. Nano-Size Characterization and Antifungal Evaluation of Essential Oil Molecules-Loaded Nanoliposomes. Molecules 2022, 27, 5728.
- Ge, Y.; Ge, M. Distribution of Melaleuca alternifolia Essential Oil in Liposomes with Tween 80 Addition and Enhancement of in Vitro Antimicrobial Effect. J. Exp. Nanosci. 2015, 11, 345–358.
- Ge, Y.; Tang, J.; Fu, H.; Fu, Y.; Wu, Y. Characteristics, Controlled-Release and Antimicrobial Properties of Tea Tree Oil Liposomes-Incorporated Chitosan-Based Electrospun Nanofiber Mats. Fibers Polym. 2019, 20, 698–708.
- Esposito, E.; Calderan, L.; Galvan, A.; Cappellozza, E.; Drechsler, M.; Mariani, P.; Pepe, A.; Sguizzato, M.; Vigato, E.; Pozza, E.D.; et al. Ex Vivo Evaluation of Ethosomes and Transethosomes Applied on Human Skin: A Comparative Study. Int. J. Mol. Sci. 2022, 23, 15112.
- Bisht, A.; Hemrajani, C.; Upadhyay, N.; Nidhi, P.; Rolta, R.; Rathore, C.; Gupta, G.; Dua, K.; Chellappan, D.K.; Dev, K.; et al. Azelaic Acid and Melaleuca alternifolia Essential Oil Co-Loaded Vesicular Carrier for Combinational Therapy of Acne. Ther. Deliv. 2022, 13, 13–29.
- da Silva, N.P.; Pereira, E.D.C.R.L.; Duarte, L.M.; de Oliveira Freitas, J.C.; de Almeida, C.G.; da Silva, T.P.; de Melo, R.C.N.; Morais Apolônio, A.C.; de Oliveira, M.A.L.; de Mello Brandão, H.; et al. Improved Anti-Cutibacterium acnes Activity of Tea Tree Oil-Loaded Chitosan-Poly(ε-Caprolactone) Core-Shell Nanocapsules. Colloids Surf. B Biointerfaces 2020, 196, 111371.
- Youn, B.H.; Kim, Y.S.; Yoo, S.; Hur, M.H. Antimicrobial and Hand Hygiene Effects of Tea Tree Essential Oil Disinfectant: A Randomised Control Trial. Int. J. Clin. Pract. 2021, 75, e14206.
- Messager, S.; Hammer, K.A.; Carson, C.F.; Riley, T.V. Assessment of the Antibacterial Activity of Tea Tree Oil Using the European EN 1276 and EN 12054 Standard Suspension Tests. J. Hosp. Infect. 2005, 59, 113–125.
- Filatov, V.A.; Kulyak, O.Y.; Kalenikova, E.I. In Vitro and in Vivo Antimicrobial Activity of an Active Plant-Based Quadrocomplex for Skin Hygiene. J. Pharm. Pharmacogn. Res. 2022, 10, 905–921.
- Kunicka-Styczyńska, A.; Sikora, M.; Kalemba, D. Lavender, Tea Tree and Lemon Oils as Antimicrobials in Washing Liquids and Soft Body Balms. Int. J. Cosmet. Sci. 2011, 33, 53–61.
- Gnatta, J.R.; de Brito Poveda, V.; Padoveze, M.C.; Graziano, K.U.; Turrini, R.N.T.; da Silva, M.J.P. Melaleuca alternifolia Essential Oil Soap: A Potential Alternative for Hand Hygiene. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1517–1520.
- Sgorbini, B.; Cagliero, C.; Argenziano, M.; Cavalli, R.; Bicchi, C.; Rubiolo, P. In Vitro Release and Permeation Kinetics of Melaleuca alternifolia (Tea Tree) Essential Oil Bioactive Compounds from Topical Formulations. Flavour Fragr. J. 2017, 32, 354–361.
- Kairey, L.; Agnew, T.; Bowles, E.J.; Barkla, B.J.; Wardle, J.; Lauche, R. Efficacy and safety of Melaleuca alternifolia (tea tree) oil for human health—A systematic review of randomized controlled trials. Front. Pharmacol. 2023, 14, 31, doi.org/10.3389/fphar.2023.1116077.
- Abozeid, D.; Fawzy, G.; Issa, M.; Abdeltawab, N.; Soliman, F. Medicinal Plants and Their Constituents in the Treatment of Acne Vulgaris. . Biointerface Res. Appl. Chem. 2023, 13, 189.
- Bassett, I.B.; Pannowitz, D.L.; Barnetson, R.S.C. A Comparative Study of Tea-Tree Oil versus Benzoylperoxide in the Treatment of Acne. Med. J. Aust. 1990, 153, 455–458.
- Enshaieh, S.; Jooya, A.; Siadat, A.H.; Iraji, F. The Efficacy of 5% Topical Tea Tree Oil Gel in Mild to Moderate Acne Vulgaris: A Randomized, Double-Blind Placebo-Controlled Study. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 22–25.
- Kwon, H.H.; Yoon, J.Y.; Park, S.Y.; Min, S.; Suh, D.H. Comparison of Clinical and Histological Effects between Lactobacillus-Fermented Chamaecyparis obtusa and Tea Tree Oil for the Treatment of Acne: An Eight-Week Double-Blind Randomized Controlled Split-Face Study. Dermatology 2014, 229, 102–109.
- Malhi, H.K.; Tu, J.; Riley, T.V.; Kumarasinghe, S.P.; Hammer, K.A. Tea Tree Oil Gel for Mild to Moderate Acne; a 12 Week Uncontrolled, Open-Label Phase II Pilot Study. Australas. J. Dermatol. 2017, 58, 205–210.
- Lubtikulthum, P.; Kamanamool, N.; Udompataikul, M. A Comparative Study on the Effectiveness of Herbal Extracts vs 2.5% Benzoyl Peroxide in the Treatment of Mild to Moderate Acne Vulgaris. J. Cosmet. Dermatol. 2019, 18, 1767–1775.
- Najafi-Taher, R.; Jafarzadeh kohneloo, A.; Eslami Farsani, V.; Mehdizade Rayeni, N.; Moghimi, H.R.; Ehsani, A.; Amani, A. A Topical Gel of Tea Tree Oil Nanoemulsion Containing Adapalene versus Adapalene Marketed Gel in Patients with Acne Vulgaris: A Randomized Clinical Trial. Arch. Dermatol. Res. 2022, 314, 673–679.
- Mazzarello, V.; Donadu, M.G.; Ferrari, M.; Piga, G.; Usai, D.; Zanetti, S.; Sotgiu, M.A. Treatment of Acne with a Combination of Propolis, Tea Tree Oil, and Aloe Vera Compared to Erythromycin Cream: Two Double-Blind Investigations. Clin. Pharmacol. 2018, 10, 175–181.
- Yadav, N.; Singh, A.; Chatterjee, A.; Belemkar, S. Evaluation of Efficacy and Safety of Perfact Face Gel and Perfact Face Tablets in Management of Acne. J. Clin. Exp. Dermatol. 2011, 2, 1000118.
- Kim, B.Y.; Shin, S. Antimicrobial and Improvement Effects of Tea Tree and Lavender Oils on Acne Lesions. J. Converg. Inf. Technol. 2013, 8, 339–345.
- Lupu, M.; Malciu, A.M.; Voiculescu, V.M. Feasibility of Reflectance Confocal Microscopy Monitoring in Oily, Acne-Prone Facial Skin Treated with a Topical Combination of Alpha and Beta-Hydroxy Acids, Anti-Inflammatory Molecules, and Herculane Thermal Water: A Blinded, One-Month Study. Life 2022, 12, 1973.
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A Review of Applications of Tea Tree Oil in Dermatology. Int. J. Dermatol. 2013, 52, 784–790.




