Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SUKHWINDER SINGH | -- | 4420 | 2023-07-05 06:26:40 | | | |
| 2 | Catherine Yang | Meta information modification | 4420 | 2023-07-05 07:17:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Singh, S.; Singh, A.; Hallan, S.S.; Brangule, A.; Kumar, B.; Bhatia, R. Chenopodium album. Encyclopedia. Available online: https://encyclopedia.pub/entry/46426 (accessed on 07 February 2026).
Singh S, Singh A, Hallan SS, Brangule A, Kumar B, Bhatia R. Chenopodium album. Encyclopedia. Available at: https://encyclopedia.pub/entry/46426. Accessed February 07, 2026.
Singh, Sukhwinder, Amandeep Singh, Supandeep Singh Hallan, Agnese Brangule, Bhupinder Kumar, Rohit Bhatia. "Chenopodium album" Encyclopedia, https://encyclopedia.pub/entry/46426 (accessed February 07, 2026).
Singh, S., Singh, A., Hallan, S.S., Brangule, A., Kumar, B., & Bhatia, R. (2023, July 05). Chenopodium album. In Encyclopedia. https://encyclopedia.pub/entry/46426
Singh, Sukhwinder, et al. "Chenopodium album." Encyclopedia. Web. 05 July, 2023.
Copy Citation
Bathua (Chenopodium album) is a rich source of extensive-ranging nutrients, including bio-active carbohydrates, flavonoids and phenolics, minerals, and vitamins that translate to countless health benefits such as anticancer, antidiabetic, anti-inflammatory, antimicrobial, and antioxidant activity. Ascaridole, an important phytoconstituent present in aerial parts of the plant, contributes to its anthelmintic property.
Chenopodium album
food applications
impact of the processing
ethnobotanical use
1. Characteristics and Cultivation of C. album
1.1. Botanical Description and Vernacular Names
C. album is a pale green, erect, and strong-smelling polymorphous annual herb of a height of up to 3.5 m, growing 3600 m above sea level. The word “Chenopodium” is derived from two Greek words, viz., “khen” (goose) and “pous” (foot), describing the goosefoot shape of most of the species belonging to the genus. The taxonomic features of the plant include kingdom, phylum, subphylum, class, order, and family, viz., Plantae, Spermatophyta, Angiospermae, Dicotyledonae, Caryophyllales, and Amaranthaceae. The leaves are simple and alternate, oval to obovate or lanceolate shaped with length and width of 1.5–8 cm and 3 cm, respectively, and are attached to the petiole. Decussate leaves are grown first, followed by alternating with long, oval onboard teethed alternate leaves. The stem is cylindrical to angular, erect, grooved, usually branched, and often reddish [1][2]. Dense inflorescence formed by the aggregation of flowers are attached to the leaf axils at the terminus of stems. Small flowers possess radial symmetry and grow on dense branched inflorescence of 10–40 cm in length. The seeds are black, smooth and shiny, horizontally flattened, and lenticular with a diameter of 0.7–1.5 mm. The cotyledons are fleshy, elliptic, elongated, 10–15 mm long and 2–3 mm wide, and shortly petiolate [3][4].
The common English names of C. album include “Melde”, “Lamb’s quarters”, Fat-hen”, “Goosefoot”, “Misbredie”, “Withondebossie”, “Umbikicane”, “Bloubossie”, “Wild spinach”, “Misbredie”, “Varkbossie” and “Pigweed”. The vernacular names used in different parts of India are “Chandan betu” (Bengali), “Pappukura” (Telgu), “Bathua Sag” (Hindi), “Katu ayamoddakam” (Malayalam), “Parupukkirai” (Tamil), “Bathava” (Gujrati) and “Bathu” [5]. The plant names according to Ayurveda, Siddha, Folk and Unani systems of medicine are “Vaastuuka”, “Paruppukeerai”, “Chilli-shaak”, and “Baathu”, respectively [6].
1.2. Cultivation Information
C. album is a widely distributed weed plant, mainly in Asia, Africa, Europe, and North America; it grows in nitrogen-rich soils. Around 21 species of the plant are found in India, particularly in Rajasthan, Kullu valley, and Shimla. The most likely companions of the weed plant are corn, potatoes, and cucurbits. The plant can be traditionally grown in nitrogen-rich soils using the hydroponic method [7][8]. The soil required for the growth of the plant should be moderately fertile; a pH of 4.5 to 8.3 is tolerable. The germination potential of freshly harvested seeds remains around 35%; however, low-temperature (0 to 5 °C) treatments have been found to increase the germinability as prolonged soaking over 20 days do. The percentage of germination is maximum for seeds lying just below the soil surface. The difference in germination optima at different temperatures reflects the different behavior of plants at varying places [1]. Interestingly, the plant is frost tolerant. The longer exposure of the plant to sunlight results in larger and more vigorous plants which discloses the reason for sparse distribution around the equator and extensive distribution in temperate regions [9].
2. Ethnobotanical and Ethnomedicinal Use of C. album
The traditional ayurvedic book Ashtang Hridaya emphasizes the importance of meshed dishes prepared by cooking greens of C. album. The ancient “Vedas” written by sages also puts the plant into the spotlight. The “Rig Veda” and “Atharva Veda” highlight the beneficial effects of C. album in the treatment of piles, clearing worms, and as a laxative. The knowledge compiled from these Vedas and edited by “Agnivesha” and “Charaka”, respectively, has led to a legendary compilation, Charaka Samhita, which, along with Sushruta Samhita, is still used by the practitioners of the traditional system of medicine. Both these books underscore the importance of C. album in improving digestive power, memory, appetite, and body strength [10][11][12]. In addition, it has been said to have purgative action and help to relieve constipation [13].
Although manual cultivation of the herbaceous pot plant, commonly known as bathua, is not widely practiced in India, its growth can be easily detected in the corners of early grain fields in the country. Since long ago, the plant has been employed in the diet as well as for the management of several diseases; the leaf extract is still used in the Ladakh region (India) for controlling painful urination [14]. Ethnomedicinal surveys revealed the utilization of decoctions prepared from different plant parts as herbal remedies for several diseases. Whole-plant decoction has been employed for anthelmintic purposes and the management of jaundice and other liver diseases in various parts of Pakistan (PAK), including Hattar, Gulla Khel, and Makerwal [15][16]. Additionally, the decoction prepared from aerial parts is known to be utilized to treat stomach diseases and gastrointestinal disorders in Gilgit-Baltistan, PAK [17]. The use of the plant for treating indigestion and constipation is also practiced in the Parbati Valley of Kullu, and Sikandra Hill Range of Mandi, Himachal Pradesh [18][19]. In addition, the anthelmintic property has also been reported in myrrh (Commiphora molmol), tulsi (Ocimum sanctum), papaya (Carica papaya), and ginkgo (Ginkgo biloba) [20].
Moreover, the treatment of kidney stones and urinary tract complications with cooked C. album leaves and/or herbal tea made from them is considered to be the appropriate treatment option in the folk medicine of Rajasthan, Toba Tek Singh, and Azad Jammu and Kashmir [21][22][23]. Additionally, people of the Shekhavati region of Rajasthan make use of cooked C. album leaves for the treatment of colic and other urinary system issues [24]. Furthermore, the tribal use of fresh leaves and flowers for vegetables and dried plant powder for diuretic purposes has been reported in Chonthra Karak (Pakistan), and Garhwal (India) [25].
Interestingly, the plant is reported to possess sexual health-promoting properties [26]. The oral consumption of whole-plant powder for the treatment of sexually related problems is practiced in Gujranwala and Lower Kurram [27][28]. In the trans-Himalayan region of India, half a spoon of whole-plant powder is used for treating headaches and seminal weakness [29]. The use of plant seeds and leaves for the treatment of unconsciousness and removal of thirst has also been reported in Mirpur (Pakistan) [30]. Interestingly, the use of amla (Emblica officinalis), black pepper (Piper nigrum), wild mint (Mentha arvensis), tamarind (Tamarindus indica), and allium (Allium odorosum) has also been reported for the treatment of kidney stones by Muslim herbalists [31].
The ethnobotanical uses of cooked plant parts (leaves and stems) further extend to the treatment of flu, gall stones, and tuberculosis. The traditional uses of the plant also include the treatment of sunstroke, sunburn, and swollen feet [32][33]. The herbal drink (fresh infusion) prepared from the whole plant is also used to treat intestinal ulceration in traditional communities of Pakistan [34]. Besides the other uses of the plant, the village peoples of Thoppampatti, Tamilnadu utilize the whole plant for anti-scorbutic uses [35]. Furthermore, the plant is also used as a blood purifier by rural communities of the Arid regions of Punjab, Pakistan [36]. The plant is used for treating skin-related problems in Dehradun, Uttarakhand [37]. The use of the whole plant in the treatment of enlarged spleen and plant roots for the treatment of rheumatism and snake poison in Islamabad is also being practiced [38]. Additionally, the erythropoiesis-stimulating activity of the bathua plant is ethnobotanically employed for the treatment of anemia in the Kumaun Himalayan region [39].
3. Nutritional and Phytochemical Profile of C. album
3.1. Vitamins and Minerals
The plant is a rich source of vital minerals and vitamins for the body in which retinol, ascorbic acid, B-complex, calcium, and potassium are predominant. The mineral content present in the leaves of C. album is much comparably higher than in other consumed vegetables such as beet, mustard leaves and spinach [40]. The concentration of minerals varies among raw and cooked vegetables. The average content of minerals/100 g of raw lamb’s quarters is known to be as follows: calcium—309 mg, magnesium—34 mg, potassium—452 mg, iron—1.2 mg, phosphorous—72 mg, sodium—43 mg, and other elements including selenium, copper, manganese, and zinc in small (less than 1 mg) quantities. Zinc and iron present in leafy vegetables are essential for a healthy immune system and combating anemias. In addition, the plant is a good reserve of retinol and ascorbic acid. The average vitamin content present/100 g of the raw plant is noted to be the following: retinoic acid—11,600 IU, ascorbic acid—80 mg, niacin—1.2 mg, and a trace amount of thiamin, riboflavin, pantothenic acid, pyridoxal, and folate (30 µg/100 g) [41]. One study utilized the thiocyanate method to determine in vitro bioavailability of iron from fresh and dehydrated leaves of Bathua and food made from it [42]. The in vitro bioavailability of iron from paratha and laddoo made from leaves was found to be 2.16 mg/100 g and 2.78 mg/100 g. In addition, the calcium present in raw as well as cooked C. album leaves has been reported to be 32 to 33% bioavailable [43].
3.2. Carbohydrates
Polysaccharides are formed by the linking of several monosaccharide units attached by glycosidic linkages. Further, the biological functions of the resulting polymers are influenced by the degree of polymerization, types of monosaccharide units attached, and the glycosidic linkages. The total carbohydrate content in the raw and cooked lamb quarters is reported to be 7.3 g and 5 g/100 g, respectively [41]. Fructose, glucose, lactose, maltose, and sucrose have been reported in young shoots and mature plants [44]. Dietary fiber is part of a plant-derived food product that is not completely digestible in the human intestines. The consumption of a fiber-rich diet is associated with a reduced risk of cardiovascular diseases. Total dietary fibers represent about 4 g of total carbohydrates in 100 g of raw vegetables. However, some studies have reported a higher content of dietary fibers in young shoots and mature plant material [40].
3.3. Protein and Amino Acids
The nutritional quality of a plant in terms of protein content is governed by the presence and proportion of essential amino acids in the plant/food product. Essential amino acids are not biosynthesized by the human body and are vital for bodily functions. High protein content and a balanced spectrum of amino acids are some of the reasons behind the consumption of C. album. The green leafy part and seeds represent the highly valuable parts for the protein content, particularly higher in lysine due to its synthesis and gathering in a soluble and protein form. The average protein content in the raw lamb’s quarters is known to be 4.2 g/100 g of material [41]. However, some studies have reported even higher protein content, such as 203 g/kg and 32.2 g/100 g in vegetation matter [44][45]. The content of isoleucine, leucine, phenylalanine, threonine, and valine in the green matter protein is reported to be even higher than that in seeds [5]. Among essential amino acids, arginine (11.29 g/kg), leucine (13.44 g/kg), lysine (10.11 g/kg), and phenylalanine (9.26 g/kg) are mainly present in the green matter, while those predominating in seeds are arginine (17.18 g/kg), leucine (7.58 g/kg) and lysine (8.07 g/kg) [45].
3.4. Fatty Acids
Quite a low yield of oils but rich amounts of essential oils are found in the leaves of wild edible plants. Essential fatty acids belonging to the ω3 series were found in the C. album. These fatty acids are known to play a valuable role in modulating human metabolism as well as prevent against coronary heart diseases [46][47]. According to the U.S. Department of Agriculture, the total lipid content in the raw lamb’s quarters is reported to be 0.8 g/100 g, with saturated fatty acids (0.059 g), monounsaturated fatty acids (1.05 g), and polyunsaturated fatty acids (0.351 g) [41]. The predominant fatty acids are 18:3ω3, 18:2ω6, and 16:0. However, unusual fatty acids such as eicosapentaenoic acid and docosahexaenoic acid are absent in the leaves [47]. The polyunsaturated fatty acid composition of a plant may provide beneficial effects towards prevention of diseases such as osteoarthritis and autoimmune disorders. Comparatively, the fatty acid content in leaves is higher compared to that of roots. Along with the salt tolerance of C. album, the total composition of fatty acids in the plant parts was reported to remain unaffected under salt stress conditions [48].
3.5. Phytochemicals
Apart from the appreciable composition of carbohydrates, protein, and fats, other phytochemicals belonging to the class of alkaloids, saponins, terpenoids, flavonoids, and phenolic compounds make C. album a versatile revitalizing source. Phenolic compounds in the plant are responsible for several biological activities, including anticancer, antihyperglycemic, anti-inflammatory, antimicrobial, and lowering of adipogenesis [6][49][50]. The high content of crude alkaloids is responsible for the spasmolytic and anesthetic activity. Additionally, the leaves are reported to contain carotenoids which possess provitamin-A activity and hence promise antioxidant activity [51].
The essential oil obtained by hydro-distillation was found to be composed of higher hydrocarbons, oxygenated and bicyclic mono-, di- and sesquiterpenoids, and fatty acids [49]. Volatile oil obtained from the plant was found to be composed of (E)-ascaridole, carvacrol, (Z)-carvyl acetate, p-cimen-80-ol, (E)-piperitol acetate, benzyl alcohol, p-mentha-1,3,8,-triene, p-cymene, α-terpinene, p-cresol, and piperitone [52]. These components were reported to account for 90.4% of total volatile oil. Phytol was identified to be the most oxygenated diterpene in the oil. In addition, some studies have reported the α-pinene as the most abundant and pinane-2-ol to be the most oxygenated monoterpene component of the essential oil [53].
Furthermore, ester compounds of hexadecenoic acid, 9-Octadecenoic acid and 9,12-Octadecadienoic acid owing to valuable activities such as antibacterial, antifungal, antioxidant, antiviral, anti-inflammatory, and nematocidal activity have been reported to be present in the roots of the plant [54][55]. Volatile organic compounds synthesized by microorganisms have shown significant plant growth-promoting activity by regulating photosynthesis and other vital functions. Interestingly, the volatile organic compound cryptomeridiol obtained from seeds shows significant plant growth-promoting activity [6][56][57]. The methanolic extract of roots has revealed the presence of a novel compound, chenoalbicin, with an alkaloid moiety attached to a cinnamic acid amide. The compound was reported to have allelopathic effects [58][59]. The chemical structures of phytochemicals identified in the different plant extracts and oil are given in Figure 1. Phytic acid, an antinutritional compound present in the leaves of the plant, acts as a major phosphorous storage compound. The phytochemical impairs the absorption of important mineral ions such as calcium, iron, and zinc, which are present in a co-administered diet [60].
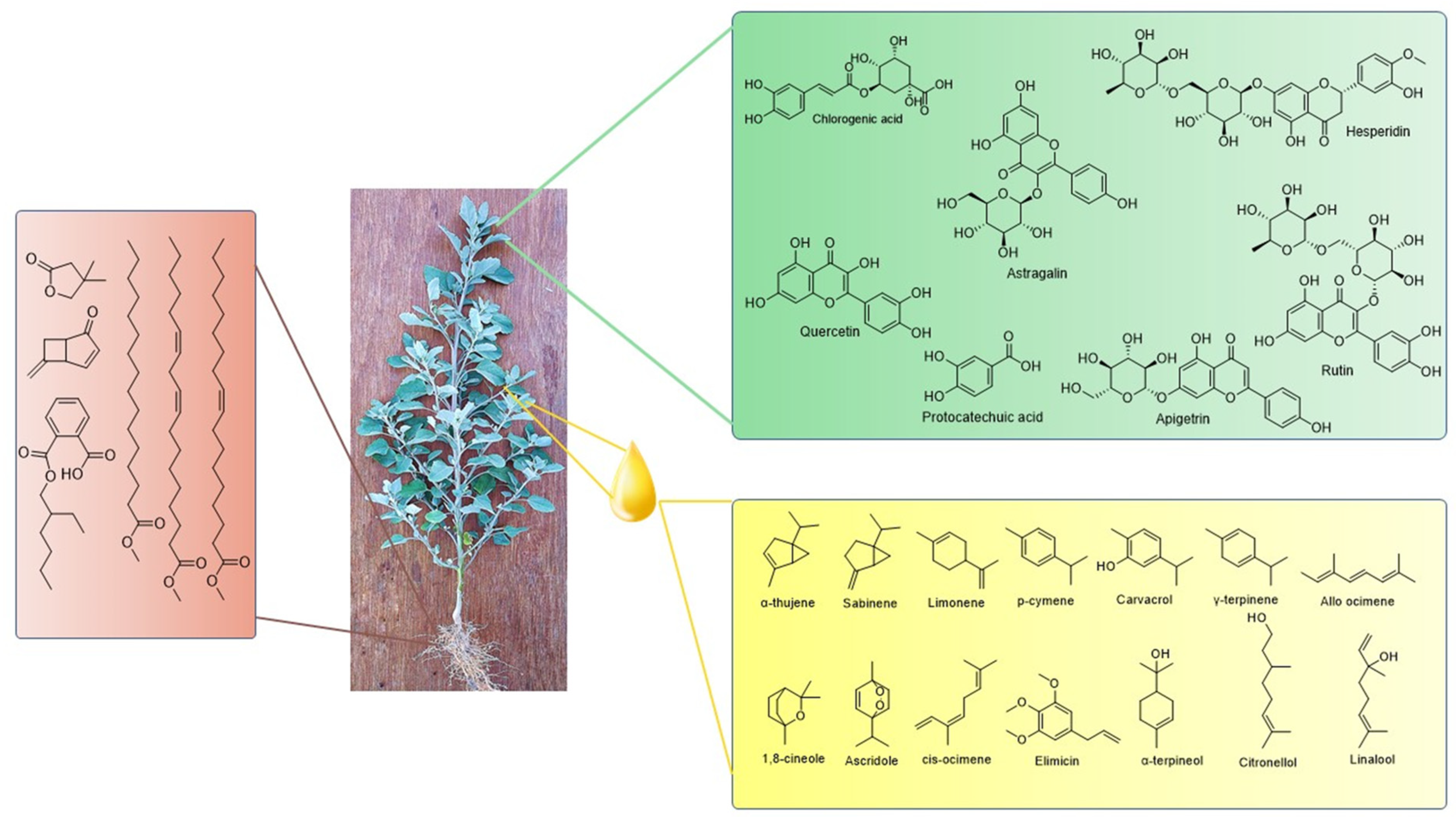
Figure 1. Biologically active phytoconstituents present in different parts of C. album.
4. Functional Activities of C. album
4.1. Antimicrobial Activities
Antimicrobial activity of different molecules is executed by several modes, including disruption/inhibition of cell wall synthesis and protein synthesis, and by binding to different functional units and inhibiting their functions. Interestingly, C. album has been reported for its valuable activity against bacteria, fungi, and nematodes. The antimicrobial activity of the C. album is discussed below.
4.1.1. Antibacterial Activity
The antibacterial activity of oil obtained by hydro-distillation of C. album leaves against Gram-positive and Gram-negative strains has been investigated [49]. Agar well diffusion, agar disc diffusion and microdilution assays revealed significant antibacterial activity against MDR bacterial strains with inhibition zones ranging from 7.0 ± 0.0 mm to 16.0 ± 6.6 mm (in the disc diffusion method) and from 7.0 ± 0.6 mm to 15.0 ± 1.0 mm (in the well diffusion method). Among the tested strains, oil was found to be most effective against Escherichia coli (MIC 1.25 mg/mL and MBC 2.5 mg/mL), Shigella dysenteriae (MIC 0.62 mg/mL and MBC 1.25 mg/mL), Shigella sonnei (MIC 1.25 mg/mL and MBC 2.5 mg/mL), Salmonella typhimurium (MIC 0.31 mg/mL and MBC 0.62 mg/mL), and Staphylococcus aureus (MIC 1.25 mg/mL and MBC 2.5 mg/mL).
In another study, the water extract of C. album exhibited significant anti-bacterial action against Gram-positive (Bacillus cereus MIC 0.5 mg/mL, Staphylococcus epidermidis MIC 0.5 mg/mL, Staphylococcus aureus MIC 1.0 mg/mL, Micrococcus cristinae MIC 0.5 mg/mL, and Streptococcus pyogens MIC 0.5 mg/mL) and Gram-negative (E. coli MIC 1.0 mg/mL, Salmonella pooni MIC 1.0 mg/mL, and Serratia marcescens MIC 1.0 mg/mL) strains [61].
Additionally, Korcan and co-authors investigated the antibacterial activity of methanolic extract and reported maximum activity against Bacillus subtilis (13 mm zone of inhibition at 100 µg/mL), and the activity was found to be increased with increasing concentration of extract [62]. Lone et al. reported the maximum inhibiting activity of the methanolic extract against S. aureus with an inhibition zone of 28 ± 0.14 mm and a mild effect against E. coli among the tested organisms [63]. Interestingly, Umar and colleagues employed the leaf extract as a reducing agent in the development of reduced graphene oxide nanoparticles displaying antimicrobial activities. The significant antibacterial activity of the plant warrants its potential as a potent antibacterial agent [64]. However, further research is needed to isolate and identify the specific molecules responsible for the action.
4.1.2. Antifungal Activity
The antifungal potential of C. album against various pathogenic phyto-fungi has attracted scientific interest in recent years. Alkooranee et al. investigated the antifungal potential of C. album roots and leaves against phytopathogenic fungi, including Alternaria alternate, Fusarium solani, Pythium aphanidermatum, Rhizoctonia solani, and Sclerotinia sclerotium. The water extract of leaves and roots was reported to have a significant mycelial growth reduction effect [65]. Furthermore, Sherazi [66] explored the antifungal activity of C. album for the management of Ascochyta rabiei, which are implicated in chickpea blight. Among different fractions of methanolic extract of C. album leaves, the n-hexane fraction exhibited the highest antifungal potential. In addition to the above, Javid and Rauf utilized the methanolic leaf extract for controlling the basal rot disease (caused by Fusarium oxysporum) of onion [67]. The chloroform fraction of methanolic extract exhibited the maximum antifungal activity by reducing 96–100% of fungal biomass. Inflorescence extract possesses the highest antifungal activity against F. oxysporum, and a possible management of the problem was achieved by employing methanolic inflorescence extract as a natural fungicide [68]. The antifungal potential of plant roots against Sclerotium rolfsii, soil-borne phytopathogenic fungi has been investigated. The methanolic extract of roots was reported to significantly reduce fungal biomass. Furthermore, the abundant compound in the extract, as identified by GC-MS, was found to be mono(2-ethylhexyl)ester of 1,2-benzenedicarboxylic acid [54].
4.2. Anthelmintic Activity
The current epidemiology, unavailability of vaccines for intestinal infections, and the resistance to chemotherapy have made it compelling to discover and develop novel anthelmintic drugs. Medicinal plants, which are continuously the source of novel medicinally useful molecules, have attracted scientific interest for the purpose. Further, Peachey et al. [69] investigated the anthelmintic potential of C. album against cyathostomins, a most important gastrointestinal nematode infecting equids. Plant extracts were prepared by drying, milling, macerating with methanol, and vacuum evaporation. Larval migration inhibition test and egg hatch test revealed the significant anthelmintic activity of C. album, with the lowest value of EC50 at 2.3 mg/mL. Lone et al. [63] reported dose- and time-dependent anthelmintic activity against Haemonchus contortus. Worm mobility inhibition assay and fecal egg count reduction assays were used to determine in vitro and in vivo anthelmintic activity. Methanolic extract possessed more significant anthelmintic activity than aqueous extract. In contrast, it has been reported that the aqueous extract is better in terms of anti-parasitic activity against H. contortus [70]. The anthelmintic potential of C. album extracts against adult Eisenia fetida, an Indian earthworm, has been investigated [71]. Among all extracts, ethyl acetate was reported to exhibit highly significant anthelmintic activity at a 10 mg/mL concentration by causing paralysis and fatality of the earthworms, with paralysis and death time reported to be 10.08 ± 1.11 and 65.28 ± 2.09, respectively. Furthermore, the reversible paralysis of body wall muscle was caused due to the GABA-mimetic action of the extract. Whole-plant extract of C. album was also reported to possess ovicidal efficacy against GI nematodes in goats with ED50 and ED90 values of methanolic, ethylacetate and chloroform extracts at 3.86 and 7.14, 2.73 and 8.31, 4.41 and 20.11 mg/mL, respectively [72].
4.3. Hepatoprotective Activity
Infections, increased alcohol consumption, anemia, malnutrition, and availability of hepatotoxic drugs over the counter have been implicated as the most common reasons behind liver diseases [73]. The conventionally used drugs for the treatment may lead to serious adverse effects. The use of natural remedies prepared from medicinal plants could be promising. In such context, the hepatoprotective potential of C. album has been intensively explored by researchers in recent years. In vitro hepatoprotective potential of aerial parts of C. album in Hep G2 cells was reported; compounds isolated from extracts including nemanolone D, nemanolone E, and substituted 5,7-dimethoxy-cyclohepta-furan-6-one derivatives showed notable activity to lower AST and ALT levels in Hep G2 cells treated with H2O2 and hepatoprotective potential against paracetamol-induced liver damage [74][75]. Moving further, biochemical marker analysis and histopathological studies revealed the hepatoprotective action of aqueous and alcoholic extract as evinced by restored levels of alkaline phosphatase, bilirubin content, and serum transaminases, and reversal of liver damage induced by the toxin. Moreover, the methanolic extract of C. album has been reported to protect the liver against ethanol-induced liver damage [76]. The liver protection potential of the plant is extended to CCl4-induced liver damage. The extracts of powdered plant material were found to alleviate the CCl4-induced elevated levels of ALT, AST, bilirubin, and total cholesterol. The methanolic extract was found to exhibit the significant hepatoprotective activity the most [77][78][79].
4.4. Antioxidant Activity
The general in-built antioxidant mechanism of the human body, which ought to equipoise between reactive oxygen species (ROS) production and antioxidant activity, is often disturbed in long lifetime, increased oxygen consumption, and production of reactive nitrogen species. The oxidative stress generated in this was leads to the pathogenic development of neurodegenerative diseases, diabetes, cancer, vascular diseases, kidney diseases, pulmonary diseases, and aging. Plant polyphenols, including flavonoids and non-flavonoids, possess significant antioxidant activity to combat radical-initiated oxidative damage [80][81][82][83][84][85][86].
The mechanism of antioxidation involves the scavenging of free radicals and/or inhibition of the production of reactive oxygen species [87][88]. C. album, due to the presence of a considerable amount of polyphenols, exhibits strong antioxidant activity. The antioxidant activity of aqueous and alcoholic extract of C. album was assessed by DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) assay, superoxide anion radical scavenging activity-riboflavin photo-oxidation method, hydroxyl-scavenging activity-deoxyribose assay, and lipid peroxidation method. DPPH assay revealed the aqueous extract to possess the highest percentage (96%) of free radical inhibition while the riboflavin photo-oxidation method and hydroxyl scavenging method evinced methanolic extract to exhibit the highest and most significant antioxidant activity [63]. Moreover, superior DPPH free radical scavenging activity has also been reported [89]. Researchers employed NBT (nitroblue tetrazolium reduction test) and ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity methods in extra to assess the antioxidant activity of C. album seed extracts. The superior DPPH free radical scavenging of aqueous extracts may occur due to the presence of more hydrogen donors to scavenge the free radical [63]. The results of the Ferric reducing antioxidant power (FRAP) assay, in addition to the ABTS assay, have corroborated the antioxidant activity of C. album [3].
4.5. Anticancer Activity
The continuous hunt for novel anticancer drugs and previously discovered anticancer molecules from plants has led to increased scientific attention towards plant extracts and the phytochemicals present in them. Phytochemicals, including taxanes, Catharanthus alkaloids, geniposide and derivatives, and other compounds such as artesunate, colchicine, and roscovitine possess the significant anticancer potential to be used in the management of cancer [90].
The anticancer potential of commonly consumed plant C. album has been assiduously scrutinized by researchers across the globe. Rana et al. investigated the in vivo anticancer potential of C. album leaves against Ehlrich ascites carcinoma (EHC) cells in Swiss albino mice. Results have revealed the statistically significant cell growth inhibition of 30.60% and 41.80% at the concentration of 200 mg/kg and 400 mg/kg of C. album extract, respectively. Moreover, the treatment was found to reinstate all the biochemical parameters, including hemoglobin content, red blood cell (RBC), and white blood cell (WBC) count in the mice. The inhibition of cell growth, a decrease in tumor weight, increase in mean survival time, and induction of apoptosis were all concluded to be contributors to the anticancer effect. Plant-sourced lectins are known to instigate apoptosis and autophagy of cancer cells [91]. In a study, lectin (175 µg/mL) isolated from C. album seeds showed strong anti-cancerous potential in hepatoma HepG2 cells as evinced by significantly decreased transcription levels of AFP and GPC3 levels by 90% and 89%, respectively [92].
Interestingly, Umar et al., 2020, formulated the reduced graphene oxide nanoparticles using an extract of C. album as a reducing agent. The anticancer activity against MCF-7 cells of the developed nanoparticles suggested a new approach for the treatment of breast cancer [64]. Moreover, tropolone-based compounds, which included two new tropolones and three known tropolone derivatives (lactarotropone, 2,9-epoxylactarotropone, and 6-lactaradien-5-oic acid γ-lactone) obtained from the aerial parts of the plant have been reported to exhibit notable in vitro anticancer activity against human tumor cell lines including HGC-27 (stomach cancer cells), MDA-MB-231 (breast cancer cells), A-549 (lung cancer cells), HCT-116 (colon cancer cells), and A2780 (ovarian cancer cells) with IC50 values ranging from 0.5 ± 0.2 to 15.5 ± 2.7 μM [74]. However, in vivo and clinical studies are needed to validate the anti-cancer activity of the obtained derivatives.
4.6. Other Activities
Type 2 diabetes, one of the major endocrine disorders, is characterized by impaired insulin secretion and/or impaired action of insulin at the cellular level. Some plants are known and ethnobotanically used in several parts of the world for their potent antidiabetic activity, C. album among them [93]. A study emphasized the antidiabetic potential of methanolic extract of the plant in male Wistar albino rat models. Treatment with a high dose of C. album extract (500 mg/kg body weight p.o) resulted in the maximum decrease in fasting blood glucose level (139.5 ± 4.8 mg/dL, p < 0.01), while mild and low doses caused a slower reduction (142.2 ± 4.1 mg/dL and 148.3 ±1.5 mg/dL, p < 0.01 respectively) in the same at the end of the experiment (after 12 h) [94]. The flavonoid fraction is more effective than the tannin, alkaloid, and saponin fractions for lowering blood glucose levels [95]. The flavonoid fraction of C. album extract at doses of 250 and 500 mg/kg was reported to act by inhibition of α-amylase (IC50—122.18 µg/mL) in a dose-dependent manner. However, some of these doses seem to be supraphysiological and out of realistic range for human nutrition. A general layout representing the important biological activities of the plant and phytochemicals responsible is given in Figure 2.
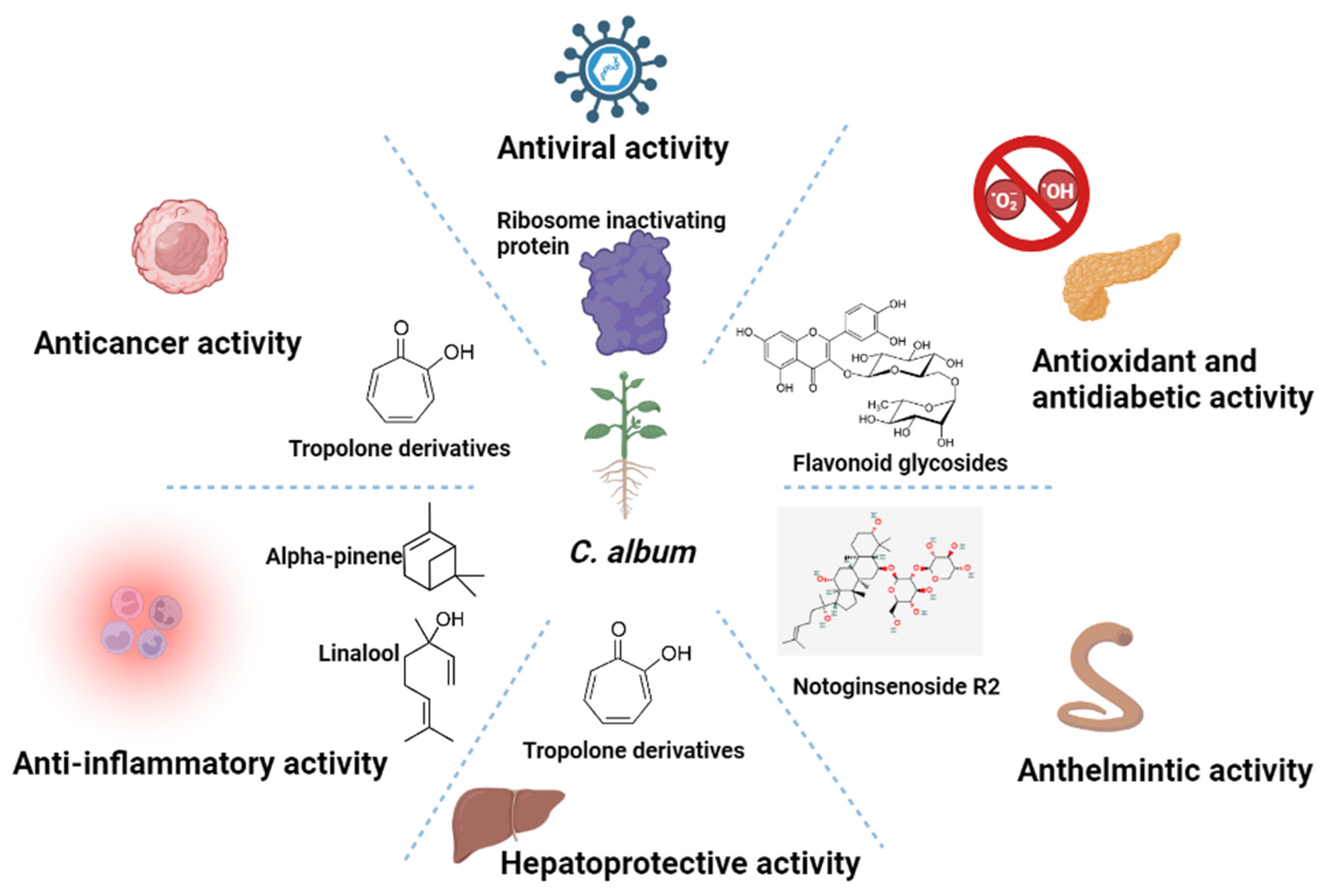
Figure 2. Figure representing important chemical components and corresponding biological activities of C. album.
Anti-nociception refers to the decrease in sensitivity of pre-existing painful stimuli. Results from the study revealed the anti-nociceptive activity of crude methanolic extract of C. album leaves [96]. The results from a study by Mushtaq and co-authors also corroborate the analgesic and anti-inflammatory activity of the plant. C. album resulted in the maximum (64%) inhibition of edema [97]. Interestingly, some studies have also reported the contraceptive action of the plant [98]. The anti-ulcer effect of plant extract has also been documented. It has been reported that alcohol exhibits positive effects against gastric ulcers by significantly decreasing the gastric secretion volume, free and total acidity, and ulcer index [99]. The antirheumatic potential of C. album aerial part extract also has been highlighted. The treatment with extract by its capacity to inhibit NF-κB significantly reduced the paw edema and normalized the level of hematological (Hb, RBC, WBC, and ESR) and biochemical (serum creatinine, total proteins, and acute-phase proteins) markers [100].
References
- CABI. Chenopodium album (Fat Hen); CABI: Wallingford, UK, 2019.
- Singh, P.; Shivhare, Y.; Singhai, A.; Sharma, A. Pharmacological and phytochemical profile of Chenopodium album Linn. Res. J. Pharm. Technol. 2010, 3, 960–963.
- Pandey, S.; Gupta, R.K. Screening of nutritional, phytochemical, antioxidant and antibacterial activity of Chenopodium album (Bathua). J. Pharmacogn. Phytochem. 2014, 3, 1–9.
- Tyagi, K.; Sharma, S.; Rashmi, R.; Kumar, S.; Khair, S. A comparative study of histo-pharmacognosy of Chenopodium album Linn. under the impact of Bicycle Industry Effluent. J. Pharm. Res. 2013, 6, 667–673.
- Poonia, A.; Upadhayay, A. Chenopodium album Linn: Review of nutritive value and biological properties. J. Food Sci. Technol. 2015, 52, 3977–3985.
- Khare, C. Indian Medicinal Plants: An Illustrated Dictionary; A Springer Live Reference; Springer: Berlin/Heidelberg, Germany, 2011.
- Arora, C.; Sahua, D.; Bhartib, D.; Tamrakara, V.; Sonia, S.; Sharma, S. Adsorption of hazardous dye crystal violet from industrial waste using low-cost adsorbent Chenopodium album. Desalination Water Treat. 2019, 167, 324–332.
- Ghirardelli, A.; Schiavon, M.; Zanin, G.; Ostapczuk, P.; Masin, R. Short-Term Responses to Salinity of Soybean and Chenopodium album Grown in Single and Mixed-Species Hydroponic Systems. Agronomy 2021, 11, 1481.
- Le, T.H.; Jia, W.; Cho, K.M.; Khaitov, B.; Park, K.W. A Review on the Status of Exotic Weed (Chenopodium album L.) in Korea and Methods to Control. Weed Turfgrass Sci. 2019, 8, 187–197.
- Jaiswal, Y.S.; Williams, L.L. A glimpse of Ayurveda—The forgotten history and principles of Indian traditional medicine. J. Tradit. Complement. Med. 2017, 7, 50–53.
- Sharma, S. Realms of Ayurveda: Scientific Excursions by Nineteen Scholars; Arnold-Heinemann: Puram, India, 1979.
- Yadav, N.; Vasudeva, N.; Singh, S.; Sharma, S.K. Medicinal properties of genus Chenopodium Linn. Indian J. Nat. Prod. Resour. 2007, 6, 131–134.
- Tripathi, D.B. Ashtang Hridyam. Nirmala Hindi Commentary; Chaukhamba Surbharti Prakashan: Delhi, India, 2007; p. 29.
- Ballabh, B.; Chaurasia, O.; Ahmed, Z.; Singh, S.B. Traditional medicinal plants of cold desert Ladakh—Used against kidney and urinary disorders. J. Ethnopharmacol. 2008, 118, 331–339.
- Hussain, K.; Shahazad, A.; Zia-ul-Hussnain, S. An ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot. Leafl. 2008, 2008, 5.
- Shah, A.; Marwat, S.K.; Gohar, F.; Khan, A.; Bhatti, K.H.; Amin, M.; Din, N.U.; Ahmad, M.; Zafar, M. Ethnobotanical study of medicinal plants of semi-tribal area of Makerwal & Gulla Khel (lying between Khyber Pakhtunkhwa and Punjab Provinces), Pakistan. Am. J. Plant Sci. 2013, 4, 98–116.
- Abbas, Q.; Hussain, A.; Khan, S.W.; Hussain, A.; Shinwari, S.; Hussain, A.; Ullah, A.; Zafar, M.; Ali, K. Floristic Diversity, Ethnobotany and Traditional Recipes of Medicinal Plants of Maruk Nallah, Haramosh Valley, District Gilgit, Gilgit Baltistan: Traditional recipes of Maruk Nallah, Haramosh Valley, District Gilgit. Proc. Pak. Acad. Sci. B. Life Environ. Sci. 2019, 56, 97–112.
- Kumar, G.; Chander, H. Traditional Usage of Ethno-medicinal Plants of Sikandra Hill Range in Mandi District of Himachal Pradesh, India. Asian J. Adv. Basic Sci. 2019, 7, 42–49.
- Sharma, P.; Samant, S. Diversity, distribution and indigenous uses of medicinal plants in Parbati Valley of Kullu district in Himachal Pradesh, Northwestern Himalaya. Asian J. Adv. Basic Sci. 2014, 2, 77–98.
- Adak, M.; Kumar, P. Herbal anthelmintic agents: A narrative review. J. Tradit. Chin. Med. 2022, 42, 641–651.
- Sharma, N.; Tanwer, B.S.; Vijayvergia, R. Study of medicinal plants in Aravali regions of Rajasthan for treatment of kidney stone and urinary tract troubles. Int. J. PharmTech Res. 2011, 3, 110–113.
- Mahmood, A.; Mahmood, A.; Malik, R.N. Indigenous knowledge of medicinal plants from Leepa valley, Azad Jammu and Kashmir, Pakistan. J. Ethnopharmacol. 2012, 143, 338–346.
- Tufail, M.; Hussain, K.; Nawaz, K.; Bhatti, K.H.; Yasin, G.; Ali, S.S. Ethnobotanical Survey of Important Wild Medicinal Plants of Tehsil Gojra, District Toba Tek Singh, Punjab, Pakistan. Ethnobot. Res. Appl. 2020, 20, 1–14.
- Katewa, S.; Galav, P. Traditional herbal medicines from Shekhawati region of Rajasthan. Indian J. Tradit. Knowl. IJTK 2005, 4, 237–245.
- Rehman, K.; Mashwani, Z.-U.-R.; Khan, M.A.; Ullah, Z.; Chaudhary, H.J. An ethno botanical perspective of traditional medicinal plants from the Khattak tribe of Chonthra Karak, Pakistan. J. Ethnopharmacol. 2015, 165, 251–259.
- Pande, M.; Pathak, A. Sexual Function Improving Effect of Chenopodium album (Bathua sag) in Normal Male Mice. Biomed. Pharmacol. J. 2015, 1, 325–332.
- Mahmood, A.; Mahmood, A.; Malik, R.N.; Shinwari, Z.K. Indigenous knowledge of medicinal plants from Gujranwala district, Pakistan. J. Ethnopharmacol. 2013, 148, 714–723.
- Hussain, W.; Ullah, M.; Dastagir, G.; Badshah, L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J. Phytomed. 2018, 8, 313–329.
- Devi, U.; Seth, M.; Sharma, P.; Rana, J. Study on ethnomedicinal plants of Kibber Wildlife Sanctuary: A cold desert in Trans Himalaya, India. J. Med. Plants Res. 2013, 7, 3400–3419.
- Mahmood, A.; Qureshi, R.A.; Mahmood, A.; Sangi, Y.; Shaheen, H.; Ahmad, I.; Nawaz, Z. Ethnobotanical survey of common medicinal plants used by people of district Mirpur, AJK, Pakistan. J. Med. Plants Res. 2011, 5, 4493–4498.
- Ahmed, M.M.; Singh, K.P. Traditional knowledge of kidney stones treatment by Muslim Maiba (herbalists) of Manipur, India. Not. Sci. Biol. 2011, 3, 12–15.
- Bano, A.; Ahmad, M.; Hadda, T.B.; Saboor, A.; Sultana, S.; Zafar, M.; Khan, M.P.Z.; Arshad, M.; Ashraf, M.A. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. J. Ethnobiol. Ethnomedicine 2014, 10, 43.
- Rahman, S.; Husen, A. Potential Role of Medicinal Plants in the Cure of Liver and Kidney Diseases. In Non-Timber Forest Products; Springer: Berlin/Heidelberg, Germany, 2021; pp. 229–254.
- Ahmad, M.; Khan, M.P.Z.; Mukhtar, A.; Zafar, M.; Sultana, S.; Jahan, S. Ethnopharmacological survey on medicinal plants used in herbal drinks among the traditional communities of Pakistan. J. Ethnopharmacol. 2016, 184, 154–186.
- Sivasankari, B.; Anandharaj, M.; Gunasekaran, P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J. Ethnopharmacol. 2014, 153, 408–423.
- Ashfaq, S.; Ahmad, M.; Zafar, M.; Sultana, S.; Bahadur, S.; Abbas, N. Medicinal Plant Biodiversity Used among the Rural Communities of Arid Regions of Northern Punjab, Pakistan; NISCAIR-CSIR: Delhi, India, 2019.
- Adhikari, B.S.; Babu, M.; Saklani, P.; Rawat, G. Medicinal plants diversity and their conservation status in Wildlife Institute of India (WII) campus, Dehradun. Ethnobot. Leafl. 2010, 2010, 6.
- Shinwari, M.I.; Khan, M.A. Folk use of medicinal herbs of Margalla hills national park, Islamabad. J. Ethnopharmacol. 2000, 69, 45–56.
- Mehra, A.; Bajpai, O.; Joshi, H. Diversity, utilization and sacred values of Ethno-medicinal plants of Kumaun Himalaya. Trop. Plant Res. 2014, 1, 80–86.
- Guerrero, J.L.G.; Torija Isasa, M.E. Nutritional composition of leaves of Chenopodium species (C. album L., C. murale L. and C. opulifolium Shraeder). Int. J. Food Sci. Nutr. 1997, 48, 321–327.
- U.S. Department of Agriculture. Lambsquarters Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169244/nutrients (accessed on 23 April 2023).
- Prasad, R.; Gupta, A.; Parihar, R.; Gangwar, K. In vitro method for predicting the bioavailability of iron from Bathua (Chenopodium album) and Fenugreek (Trigonella foenum graecum) leaves in Indian cookies. J. Appl. Nat. Sci. 2014, 6, 701–706.
- Amalraj, A.; Pius, A. Bioavailability of calcium and its absorption inhibitors in raw and cooked green leafy vegetables commonly consumed in India—An in vitro study. Food Chem. 2015, 170, 430–436.
- Gqaza, B.M.; Njume, C.; Goduka, N.I.; George, G. Nutritional assessment of Chenopodium album L.(Imbikicane) young shoots and mature plant-leaves consumed in the Eastern Cape Province of South Africa. Int. Proc. Chem. Biol. Environ. Eng. 2013, 53, 97–102.
- Gesinski, K.; Nowak, K. Comparative analysis of the biological value of protein of Chenopodium quinoa Willd. and Chenopodium album L. Part I. Amino acid composition of the seed protein. Acta Sci. Polonorum. Agric. 2011, 10, 57–65.
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; El-Sabrout, K.; Alqaisi, O.; Dawood, M.A.; Soomro, H.; Abdelnour, S.A. Nutritional significance and health benefits of omega-3,-6 and-9 fatty acids in animals. Anim. Biotechnol. 2022, 33, 1678–1690.
- Guil-Guerrero, J.L.; Rodríguez-García, I. Lipids classes, fatty acids and carotenes of the leaves of six edible wild plants. Eur. Food Res. Technol. 1999, 209, 313–316.
- Ivanova, T.; Maiorova, O.; Orlova, Y.V.; Kuznetsova, E.; Khalilova, L.; Myasoedov, N.; Balnokin, Y.V.; Tsydendambaev, V. Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russ. J. Plant Physiol. 2016, 63, 763–775.
- Khomarlou, N.; Aberoomand-Azar, P.; Lashgari, A.P.; Tebyanian, H.; Hakakian, A.; Ranjbar, R.; Ayatollahi, S.A. Essential oil composition and in vitro antibacterial activity of Chenopodium album subsp. Striatum. Acta Biol. Hung. 2018, 69, 144–155.
- Liu, M.; Zhu, K.; Yao, Y.; Chen, Y.; Guo, H.; Ren, G.; Yang, X.; Li, J. Antioxidant, anti-inflammatory, and antitumor activities of phenolic compounds from white, red, and black Chenopodium quinoa seed. Cereal Chem. 2020, 97, 703–713.
- Sangeetha, R.K.; Baskaran, V. Carotenoid composition and retinol equivalent in plants of nutritional and medicinal importance: Efficacy of β-carotene from Chenopodium album in retinol-deficient rats. Food Chem. 2010, 119, 1584–1590.
- Jardim, C.M.; Jham, G.N.; Dhingra, O.D.; Freire, M.M. Composition and antifungal activity of the essential oil of the Brazilian Chenopodium ambrosioides L. J. Chem. Ecol. 2008, 34, 1213–1218.
- Usman, L.; Hamid, A.; Muhammad, N.; Olawore, N.; Edewor, T.; Saliu, B. Chemical constituents and anti-inflammatory activity of leaf essential oil of Nigerian grown Chenopodium album L. EXCLI J. 2010, 9, 181.
- Ali, A.; Javaid, A.; Shoaib, A. GC-MS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii. Planta Daninha 2017, 35, e017164713.
- Dos Santos Lima, L.A.R.; Johann, S.; Cisalpino, P.S.; Pimenta, L.P.S.; Boaventura, M.A.D. In vitro antifungal activity of fatty acid methyl esters of the seeds of Annona cornifolia A. St.-Hil.(Annonaceae) against pathogenic fungus Paracoccidioides brasiliensis. Rev. Soc. Bras. Med. Trop. 2011, 44, 777–780.
- Poonia, A. Bioactive Compounds of Fat-Hen (Chenopodium album L.). In Bioactive Compounds in Underutilized Vegetables and Legumes. Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020; pp. 1–11.
- Bera, B.; Mukherjee, K.; Ganguly, S. Chemical investigation of the seeds of diploid cytotypes of Chenopodium album. Fitoterapia 1991, 62, 178.
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Di Marino, C.; Golino, A.; Previtera, L.; Zarrelli, A. Cinnamic acid amides from Chenopodium album: Effects on seeds germination and plant growth. Phytochemistry 2003, 64, 1381–1387.
- Cutillo, F.; D’Abrosca, B.; DellaGreca, M.; Zarrelli, A. Chenoalbicin, a novel cinnamic acid amide alkaloid from Chenopodium album. Chem. Biodivers. 2004, 1, 1579–1583.
- Sood, P.; Modgil, R.; Sood, M.; Chuhan, P. Anti-nutrient profile of different Chenopodium cultivars leaves. Ann. Food Sci. Technol. 2012, 13, 68–74.
- Adedapo, A.; Jimoh, F.; Afolayan, A. Comparison of the nutritive value and biological activities of the acetone, methanol and water extracts of the leaves of Bidens pilosa and Chenopodium album. Acta Pol. Pharm 2011, 68, 83–92.
- Korcan, S.E.; Aksoy, O.; Erdoğmuş, S.F.; Ciğerci, İ.H.; Konuk, M. Evaluation of antibacterial, antioxidant and DNA protective capacity of Chenopodium album’s ethanolic leaf extract. Chemosphere 2013, 90, 374–379.
- Lone, B.A.; Chishti, M.; Bhat, F.A.; Tak, H.; Bandh, S.A.; Khan, A. Evaluation of anthelmintic antimicrobial and antioxidant activity of Chenopodium album. Trop. Anim. Health Prod. 2017, 49, 1597–1605.
- Umar, M.F.; Ahmad, F.; Saeed, H.; Usmani, S.A.; Owais, M.; Rafatullah, M. Bio-Mediated Synthesis of Reduced Graphene Oxide Nanoparticles from Chenopodium album: Their Antimicrobial and Anticancer Activities. Nanomaterials 2020, 10, 1096.
- Alkooranee, J.T.; Al-khshemawee, H.H.; Al-badri, M.A.K.; Al-srai, M.S.; Daweri, H.H. Antifungal activity and GC-MS detection of leaves and roots parts of Chenopodium album extract against some phytopathogenic fungi. Indian J. Agric. Res. 2020, 54, 117–121.
- Sherazi, A.; Jabeen, K.; Iqbal, S.; Yousaf, Z. Management of Ascochyta rabiei by Chenopodium album extracts. Planta Daninha 2016, 34, 675–680.
- Javaid, A.; Rauf, S. Management of basal rot disease of onion with dry leaf biomass of Chenopodium album as soil amendment. Int. J. Agric. Biol. 2015, 17, 142–148.
- Rauf, S.; Javaid, A. Antifungal activity of different extracts of Chenopodium album against Fusarium oxysporum f. sp. cepae, the cause of onion basal rot. Int. J. Agric. Biol. 2013, 15, 367–371.
- Peachey, L.; Pinchbeck, G.; Matthews, J.; Burden, F.; Mulugeta, G.; Scantlebury, C.; Hodgkinson, J. An evidence-based approach to the evaluation of ethnoveterinary medicines against strongyle nematodes of equids. Vet. Parasitol. 2015, 210, 40–52.
- Suleman, M.; Hassan, A.U.; Abbas, F.F.I. Antibacterial, Antiparasitic and Phytochemical Activities of Chenopodium album (Bathua) Plant Extract. Bangladesh J. Bot. 2021, 50, 417–421.
- Choudhary, N.; Khatik, G.L.; Choudhary, S.; Singh, G.; Suttee, A. In vitro anthelmintic activity of Chenopodium album and in-silico prediction of mechanistic role on Eisenia foetida. Heliyon 2021, 7, e05917.
- Sachan, A.; Shanker, D.; Jaiswal, A.K.; Sudan, V. In vitro ovicidal assessment of methanol, ethyl acetate and chloroform extracts of Annona squamosa and Chenopodium album against caprine gastrointestinal nematodiosis. J. Parasit. Dis. 2015, 39, 62–66.
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171.
- Ma, Q.-G.; Wei, R.-R.; Zhang, X.-D.; Sang, Z.-P.; Dong, J.-H.; Lu, Q.-X.; Huang, H.-F.; Guo, D.-M.; Jiang, L. Tropolone derivatives with hepatoprotective and antiproliferative activities from the aerial parts of Chenopodium album Linn. Fitoterapia 2020, 146, 104733.
- Vijay, N.; Padmaa, M. Hepatoprotective activity of Chenopodium album Linn. against paracetamol induced liver damage. Pharmacologyonline 2011, 3, 312–328.
- Karwani, G.; Sisodia, S.S. Hepatoprotective activity of Chenopodium album Linn. in Ethanol induced Hepatotoxicity in Rats. Res. J. Pharm. Technol. 2015, 8, 669–673.
- Karwani, G.; Sisodia, S.S. Hepatoprotective activity of Chenopodium album Linn. in carbon tetrachloride induced hepatotoxicity rats. Res. J. Pharmacol. Pharmacodyn. 2015, 7, 29–34.
- Nayak, D.P.; Dinda, S.; Swain, P.; Kar, B.; Patro, V. Hepatoprotective activity against CCl4-induced hepatotoxicity in rats of Chenopodium album aerial parts. J. Phytother. Pharmacol. 2012, 1, 33–41.
- Parkash, J.; Patel, K.R. Hepatoprotective activity of Chenopodium album leaves extract in CCl4 induced hepatotoxicity in rats. J. Drug Deliv. Ther. 2015, 5, 88–93.
- Stagos, D. Antioxidant Activity of Polyphenolic Plant Extracts; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020.
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283.
- Ahangarpour, A.; Sayahi, M.; Sayahi, M. The antidiabetic and antioxidant properties of some phenolic phytochemicals: A review study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 854–857.
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197.
- Chen, Q.; Wang, Q.; Zhu, J.; Xiao, Q.; Zhang, L. Reactive oxygen species: Key regulators in vascular health and diseases. Br. J. Pharmacol. 2018, 175, 1279–1292.
- Yaribeygi, H.; Farrokhi, F.R.; Rezaee, R.; Sahebkar, A. Oxidative stress induces renal failure: A review of possible molecular pathways. J. Cell. Biochem. 2018, 119, 2990–2998.
- Dua, K.; Malyla, V.; Singhvi, G.; Wadhwa, R.; Krishna, R.V.; Shukla, S.D.; Shastri, M.D.; Chellappan, D.K.; Maurya, P.K.; Satija, S. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: An emerging need for novel drug delivery systems. Chem.-Biol. Interact. 2019, 299, 168–178.
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species—A review. Molecules 2020, 25, 917.
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123.
- Nengroo, Z.; Rauf, A. Fatty acid composition and antioxidant activity of Angelica glauca and Chenopodium album seed extracts from Kashmir. Grasas Y Aceites 2021, 72, e393.
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533.
- Rana, S.; Rahman, S.; Sana, S.; Biswas, T.K.; Hashem, A.K.M.; Parvin, S.; Mazumder, K. Anticancer potential of Chenopodium album leaf extract against Ehrlich ascites carcinoma cells in Swiss albino mice. Future J. Pharm. Sci. 2020, 6, 65.
- Javed, M.; Bilal, M.; Tabassum, B.; Malik, A.; Adeyinka, O.S.; Tariq, M.; Nasir, I.A. Purification and functional characterization of lectin from Chenopodium album. J. Proteins Proteom. 2022, 13, 55–62.
- Nepal, A.; Chakraborty, M. An overview on medicinal plants of Sikkim Himalayas region with emphasis on antidiabetic: A review. J. Pharmacogn. Phytochem. 2021, 10, 215–217.
- Kant, S. Pharmacological evaluation of antidiabetic and antihyperlipidemic activity of Chenopodium album root extract in male Wistar albino rat models. Int. J. Green Pharm. 2018, 12, 115–122.
- Choudhary, N.; Prabhakar, P.K.; Khatik, G.L.; Chamakuri, S.R.; Tewari, D.; Suttee, A. Evaluation of Acute toxicity, In-vitro, In-vivo Antidiabetic Potential of the Flavonoid Fraction of the plant Chenopodium album L. Pharmacogn. J. 2021, 13, 765–779.
- Magama, S.; Asita, A.O. Evaluation of Chenopodium album Linn. crude methanolic leaf extract for central antinociceptive activity in albino mice using the hot plate test. Int. J. Sci. 2017, 6, 36–44.
- Mushtaq, A.; Rashid, S.; Jamil, M.; Anwar, R.; Khawaja, N.R. Anti-nociceptive and anti-inflammatory activity of Trapa bispinosa, Chenopodium album and Cuscuta reflexa. Int. J. Biol. Pharm. Allied Sci. 2017, 6, 608–622.
- Kumar, S.; Chatterjee, R.; Dolai, S.; Adak, S.; Kabir, S.N.; Banerjee, S.; Mondal, N.B. Chenopodium album seed extract-induced sperm cell death: Exploration of a plausible pathway. Contraception 2008, 77, 456–462.
- Nigam, V.; Paarakh, P.M. Anti-ulcer effect of Chenopodium album Linn. against gastric ulcers in rats. Int. J. Pharm. Sci. Drug Res. 2011, 3, 319–322.
- Arora, S.K.; Itankar, P.R.; Verma, P.R.; Bharne, A.P.; Kokare, D.M. Involvement of NFκB in the antirheumatic potential of Chenopodium album L., aerial parts extracts. J. Ethnopharmacol. 2014, 155, 222–229.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.1K
Revisions:
2 times
(View History)
Update Date:
05 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




