Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chih-Yi Ho | -- | 2484 | 2023-07-04 07:10:58 | | | |
| 2 | Rita Xu | Meta information modification | 2484 | 2023-07-04 07:48:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ho, C.; Chen, J.Y.; Hsu, W.; Yu, S.; Chen, W.; Chiu, S.; Yang, H.; Lin, S.; Wu, C. Genetics of Female Pattern Hair Loss. Encyclopedia. Available online: https://encyclopedia.pub/entry/46371 (accessed on 07 February 2026).
Ho C, Chen JY, Hsu W, Yu S, Chen W, Chiu S, et al. Genetics of Female Pattern Hair Loss. Encyclopedia. Available at: https://encyclopedia.pub/entry/46371. Accessed February 07, 2026.
Ho, Chih-Yi, Jeff Yi-Fu Chen, Wen-Li Hsu, Sebastian Yu, Wei-Chiao Chen, Szu-Hao Chiu, Hui-Ru Yang, Sheng-Yao Lin, Ching-Ying Wu. "Genetics of Female Pattern Hair Loss" Encyclopedia, https://encyclopedia.pub/entry/46371 (accessed February 07, 2026).
Ho, C., Chen, J.Y., Hsu, W., Yu, S., Chen, W., Chiu, S., Yang, H., Lin, S., & Wu, C. (2023, July 04). Genetics of Female Pattern Hair Loss. In Encyclopedia. https://encyclopedia.pub/entry/46371
Ho, Chih-Yi, et al. "Genetics of Female Pattern Hair Loss." Encyclopedia. Web. 04 July, 2023.
Copy Citation
Pattern hair loss can occur in both men and women, and the underlying molecular mechanisms have been continuously studied in recent years. Male androgenetic alopecia (M-AGA), also termed male pattern hair loss, is the most common type of hair loss in men. M-AGA is considered an androgen-dependent trait with a background of genetic predisposition.
female pattern hair loss
genetic factors
single nucleotide polymorphism (SNP)
1. Introduction
Female pattern hair loss (FPHL) is commonly seen in women. Although in most cases it is a non-scarring, non-inflammatory condition, loss of hair density often leads to disfigurement, afflicting most patients. The disease is clinically characterized by gradual loss of hair in the central and forehead regions of the scalp, but preservation of the forehead hairline. (Figure 1) This pattern differs from that seen in male androgenetic alopecia (M-AGA), which is often characterized by a receding frontal hairline, followed by baldness and possibly central hair loss. Although the appearance may be different, the final follicular changes in both disorders are similar, such as perifollicular microinflammation, increased number of atrophic follicles, reduced sebaceous gland volume, and reduced anagen-to-telogen ratio [1][2]. Given that these two diseases eventually lead to typical follicular miniaturization and hair loss, it has long been believed that the pathogenesis of female pattern hair loss should be similar to that of M-AGA. It has been recognized that M-AGA is due to a genetically determined androgen-dependent trait; however, the exact genetic cause of FPHL remains unclear, and the direct relationship between androgens and FPHL is yet to be elucidated. So far, accumulating studies have suggested that the pathogenesis of these two diseases is not identical.
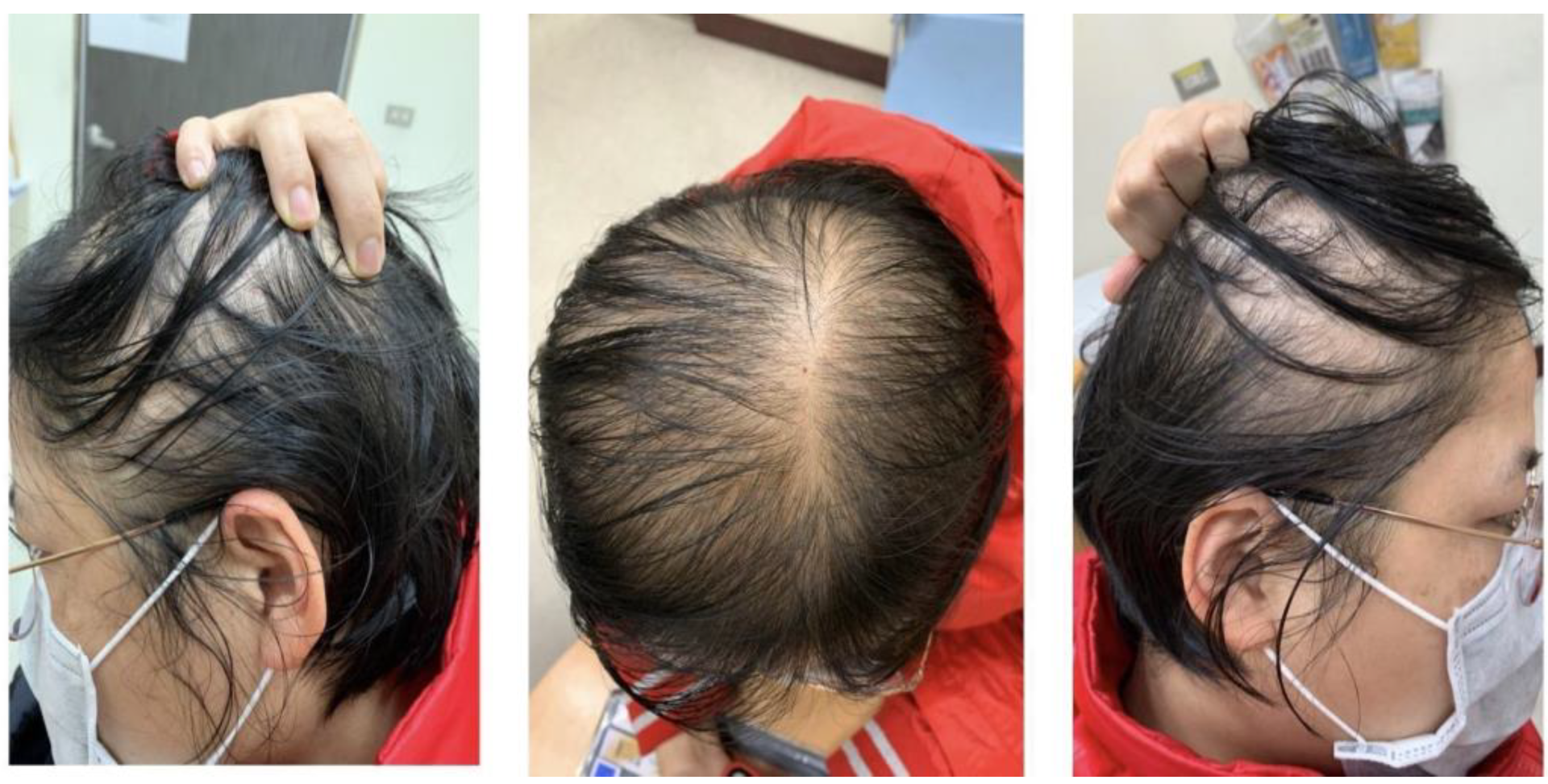
Figure 1. Hair manifestations in women with FPHL.
In clinical practice, several classification systems are used to grade the severity of FPHL. Three commonly used grading methods are the Ludwig classification, Olsen classification, and Sinclair classification (Figure 2). First proposed in 1977, the Ludwig classification is a widely recognized system that divides FPHL into three grades, focusing on the central portion of the scalp and the frontal hairline. It provides an intuitive method to assess the severity of hair thinning and helps determine appropriate treatment strategies [3]. Olsen’s classification is based on the density of the hair and the visibility of the scalp. It takes into account preservation of the anterior hairline, vertex involvement, and general degree of hair loss, with emphasis on forehead protrusion [4]. Sinclair’s classification evaluates hair thinning along the midline and further ranks the severity of hair loss into five grades [5]. Compared with previous methods, this grading system provides a finer classification, making it potentially suitable for the early detection of FPHL. These grading systems, including those of Ludwig, Olsen, and Sinclair, are valuable tools for healthcare professionals to assess and classify the severity of FPHL. Using these grading methods, individualized treatment plans can be formulated and the progress of the disease can be monitored effectively.
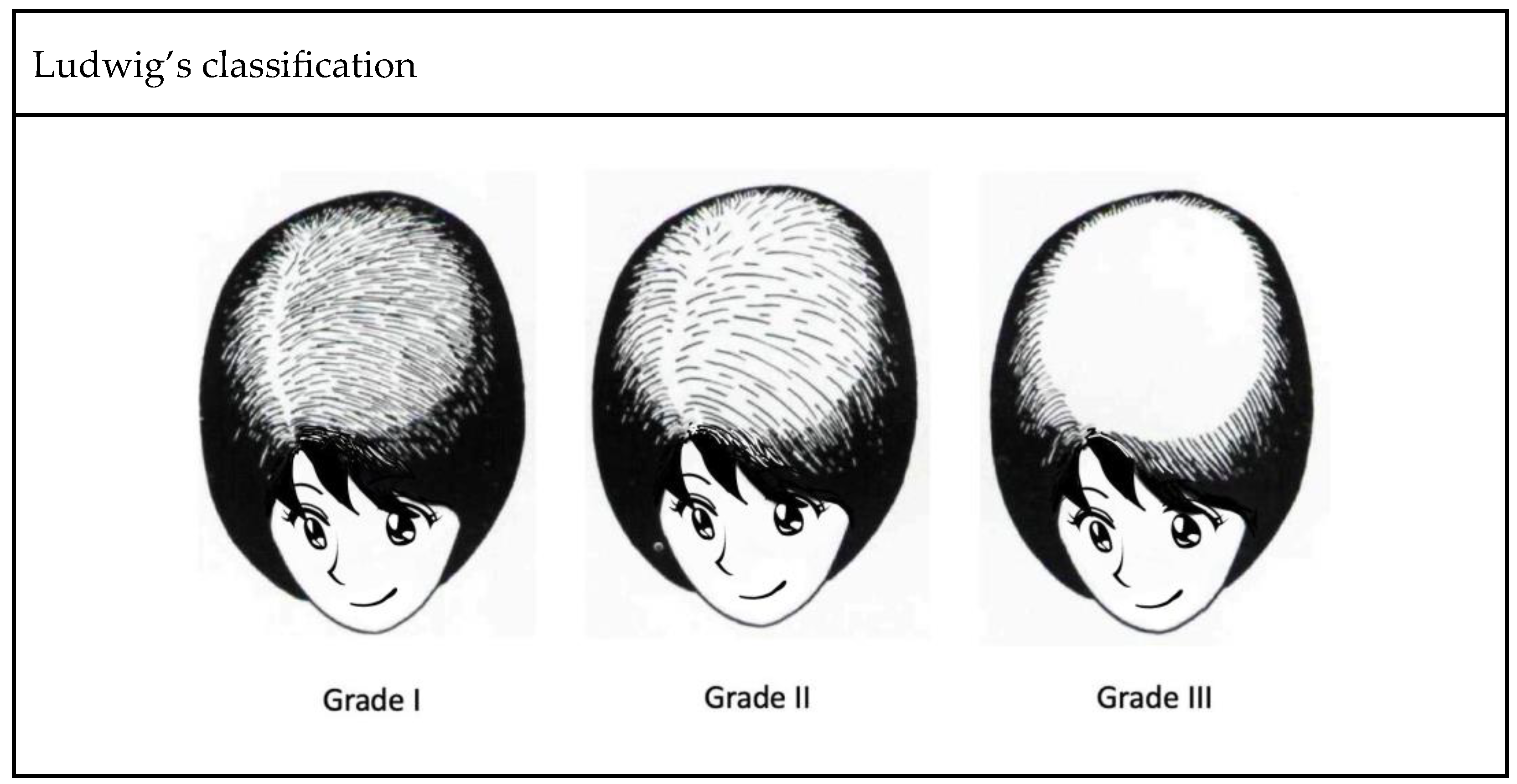
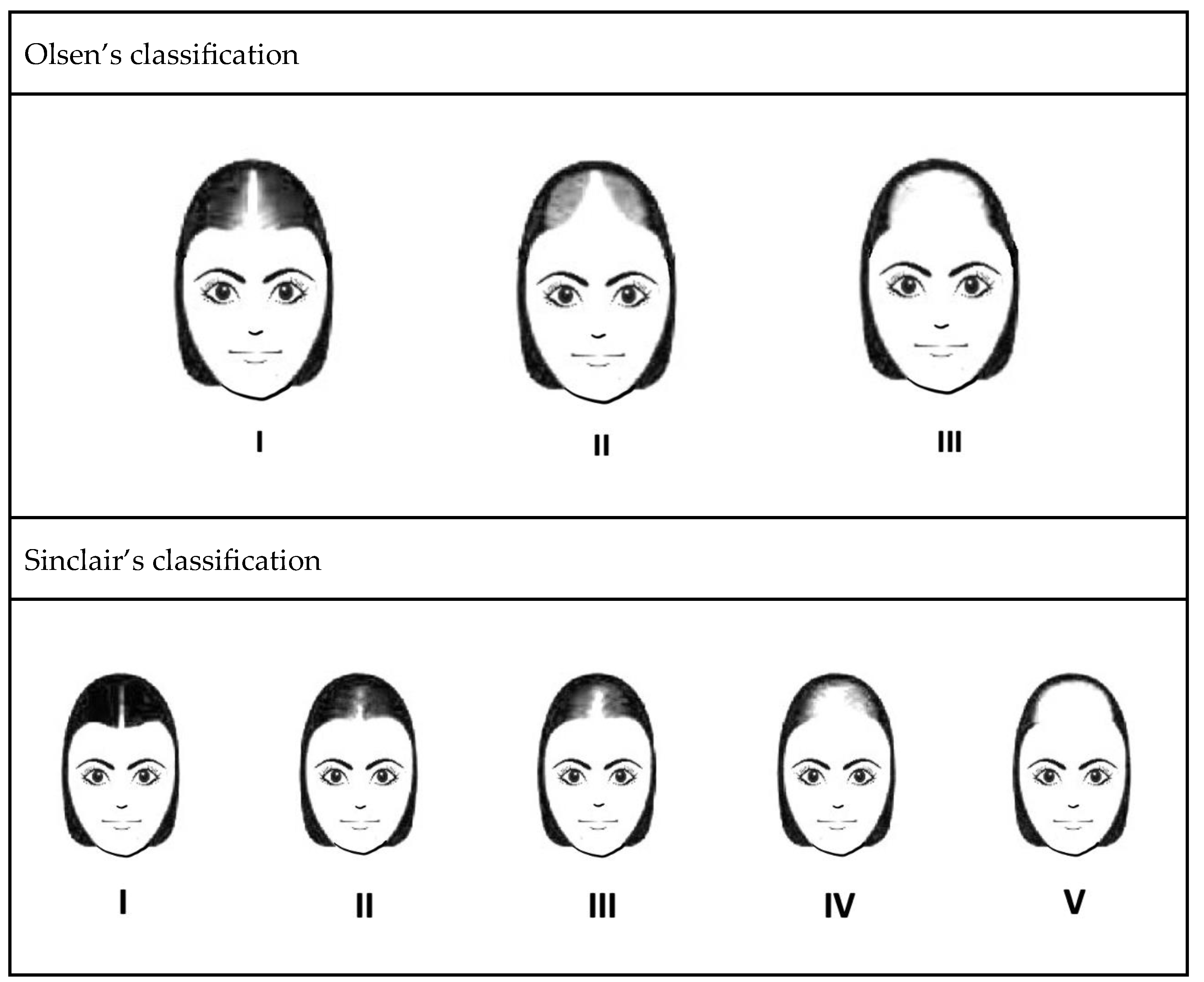
Figure 2. Three well-recognized classification systems of FPHL.
FPHL is not only a common hair problem, it also profoundly impacts patients’ psychological and social well-being [6]. FPHL could cause severe psychological stress, leading to depression, anxiety, and damaged self-esteem, which may further affect patients’ interpersonal relationships, work, and quality of life [7]. A previous study indicated that FPHL could negatively impact patients’ functioning, emotions, self-confidence, and stigmatization, and some patients may even endure psychological disturbance, such as dysmorphophobia or affective disorder [8]. The article presents several strategies of interventions, such as adopting positive behaviors and attitudes to reduce the negative impact of hair loss. Nonetheless, some patients may experience maladaptive responses, including aimless rumination and anger towards others, which may deteriorate their personal lives, such as marital relationships. Consequently, hair loss and the associated maladaptive responses have a significant impact on the patients. Therefore, understanding FPHL helps in clinical treatment and effectively improves patients’ quality of life.
2. Genetics of FPHL
2.1. Mode of Inheritance and Genome-Wide Association Studies (GWAS)
M-AGA is considered a heritable disease [9]. A study of 572 men by MP Birch et al. showed that men with bald fathers were five times more likely to develop M-AGA than men with non-bald fathers [10]. In a case–control study conducted by Jungyoon Ohn et al. in 2022, the proportion of individuals with a positive family history of pattern hair loss is higher in patients with early onset female pattern hair loss compared to those without the disease, with rates of 71.4% versus 51.0% (p = 0.004), respectively. Furthermore, a higher correlation of maternal family history was found in patients with early onset FPHL compared to unaffected women, with rates of 33.3% versus 9.1% (p < 0.001), respectively [11]. Although the contribution of the paternal side of family history was more frequently implicated in previous studies, it was not significantly relevant in those studies. Although female hair loss is mainly determined by genetic factors, the detailed regulation of the genetic factors remains to be studied.
Genome-wide association studies (GWAS) are a method for identifying genomic variants that are statistically associated with disease risk or specific traits. Genome-wide linkage or association studies of FPHL have not been evaluated and reported in detail, and current studies mainly focus on M-AGA susceptibility loci and genes of sex steroid-related hormone pathways.
2.2. Correlation between Nutrition and FPHL
Despite the fact that data supporting the relationship between hair follicle turnover and nutritional deficiencies in FPHL patients are still lacking, many aspects of FPHL treatment are related to nutritional supplementation. A recent study by Piccini [12] clarified that affected intermediate/miniaturized hair follicles in FPHL display relative nutritional deficiency and dormant metabolism, but the function of absorbing nutrients is not impaired, thus suggesting the potential of nutritional supplementation as an adjunctive therapy for FPHL.
The effects of vitamin D on FPHL are still controversial. Vitamin D receptors (VDR) are known to be expressed in follicular keratinocytes and dermal papilla cells and play a role in hair growth regulation. Marwa M T Fawzi et al. [13] demonstrated a crucial role of VDR in the pathogenesis of alopecia areata and AGA by reporting lower serum and tissue VDR levels in alopecia areata and AGA patients compared to the control group. Recently, Iman Seleit et al. [14] conducted a case–control study identifying VDR gene types on 30 FPHL patients with 30 age-matched healthy female subjects as the control group and found that Taq-1 and Cdx-1 from VDR genes could be considered risk factors for FPHL. The genotypes of CC, TC, and T alleles of Taq-1 are associated with FPHL patients, and the CC genotype increases the risk of disease by 12.6 times. The genotypes of AA, GA, and G allele are also associated with FPHL, and the AA genotype increases the risk of disease by 7.5 times. On the other hand, Banihashemi et al. [15] proposed that the concentration of vitamin D3 in the blood is related to the incidence of FPHL, and the vitamin D3 levels were significantly lower in serum samples with FPHL. There is still a lack of relevant research on whether vitamin D supplementation could promote hair growth in FPHL patients with vitamin D deficiency.
Nutrient deficiencies associated with FPHL have been increasingly implicated in recent studies, in which the nutritional/metabolite profile of hair follicles may vary from one patient to another, likely due to differences in dietary, hormones, and genetic background. A low abundance of TCA cycle products, including citric, malic acid and lactate, from aerobic glycolysis were found in occipital or parietal intermediate hair follicles in some patients, whereas L-glutamine was increased in other patients [12]. These findings show that FPHL women may differ in the metabolism of nutrients compared with women without the disease, but whether this result can be confirmed through genetic correlation studies remains to be seen. At present, studies on the genetic aspects of VDR-associated FPHL are still relatively limited, while the impact of other nutrients on FPHL may deserve further investigation.
2.3. Polycystic Ovary Syndrome (PCOS) with FPHL
PCOS is a common cause of infertility in women of reproductive age, and FPHL is known to be associated with hyperandrogenism, especially in PCOS patients [16]. The HSD3B1 gene encodes the 3β-hydroxysteroid dehydrogenase-1 (3βHSD1) enzyme, which catalyzes the conversion of adrenal androgen precursors to the most potent androgen, dihydrotestosterone. Genetic variants of HSD3B1 1245C are known to result in increased androgen expression in peripheral tissues. This finding is supported by a genetic study from Taiwan, in which, among 472 recruited PCOS patients, those who had the HSD3B1 1245C allele were more likely to develop FPHL [17]. PCOS patients with FPHL are not only physically afflicted but suffer profound social and psychological difficulties. However, the exact pathogenic mechanism remains to be studied. It is hopeful that advanced studies on the genetic aspects should facilitate treatment, early screening, and diagnosis of PCOS with FPHL in the future.
2.4. Dickkopf WNT Signaling Pathway Inhibitor 1
DKK-1 (Dickkopf WNT signaling pathway inhibitor 1) gene encodes a member of the Dickkopf family of proteins. When mutated or overexpressed, WNT is a proto-oncoprotein that can promote cell proliferation and transformation. A study in 2019 revealed that the expression of DKK-1 was significantly increased in patients with AGA and alopecia areata (AA), suggesting that DKK-1 plays a certain role in these hair-related diseases [18].
Kwack et al. conducted a series of studies on the expression of Dickkopf 1 (DKK-1) induced by Ectodysplasin-A2 (EDA-A2). They used siRNA transfection and DKK-1 ELISA, among other methods, and found that DKK-1 expression was increased in the human balding dermal papilla cells treated with EDA-A2, eventually leading to apoptosis [19].
Furthermore, R-spondin 1 (RSPO1), a secreted Wnt signaling agonist that possesses an antagonizing effect against DKK-1, was found to be beneficial to hair condition. A recent study reported that a conditioner containing 2% watercress extract (WCE) improved hair loss symptoms by increasing hair thickness and density through its ability to inhibit DKK-1 secretion and antagonize DKK-1 through RSPO1 [20].
At present, most studies on DKK-1 focus on patients with AGA and AA, but there is increasing evidence that DKK1 influences hair follicle growth. Thus, more in-depth studies on the involvement of DKK1-related pathways in FPHL should shed more light on the pathogenesis of the disease.
2.5. Prediction of Dermal Sheath Cup Cell Therapy
The dermal sheath cup (DSC), a tissue located at the base of the dermal sheath (DS), has been shown to contribute to hair follicle regeneration [21].
Hair follicle shrinkage is well established in the mechanism of hair loss in both men and women, and DSC cells may play a role in treating both diseases. Based on these observations, Tsuboi et al. conducted a clinical study of autologous cell-based therapy, demonstrating that transplantation of DSC cells contributed to increased hair density and hair diameter [22].
However, the outcome of DSC cell transplantation varies among patients, prompting Yoshida et al. [21] to screen for changes in gene expression in DSC cells associated with the treatment outcome. They found that negative treatment response to DSC cells can be predicted by detecting markers such as CALD1, ACTA2, and two smooth muscle cell markers MYOCD and SRF. These findings provide potential markers that can help in preclinical assessments of DSC cells.
2.6. Sex Steroid Hormones Gene Polymorphism
It is conceivable that sex hormone-related genes are likely involved in regulating FPHL. For example, similar miniaturizing of terminal hair in M-AGA is caused by the binding of dihydrotestosterone to androgen receptors on susceptible hair follicles, leading to the upregulation of genes accounting for hair loss [23]. It is believed that androgen-secreting adrenal or ovarian tumors can result in hyperandrogenism. Women with polycystic ovary syndrome and ovarian androgen-secreting tumors may develop early-onset FPHL, a common feature of these disorders [16]. However, as reported in 2020, the role of androgen in FPHL remains unclear, as some patients with FPHL have normal androgen concentrations [24][25]. This result suggests that the pathophysiology of the disease has yet to be fully understood.
In the genetic research of FPHL, some studies evaluated specific SNPs of CYP19A1 and ESR2 genes, both of which are related to the transformation of sex hormones [26]. Previous genetic studies have identified associations between FPHL and the ESR2 and CYP19A1 genes. ESR2 encodes estrogen receptor beta, the target receptor for estrogen. Variations in this gene may disrupt estrogen signaling and downstream molecular pathways, leading to disease progression. A study by L. Yip et al. [27] analyzed 484 FPHL patients (Sinclair scale 3–5) and 471 controls (Sinclair scale 0) and found a significant association between FPHL and SNPs rs10137185, rs17101774, and rs2022748. However, S. Redler et al. found discordant results for rs101371856 in German and British populations [28]. CYP19A1 encodes aromatase, an enzyme responsible for converting androgens into estrogens. Genetic variants of CYP19A1 may disrupt the balance between estrogen and androgen levels, resulting in this disorder L. Yeh et al. A GWAS analysis of FPHL and CYP19A1, including 484 Australian FPHL patients (Sinclair grade 3–5) and 471 controls, found a significant association between FPHL and the SNP rs4646 CC genotype [29]. Likewise, S. Redler et al. analyzed the association between CYP19A1 and FPHL in 145 UK patients and 53 German patients. They studied four SNPs (rs4646, rs16964189, rs2470158, and rs28757184) but found no significant association between the two groups [30]. A recent study in a Chinese Han population, including 200 FPHL patients and 200 controls, revealed significant differences in the allele frequency and distribution of CYP19A1 SNPs rs6493497 and rs7176005 associated with FPHL [31]. In 2021, a study in a Polish population analyzed the association between 13 CYP19A1 and 11 ESR2 gene SNPs and FPHL. The study included 117 FPHL patients and 128 healthy controls, but no significant differences were observed [26]. To sum up, previous studies have shown an association between FPHL and CYP19A1 and ESR2. However, differences in race and sample size may have influenced the findings, and further genetic analysis are needed to confirm their relationship.
Follicle miniaturization can be affected by dihydrotestosterone (DHT), and testosterone (T) can be converted into DHT or β-estradiol (E2) through 5α-reductase (5α-R) and aromatase, respectively. Therefore, these two enzymes will determine the concentration of DHT and E2, and their ratio will affect hair volume. A study quantifying aromatase and 5α-R isozyme mRNA levels in the hair plucked from young women with FPHL found 5α-R3 mRNA in human hair, but the increase of 5α-R isozyme in FPHL patients was inconsistent [25]. Such results could explain why patients respond inconsistently to medications and also provide new directions for more specific treatments in the future.
Hormone-related treatments are frequently used in FPHL patients, and E2 supplementation has been shown to increase hair growth in ovariectomized (OVX) mice lacking E2 and exhibiting alopecia similar to FPHL [32]; however, the underlying therapeutic mechanism has yet to be elucidated. A Japanese study showed that genes related to the angiopoietin 2 (ANGPT2) pathway were upregulated in mice given E2, and OVX mice treated with ANGPT2 had higher hair density than the control mice. They concluded that the reversal of hair loss is modulated by the estradiol-ANGPT2 axis [33]. Although considerable progress has been made in the past few years toward a better understanding of hormone-related genes in FPHL, there is still a lack of results with significant correlations. In particular, SNP-related studies on the disease-causing, sex hormone metabolism genes are often inconsistent with the findings in M-AGA.
References
- Bertoli, M.J.; Sadoughifar, R.; Schwartz, R.A.; Lotti, T.M.; Janniger, C.K. Female pattern hair loss: A comprehensive review. Dermatology 2020, 33, e14055.
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9–17.
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977, 97, 247–254.
- Olsen, E.A. The midline part: An important physical clue to the clinical diagnosis of androgenetic alopecia in women. J. Am. Acad. Dermatol. 1999, 40, 106–109.
- Biondo, S.; Goble, D.; Sinclair, R. Women who present with female pattern hair loss tend to underestimate the severity of their hair loss. Br. J. Dermatol. 2004, 150, 750–752.
- Starace, M.; Orlando, G.; Alessandrini, A.; Piraccini, B.M. Female Androgenetic Alopecia: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2020, 21, 69–84.
- Davis, D.S.; Callender, V.D. Review of quality of life studies in women with alopecia. Int. J. Womens Dermatol. 2018, 4, 18–22.
- Schmidt, S.; Fischer, T.W.; Chren, M.M.; Strauss, B.M.; Elsner, P. Strategies of coping and quality of life in women with alopecia. Br. J. Dermatol. 2001, 144, 1038–1043.
- Heilmann-Heimbach, S.; Hochfeld, L.M.; Paus, R.; Nöthen, M.M. Hunting the genes in male-pattern alopecia: How important are they, how close are we and what will they tell us? Exp. Dermatol. 2016, 25, 251–257.
- Birch, M.P.; Messenger, A.G. Genetic factors predispose to balding and non-balding in men. Eur. J. Dermatol. 2001, 11, 309–314.
- Ohn, J.; Son, H.Y.; Yu, D.A.; Kim, M.S.; Kwon, S.; Park, W.S.; Kim, J.I.; Kwon, O. Early onset female pattern hair loss: A case-control study for analyzing clinical features and genetic variants. J. Dermatol. Sci. 2022, 106, 21–28.
- Piccini, I.; Sousa, M.; Altendorf, S.; Jimenez, F.; Rossi, A.; Funk, W.; Bíró, T.; Paus, R.; Seibel, J.; Jakobs, M.; et al. Intermediate Hair Follicles from Patients with Female Pattern Hair Loss Are Associated with Nutrient Insufficiency and a Quiescent Metabolic Phenotype. Nutrients 2022, 14, 3357.
- Fawzi, M.M.; Mahmoud, S.B.; Ahmed, S.F.; Shaker, O.G. Assessment of vitamin D receptors in alopecia areata and androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 318–323.
- Seleit, I.; Bakry, O.A.; Badr, E.; Mabrouk, M. Vitamin D Receptor Gene Polymorphisms Taq-1 and Cdx-1 in Female Pattern Hair Loss. Indian J. Dermatol. 2020, 65, 259–264.
- Banihashemi, M.; Nahidi, Y.; Meibodi, N.T.; Jarahi, L.; Dolatkhah, M. Serum Vitamin D3 Level in Patients with Female Pattern Hair Loss. Int. J. Trichol. 2016, 8, 116–120.
- Jiang, V.S.; Hawkins, S.D.; McMichael, A. Female pattern hair loss and polycystic ovarian syndrome: More than just hirsutism. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 535–540.
- Tu, Y.A.; Lin, S.J.; Chen, P.L.; Chou, C.H.; Huang, C.C.; Ho, H.N.; Chen, M.J. HSD3B1 gene polymorphism and female pattern hair loss in women with polycystic ovary syndrome. J. Med. Assoc. 2019, 118, 1225–1231.
- Mahmoud, E.A.; Elgarhy, L.H.; Hasby, E.A.; Mohammad, L. Dickkopf-1 Expression in Androgenetic Alopecia and Alopecia Areata in Male Patients. Am. J. Dermatol. 2019, 41, 122–127.
- Kwack, M.H.; Jun, M.S.; Sung, Y.K.; Kim, J.C.; Kim, M.K. Ectodysplasin-A2 induces dickkopf 1 expression in human balding dermal papilla cells overexpressing the ectodysplasin A2 receptor. Biochem. Biophys. Res. Commun. 2020, 529, 766–772.
- Hashimoto, M.; Kawai, Y.; Masutani, T.; Tanaka, K.; Ito, K.; Iddamalgoda, A. Effects of watercress extract fraction on R-spondin 1-mediated growth of human hair. Int. J. Cosmet. Sci. 2022, 44, 154–165.
- Yoshida, Y.; Takahashi, M.; Yamanishi, H.; Nakazawa, Y.; Kishimoto, J.; Ohyama, M. Changes in the Expression of Smooth Muscle Cell-Related Genes in Human Dermal Sheath Cup Cells Associated with the Treatment Outcome of Autologous Cell-Based Therapy for Male and Female Pattern Hair Loss. Int. J. Mol. Sci. 2022, 23, 7125.
- Tsuboi, R.; Niiyama, S.; Irisawa, R.; Harada, K.; Nakazawa, Y.; Kishimoto, J. Autologous cell-based therapy for male and female pattern hair loss using dermal sheath cup cells: A randomized placebo-controlled double-blinded dose-finding clinical study. J. Am. Acad. Dermatol. 2020, 83, 109–116.
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.D.; Ortiz-López, R.; Ocampo-Candiani, J.; Rojas-Martínez, A. Genetic and molecular aspects of androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263–268.
- Bhat, Y.J.; Saqib, N.U.; Latif, I.; Hassan, I. Female Pattern Hair Loss-An Update. Indian Dermatol. Online J. 2020, 11, 493–501.
- Sánchez, P.; Serrano-Falcón, C.; Torres, J.M.; Serrano, S.; Ortega, E. 5α-Reductase isozymes and aromatase mRNA levels in plucked hair from young women with female pattern hair loss. Arch. Dermatol. Res. 2018, 310, 77–83.
- Łukasik, A.; Kozicka, K.; Pisarek, A.; Wojas-Pelc, A. The role of CYP19A1 and ESR2 gene polymorphisms in female androgenetic alopecia in the Polish population. Postep. Dermatol. Alergol. 2022, 39, 708–713.
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Association analysis of oestrogen receptor beta gene (ESR2) polymorphisms with female pattern hair loss. Br. J. Dermatol. 2012, 166, 1131–1134.
- Redler, S.; Birch, P.; Drichel, D.; Hofmann, P.; Dobson, K.; Böhmer, A.C.; Becker, J.; Giehl, K.A.; Tazi-Ahnini, R.; Kruse, R.; et al. The oestrogen receptor 2 (ESR2) gene in female-pattern hair loss: Replication of association with rs10137185 in German patients. Br. J. Dermatol. 2014, 170, 982–985.
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Gene-wide association study between the aromatase gene (CYP19A1) and female pattern hair loss. Br. J. Dermatol. 2009, 161, 289–294.
- Redler, S.; Birch, M.P.; Drichel, D.; Dobson, K.; Brockschmidt, F.F.; Tazi-Ahnini, R.; Giehl, K.A.; Kluck, N.; Kruse, R.; Lutz, G.; et al. Investigation of variants of the aromatase gene (CYP19A1) in female pattern hair loss. Br. J. Dermatol. 2011, 165, 703–705.
- Rui, W.; Sheng, Y.; Hu, R.; Miao, Y.; Han, Y.; Guo, X.; Qi, S.; Xu, F.; Xu, J.; Yang, Q. Association of Single Nucleotide Polymorphisms in the CYP19A1 Gene with Female Pattern Hair Loss in a Chinese Population. Dermatology 2015, 231, 239–244.
- Endo, Y.; Takahashi, M.; Obayashi, Y.; Serizawa, T.; Murakoshi, M.; Ohyama, M. The ovariectomized mouse simulates the pathophysiology of postmenopausal female pattern hair loss. J. Dermatol. Sci. 2017, 87, 79–82.
- Endo, Y.; Obayashi, Y.; Ono, T.; Serizawa, T.; Murakoshi, M.; Ohyama, M. Reversal of the hair loss phenotype by modulating the estradiol-ANGPT2 axis in the mouse model of female pattern hair loss. J. Dermatol. Sci. 2018, 91, 43–51.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
04 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




