
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zuamí Villagrán | -- | 3785 | 2023-07-01 07:26:29 | | | |
| 2 | Alfred Zheng | Meta information modification | 3785 | 2023-07-03 05:12:20 | | | | |
| 3 | Alfred Zheng | Meta information modification | 3785 | 2023-07-03 05:12:52 | | | | |
| 4 | Alfred Zheng | Meta information modification | 3785 | 2023-07-03 08:46:53 | | |
Video Upload Options
Worldwide, the fungus known as huitlacoche (Ustilago maydis (DC.) Corda) is a phytopathogen of maize plants that causes important economic losses in different countries. Conversely, it is an iconic edible fungus of Mexican culture and cuisine, and it has high commercial value in the domestic market, though recently there has been a growing interest in the international market. Huitlacoche is an excellent source of nutritional compounds such as protein, dietary fiber, fatty acids, minerals, and vitamins. It is also an important source of bioactive compounds with health-enhancing properties. Furthermore, scientific evidence shows that extracts or compounds isolated from huitlacoche have antioxidant, antimicrobial, anti-inflammatory, antimutagenic, antiplatelet, and dopaminergic properties.
1. Introduction
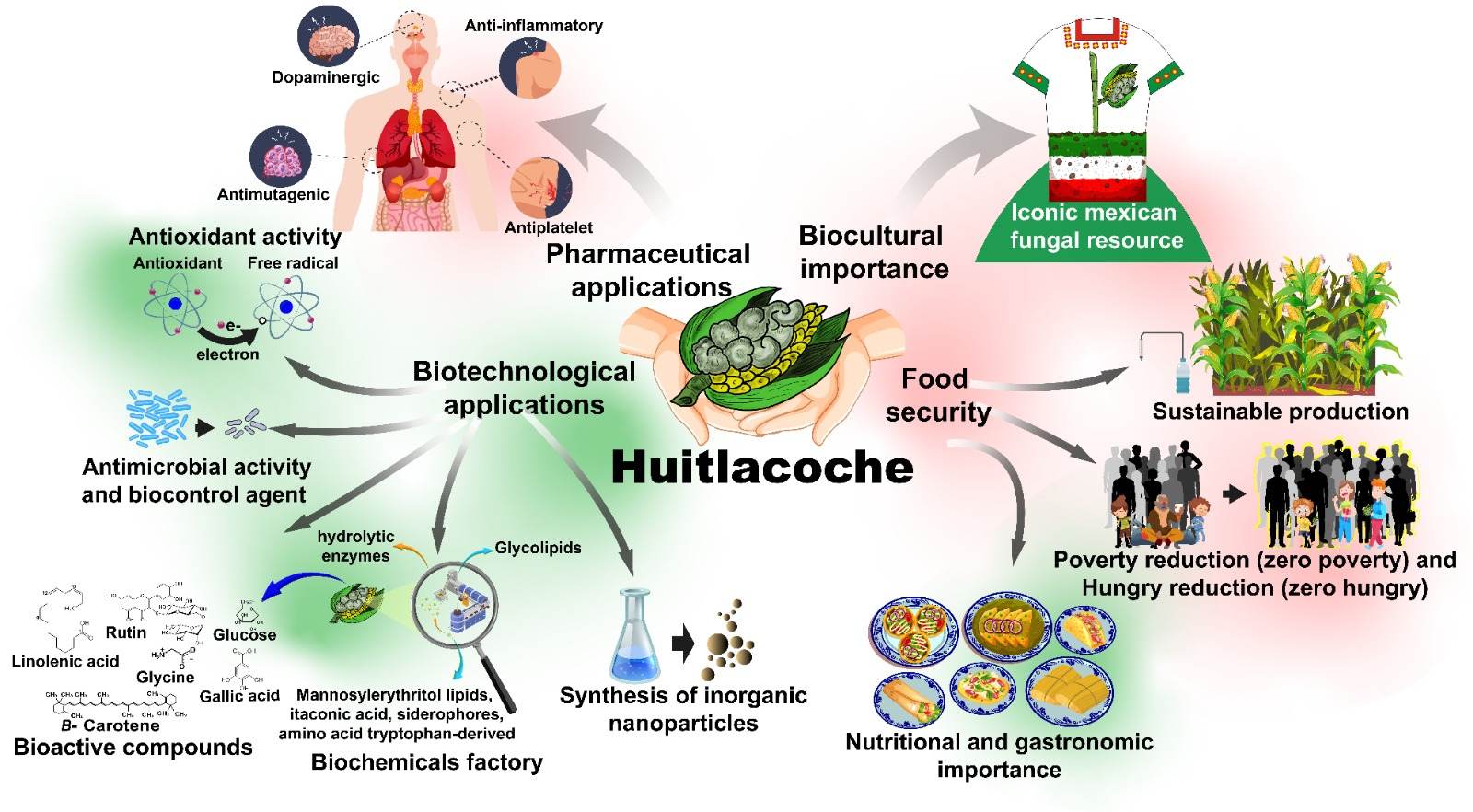




2. Potential Technological Applications of Huitlacoche
2.1. Antioxidant Capacity
| Bioactive Extracts or Compounds | Extraction Method | Method of Antioxidant Capacity | Reference | |||
|---|---|---|---|---|---|---|
| ABTS• | DPPH• | FRAP | ORAC | |||
| Hydroethanolic extract | Maceration | 45.26 a | 13.16 a | ND | ND | [38] |
| Hydroethanolic extract | UAE | 26.45 a | 22.5 a | ND | ND | [38] |
| Methanolic extract | Stirring | ND | 56–74 b | ND | ND | [42] |
| Methanol-Water | Shaking | ND | 186.44 c | ND | ND | [13] |
| Ethanolic extract | Magnetic stirring | 200–312 d | 30–165 d | 117–215 d | ND | [41] |
| Methanol-Water | Shaking | 1652.42 e | 9.50 e | 64.8 e | ND | [24] |
| Methanolic extract | Shaking | ND | ND | ND | 41–76 e | [34] |
2.2. Development of Potential Functional Foods
2.3. Biocontrol Agent for Wine Production
2.4. Antimicrobial Activity
2.5. Miscellaneous Applications
2.6. Synthesis of Inorganic Nanoparticles
2.7. Bioremediation
2.8. Other Investigated Applications
3. Conclusions
In summary huitlacoche is one of the most important edible fungi with biocultural significance in Mexico; currently, it is traditionally consumed by diverse ethnic groups, and it is also used in a wide variety of food dishes throughout the country. Moreover, this fungal resource is a crop with agro-alimentary importance and is a functional food with commercial value. Evidence shows that huitlacoche is a valuable food source with high nutritional value and bioactive compounds that can stabilizing and capping agents for inorganic nanoparticle synthesis, involved in the remotion of heavy metals from aqueous media, biocontrol agents for wine production, and also have industrial potential, e.g., by producing biosurfactant compounds and enzymes [60]
References
- Galicia-García, P.R.; Silva-Rojas, H.V.; Mendoza-Onofre, L.E.; Zavaleta-Mancera, H.A.; Córdova-Téllez, L.; Espinosa-Calderón, A. Selection of aggressive pathogenic and solopathogenic strains of Ustilago maydis to improve Huitlacoche production. Acta Bot. Bras. 2016, 30, 683–692.
- Haro-Luna, M.X.; Ruan-Soto, F.; Guzmán-Dávalos, L. Traditional knowledge, uses, and perceptions of mushrooms among the Wixaritari and mestizos of Villa Guerrero, Jalisco, Mexico. IMA Fungus 2019, 10, 16.
- Reyes-López, R.C.; Montoya, A.; Kong, A.; Cruz-Campuzano, E.A.; Caballero-Nieto, J. Folk classification of wild mushrooms from San Isidro Buensuceso, Tlaxcala, Central Mexico. J. Ethnobiol. Ethnomed. 2020, 16, 53.
- Méndez, R.M.; Ruan-Sotoh, F.; Cano-Contreras, E.J. Conocimiento tradicional de Ustilago maydis en cuatro grupos Mayenses del sureste de México. Etnobiología 2008, 6, 9–23.
- Santiago, F.H.; Moreno, J.P.; Cázares, B.X.; Suárez, J.J.A.; Trejo, E.O.; de Oca, G.M.M.; Aguilar, I.D. Traditional knowledge and use of wild mushrooms by Mixtecs or Ñuu savi, the people of the rain, from Southeastern Mexico. J. Ethnobiol. Ethnomed. 2016, 12, 35.
- Haro-Luna, M.X.; Ruan-Soto, F.; Guzmán-Dávalos, L. Traditional knowledge, uses, and perceptions of mushrooms among the Wixaritari and mestizos of Villa Guerrero, Jalisco, Mexico. IMA Fungus 2019, 10, 16.
- Molina-Castillo, S.; Espinoza-Ortega, A.; Thomé-Ortiz, H.; Moctezuma-Pérez, S. Gastronomic diversity of wild edible mushrooms in the Mexican cuisine. Int. J. Gastron. Food Sci. 2023, 31, 100652
- Patel, S.; Rauf, A.; Khan, H. The relevance of folkloric usage of plant galls as medicines: Finding the scientific rationale. Biomed. Pharmacother. 2018, 97, 240–247.
- Monroy-Gutiérrez, T.; Valle-Guadarrama, S.; Espinosa-Solares, T.; Martínez-Damián, M.T.; Pérez-López, A. Effect of microperforation and temperature on quality of modified atmosphere packaged huitlacoche (Ustilago maydis). CYTA-J. Food 2013, 11, 309–317.
- Galicia-García, P.R.; Silva-Rojas, H.V.; Mendoza-Onofre, L.E.; Zavaleta-Mancera, H.A.; Córdova-Téllez, L.; Espinosa-Calderón, A. Selection of aggressive pathogenic and solopathogenic strains of Ustilago maydis to improve Huitlacoche production. Acta Bot. Bras. 2016, 30, 683–692
- Munkacsi, A.B.; Stoxen, S.; May, G. Ustilago maydis populations tracked maize through domestication and cultivation in the Americas. Proc. R. Soc. B Biol. Sci. 2008, 275, 1037–1046.
- Molina-Castillo, S.; Espinoza-Ortega, A.; Thomé-Ortiz, H.; Moctezuma-Pérez, S. Gastronomic diversity of wild edible mushrooms in the Mexican cuisine. Int. J. Gastron. Food Sci. 2023, 31, 100652.
- Aydoğdu, M.; Gölükçü, M. Nutritional value of huitlacoche, maize mushroom caused by Ustilago maydis. Food Sci. Technol. 2017, 37, 531–535.
- Haro-Luna, M.X.; Ruan-Soto, F.; Guzmán-Dávalos, L. Traditional knowledge, uses, and perceptions of mushrooms among the Wixaritari and mestizos of Villa Guerrero, Jalisco, Mexico. IMA Fungus 2019, 10, 16.
- Mayett, Y.; Martínez-Carrera, D.; Sánchez, M.; Macías, A.; Mora, S.; Estrada, A. Consumption of edible mushrooms in developing countries: The case of Mexico. In Science and Cultivation of Edible and Medicinal Fungi; International Society for Mushroom Science: Las Vegas, NV, USA, 2004; pp. 687–696.
- Méndez, R.M.; Ruan-Sotoh, F.; Cano-Contreras, E.J. Conocimiento tradicional de Ustilago maydis en cuatro grupos Mayenses del sureste de México. Etnobiología 2008, 6, 9–23.
- Santiago, F.H.; Moreno, J.P.; Cázares, B.X.; Suárez, J.J.A.; Trejo, E.O.; de Oca, G.M.M.; Aguilar, I.D. Traditional knowledge and use of wild mushrooms by Mixtecs or Ñuu savi, the people of the rain, from Southeastern Mexico. J. Ethnobiol. Ethnomed. 2016, 12, 35.
- Wu, H.C.; His, H.Y.; Hsiao, G.; Yen, C.H.; Leu, J.Y.; Wu, C.C.; Chang, S.H.; Huang, S.J.; Lee, T.H. Chemical constituents and bioactive principles from the Mexican truffle and fermented products of the derived fungus Ustilago maydis MZ496986. J. Agric. Food Chem. 2023, 71, 1122–1131.
- Haro-Luna, M.X.; Ruan-Soto, F.; Blancas, J.; Guzmán-Dávalos, L. The cultural role played by the ethnomycological knowledge of wild mushrooms for the peoples of highlands and lowlands in Tlaltenango, Zacatecas, Mexico. Mycologia 2022, 114, 645–660.
- Dahl, K. Corn soot woman’s timeless lesson: Eat your smut. Etnobiología 2009, 7, 94–99.
- Guzmán, G. Fungi in the Maya culture: Past, present and future. In The Lowland Maya Area; Food Products Press: Nueva York, NY, USA, 2003; pp. 315–325.
- Valadez-Azúa, R.; Moreno-Fuentes, A.; Gómez-Álvarez, G. Cujtlacochi. El Cuitlacoche; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2011; ISBN 9786070221439.
- Castañeda de León, V.; Martínez-Carrera, D.; Morales, P.; Sobal, M.; Gil-Muñoz, A.; Severiano-Pérez, P.; Leal-Lara, H. Productivity and flavor of diverse genotypes of Ustilago maydis “cuitlacoche” for human consumption. Fungal Biol. 2019, 123, 481–488.
- González-Cervantes, M.E. Caracterización fisicoquímica y funcional de una pasta elaborada con sémola de trigo y harina de hongo huitlacoche (Ustilago maydis). Investig. Desarro Cienc. Technol. Aliment. 2022, 7, 172–178.
- Molina-Castillo, S.; Espinoza-Ortega, A.; Thomé-Ortiz, H.; Moctezuma-Pérez, S. Gastronomic diversity of wild edible mushrooms in the Mexican cuisine. Int. J. Gastron. Food Sci. 2023, 31, 100652.
- León-Ramírez, C.G.; Sánchez-Arreguín, J.A.; Ruiz-Herrera, J. Ustilago maydis, a delicacy of the aztec cuisine and a model for research. Nat. Resour. 2014, 05, 256–267.
- Aydo˘gdu, M.; Gölükçü, M. Nutritional value of huitlacoche, maize mushroom caused by Ustilago maydis. Food Sci. Technol. 2017, 37, 531–535.
- Patel, S. Nutrition, safety, market status quo appraisal of emerging functional food corn smut (huitlacoche). Trends Food Sci. Technol. 2016, 57, 93–102.
- Martinez-Medina, G.A.; Chávez-González, M.L.; Verma, D.K.; Prado-Barragán, L.A.; Martínez-Hernández, J.L.; Flores-Gallegos, A.C.; Thakur, M.; Srivastav, P.P.; Aguilar, C.N. Bio-funcional components in mushrooms, a health opportunity: Ergothionine and huitlacohe as recent trends. J. Funct. Foods 2021, 77, 104326.
- Guzmán, G. Diversity and use of traditional mexican medicinal fungi. A review. Int. J. Med. Mushrooms 2008, 10, 209–217.
- Wu, H.C.; His, H.Y.; Hsiao, G.; Yen, C.H.; Leu, J.Y.; Wu, C.C.; Chang, S.H.; Huang, S.J.; Lee, T.H. Chemical constituents and bioactive principles from the Mexican truffle and fermented products of the derived fungus Ustilago maydis MZ496986. J. Agric. Food Chem. 2023, 71, 1122–1131.
- Bautista-González, J.A.; Moreno-Fuentes, A. Los hongos medicinales de México. In La Etnomicología en México; Cromo Edit: Tamaulipas, Mexico, 2015; pp. 14–176.
- Dahl, K. Corn soot woman’s timeless lesson: Eat your smut. Etnobiología 2009, 7, 94–99.
- Valdez-Morales, M.; Barry, K.; Fahey, G.C.; Domínguez, J.; de Mejia, E.G.; Valverde, M.E.; Paredes-López, O. Effect of maize genotype, developmental stage, and cooking process on the nutraceutical potential of huitlacoche (Ustilago maydis). Food Chem. 2010, 119, 689–697.
- Estrada, A.F.; Brefort, T.; Mengel, C.; Díaz-Sánchez, V.; Alder, A.; Al-Babili, S.; Avalos, J. Ustilago maydis accumulates -carotene at levels determined by a retinal-forming carotenoid oxygenase. Fungal Genet. Biol. 2009, 46, 803–813.
- Montoya, A.; Estrada-Torres, A.; Caballero, J. Comparative ethnomycological survey of three localities from La Malinche Volcano, Mexico. J. Ethnobiol. 2002, 22, 103–131.
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285.
- López-Martínez, L.X.; Aguirre-Delgado, A.; Saenz-Hidalgo, H.K.; Buenrostro-Figueroa, J.J.; García, H.S.; Baeza-Jiménez, R. Bioactive ingredients of huitlacoche (Ustilago maydis), a potential food raw material. Food Chem. Mol. Sci. 2022, 4, 100076.
- González-Cervantes, M.E.; Hernández-Uribe, J.P.; Gómez-Aldapa, C.A.; Navarro-Cortez, R.O.; Palma-Rodríguez, H.M.; Vargas-Torres, A. Physicochemical, functional, and quality properties of fettuccine pasta added with huitlacoche mushroom (Ustilago maydis). J. Food Process. Preserv. 2021, 45, e15825.
- Valdez-Morales, M.; Céspedes-Carlos, L.; Valverde, M.E.; Ramírez-Chávez, E.; Paredes-López, O. Phenolic compounds, antioxidant activity and lipid profile of huitlacoche mushroom (Ustilago maydis) produced in several maize genotypes at different stages of development. Plant Foods Hum. Nutr. 2016, 71, 436–443.
- Salazar-López, J.M.; Martínez-Saldaña, M.C.; Reynoso-Camacho, R.; Chávez-Morales, R.M.; Sandoval-Cardozo, M.L.; Guevara-Lara, F. Antioxidant capacity and phytochemical characterization of ethanolic extracts from raw and cooked huitlacoche (Ustilago maydis-Zea mays). Rev. Mex. Cienc. Farm. 2017, 48, 37–47.
- Rosalba Beas, F.; Guadalupe Loarca, P.; Salvador Horacio Guzmán, M.; Rodriguez, M.G.; Nora Lilia Vasco, M.; Fidel Guevara, L. Nutraceutic potential of bioactive components present in huitlacoche from the central zone of Mexico. Rev. Mex. Cienc. Farm. 2011, 42, 36–44.
- Cortes-Sánchez, A.; Hernández-Sánchez, H.; Jaramillo-Flores, M. Production of glycolipids with antimicrobial activity by Ustilago maydis FBD12 in submerged culture. Afr. J. Microbiol. Res. 2011, 5, 2512–2523.
- Amador-Rodríguez, K.Y.; Martínez-Bustos, F.; Pérez-Cabrera, L.E.; Posadas-Del-Río, F.A.; Chávez-Vela, N.A.; Sandoval-Cardoso, M.L.; Guevara-Lara, F. Effect of huitlacoche (Ustilago maydis DC Corda) paste addition on functional, chemical and textural properties of tortilla chips. Food Sci. Technol. 2015, 35, 452–459.
- Amador-Rodríguez, K.Y.; Pérez-Cabrera, L.E.; Guevara-Lara, F.; Chávez-Vela, N.A.; Posadas-Del Río, F.A.; Silos-Espino, H.; Martínez-Bustos, F. Physicochemical, thermal, and rheological properties of nixtamalized blue-corn flours and masas added with huitlacoche (Ustilago maydis) paste. Food Chem. 2019, 278, 601–608.
- Nieter, A.; Kelle, S.; Takenberg, M.; Linke, D.; Bunzel, M.; Popper, L.; Berger, R.G. Heterologous production and characterization of a chlorogenic acid esterase from Ustilago maydis with a potential use in baking. Food Chem. 2016, 209, 1–9.
- Santos, A.; Navascués, E.; Bravo, E.; Marquina, D. Ustilago maydis killer toxin as a new tool for the biocontrol of the wine spoilage yeast Brettanomyces bruxellensis. Int. J. Food Microbiol. 2011, 145, 147–154.
- Kirkpatrick, C.L.; Parsley, N.C.; Bartges, T.E.; Cooke, M.E.; Evans, W.S.; Heil, L.R.; Smith, T.J.; Hicks, L.M. Fungal Secretome Analysis via PepSAVI-MS: Identification of the bioactive peptide KP4 from Ustilago maydis. J. Am. Soc. Mass Spectrom. 2018, 29, 859–865.
- Yang, X.L.; Awakawa, T.; Wakimoto, T.; Abe, I. Induced production of the novel glycolipid ustilagic acid C in the plant pathogen Ustilago maydis. Tetrahedron Lett. 2013, 54, 3655–3657.
- Becker, F.; Stehlik, T.; Linne, U.; Bölker, M.; Freitag, J.; Sandrock, B. Engineering Ustilago maydis for production of tailor-made mannosylerythritol lipids. Metab. Eng. Commun. 2021, 12, e00165.
- Kurz, M.; Eder, C.; Isert, D.; Li, Z.; Paulus, E.F.; Schiell, M.; Toti, L.; Vértesy, L.; Wink, J.; Seibert, G. Ustilipids, acylated -D-mannopyranosyl D-erythritols from Ustilago maydis and Geotrichum candidum. J. Antibiot. 2003, 56, 91–101.
- Wang, S.Q.; Wang, X.N.; Li, Y.Y.; Di, X.X.; Lou, H.X. Identification of purine-derived compounds, ustilagomaydisin A-C, from the plant pathogen Ustilago maydis and their modulating effects on multidrug-resistant (MDR) tumors. Phytochem. Lett. 2014, 10, 193–197.
- Juárez-Montiel, M.; Romero-Maldonado, A.; Monreal-Escalante, E.; Becerra-Flora, A.; Korban, S.S.; Rosales-Mendoza, S.; Jiménez-Bremont, J.F. The corn smut (‘Huitlacoche’) as a new platform for oral vaccines. PLoS ONE 2015, 10, e0133535.
- Monreal-Escalante, E.; Navarro-Tovar, G.; León-Gallo, A.; Juárez-Montiel, M.; Becerra-Flora, A.; Jiménez-Bremont, J.F.; Rosales-Mendoza, S. The corn smut-made cholera oral vaccine is thermostable and induces long-lasting immunity in mouse. J. Biotechnol. 2016, 234, 1–6.
- Cortés-Camargo, S.; Jiménez-Rosales, A.; Acuña-Avila, P.E. Green synthesis of Ag NPs Using Ustilago maydis as reducing and atabilizing agent. J. Nanotechnol. 2022, 2022, 2494882.
- Bakur, A.; Niu, Y.; Kuang, H.; Chen, Q. Synthesis of gold nanoparticles derived from mannosylerythritol lipid and evaluation of their bioactivities. AMB Express 2019, 9, 62
- Serrano-Gómez, J.; Olguín, M.T. Separation of Cr(VI) from aqueous solutions by adsorption on the microfungus Ustilago maydis. Int. J. Environ. Sci. Technol. 2015, 12, 2559–2566.
- Sargin, I.; Arslan, G.; Kaya, M. Microfungal spores (Ustilago maydis and U. digitariae) immobilised chitosan microcapsules for heavy metal removal. Carbohydr. Polym. 2016, 138, 201–209.
- Merkeviˇci ¯ ute-Venslov˙ e, L.; Venslovas, E.; Mankeviˇcien ˙ e, A.; Šlepetien ˙ e, A.; Ceseviˇcien ˙ e, J. Effect of Ustilago maydis on the nutritive value and aerobic deterioration of maize silage. Agronomy 2022, 13, 111.
- Villagrán, Z.; Martínez-Reyes, M.; Gómez-Rodríguez, H.; Ríos-García, U.; Montalvo-González, E.; Ortiz-Basurto, R.I.; Anaya-Esparza, L.M.; Pérez-Moreno, J. Huitlacoche (Ustilago maydis), an Iconic Mexican Fungal Resource: Biocultural Importance, Nutritional Content, Bioactive Compounds, and Potential Biotechnological Applications. Molecules 2023, 28, 4415.




