Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | XIAO DIE CHEN | -- | 2326 | 2023-06-30 09:25:24 | | | |
| 2 | Dean Liu | -3 word(s) | 2323 | 2023-07-03 02:15:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, X.; Li, Y.; Xia, H.; Chen, Y.H. Monocytes in Tumorigenesis and Tumor Immunotherapy. Encyclopedia. Available online: https://encyclopedia.pub/entry/46258 (accessed on 08 February 2026).
Chen X, Li Y, Xia H, Chen YH. Monocytes in Tumorigenesis and Tumor Immunotherapy. Encyclopedia. Available at: https://encyclopedia.pub/entry/46258. Accessed February 08, 2026.
Chen, Xiaodie, Yunqing Li, Houjun Xia, Youhai H. Chen. "Monocytes in Tumorigenesis and Tumor Immunotherapy" Encyclopedia, https://encyclopedia.pub/entry/46258 (accessed February 08, 2026).
Chen, X., Li, Y., Xia, H., & Chen, Y.H. (2023, June 30). Monocytes in Tumorigenesis and Tumor Immunotherapy. In Encyclopedia. https://encyclopedia.pub/entry/46258
Chen, Xiaodie, et al. "Monocytes in Tumorigenesis and Tumor Immunotherapy." Encyclopedia. Web. 30 June, 2023.
Copy Citation
Monocytes are highly plastic innate immune cells that display significant heterogeneity during homeostasis, inflammation, and tumorigenesis. Tumor-induced systemic and local microenvironmental changes influence the phenotype, differentiation, and distribution of monocytes. Meanwhile, monocytes and their related cell subsets perform an important regulatory role in the development of many cancers by affecting tumor growth or metastasis. Thanks to recent advances in single-cell technologies, the nature of monocyte heterogeneity and subset-specific functions have become increasingly clear, making it possible to systematically analyze subset-specific roles of monocytes in tumorigenesis.
monocytes
tumorigenesis
tumor microenvironment
1. Introduction
Monocytes are short-lived mononuclear phagocytes that circulate in the blood and efficiently extravasate into tissues. Under normal physiological conditions, monocytes originate from common myeloid progenitors (CMPs) in bone marrow, where CMPs further develop into granulocyte and monocyte precursors (GMPs), then monocyte and dendritic cell precursors (MDPs). After a series of developmental processes, MDPs give rise to common monocyte progenitors (cMoPs) that subsequently turn into classical monocytes [1][2][3]. Mature monocytes are a heterogeneous population of immune cells which can be divided into two categories in mice based on surface markers, namely, Ly6ChiCX3CR1lowCCR2+ (classical) and Ly6ClowCX3CR1hiCCR2− (non-classical) cells, and three categories in humans, including CD14hiCD16low/− (classical or inflammatory), CD14lowCD16hi (non-classical or patrolling), and CD14hi/midCD16+ (intermediate) [1][4].
Tumorigenesis can result from genetic mutations along with uncontrolled tumor cell growth and immune tolerance. Monocytes and their derived cells, such as myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs), are frequently found in the tumor microenvironment (TME) and are involved in tumor development, angiogenesis, metastatic spread, chemotherapy resistance, and immune suppression. However, the biological functions and clinical implications of different monocyte subsets are far from fully elucidated. Recently, advances in single-cell sequencing, cytometry by time of flight (Cytof), and other techniques have helped identify previously unrecognized or indistinguishable subsets of monocytes. Their fates and specific functions in TME are being elucidated, which significantly enrich the understanding of how monocytes and their subsets directly affect the biological processes of tumorigenesis.
2. The Global Effects of TME on Monocytes
Monocytes originate from bone marrow and migrate to inflammatory sites to perform their functions, where they can locally differentiate into macrophages or dendritic cells. In general, monocytes and monocyte-derived cells serve three main roles in the immune system, which are phagocytosis, antigen presentation, and cytokine production. In defense, tumor cells remodel them in TME in favor of tumourigenesis by affecting their numbers, phenotype, differentiation, and function.
2.1. The Effect of TME on the Population and Phenotype of Monocytes
Inflammation is believed as one of the inevitable consequences during tumorigenesis, leading to the recruitment of inflammatory cells, such as monocytes, to tumor sites via the bloodstream. This explains the significant increases in the number and proportion of monocytes in cancer patients. For example, a higher proportion of classical and intermediate monocytes in the peripheral blood of non-small cell lung carcinoma (NSCLC) patients is observed and compared with those of healthy donors. The counts of monocytes are especially high in patients with histories of smoking, drinking, and liver metastasis [5]. A higher count of CD163-expressing intermediate monocytes is seen in breast cancer patients compared with healthy women [6]. This change seems cancer-type-dependent as the level of intermediate monocytes is significantly lower in squamous cell carcinoma of the head and neck (SCCHN) than that in healthy donors [7]. Similarly, a significant expansion of non-classical monocytes is observed in endometrial and breast cancer patients when compared with healthy controls [8], while a marked decrease in monocytes was detected in both cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC) patients before and after surgical procedures [9].
Given the fact that monocyte counts vary across different cancer types, the proportion of monocytes in peripheral blood that correlate with inflammation status may serve to be a prognostic indicator of cancer prognosis. A study highlights the role of peripheral blood absolute monocyte count (AMC) as a potential prognostic marker. In the study, the AMC level of multiple myeloma goes beyond the defined range, indicating inferior overall survival in patients [10]. Signal regulatory protein α (SIRPα) is a receptor-like transmembrane protein that suppresses both macrophage phagocytic function and inflammatory signaling. The SIRPα-CD47 interaction has been identified as a “self” signal for normal cells to avoid auto-attack by phagocytes. Notably, tumor cells have been shown to protect themselves by expressing high levels of CD47 [11]. Furthermore, more in-depth clinical research reports that increased numbers of CD14+SIRPαhi monocytes are associated with inferior survival of follicular lymphoma, whilst an increased number of CD14−SIRPαlow subsets is correlated with better survival [12]. Changes in the number of monocytes can be used to predict patients’ responsiveness to anti-tumor therapy before surgery. This is because more CD163-expressing monocytes expand and are recruited into tumor sites after neoadjuvant chemotherapy (NAC). A significantly lower amount of CD14lowCD16hiHLA-DR+ non-classical monocytes in patients is commonly associated with no noticeable clinical responses to NAC [6].
Phenotypes of monocytes are shaped diversely within TME. Currently, next-generation sequencing (NGS) has aided in relating alterations in the transcriptomic landscape of cancer-related monocytes to their phenotypic characteristics. It is noted that upregulated regulators of inflammation and monocyte migration may be correlated with elevated expression of key factors, such as immune regulatory receptors, pro-apoptotic molecules, and pro-angiogenic factors [6][8]. Serum levels of monocyte-derived cytokines, such as interleukin (IL)-6, granulocyte colony-stimulating factor (G-CSF), and granulocyte–macrophage colony-stimulating factor (GM-CSF), are also increased in lung cancer patients, particularly, G-CSF levels are positively correlated with lung cancer severity [13]. Circulating monocytes from pancreatic cancer patients show constitutive phosphorylation of signal transducer and activator of transcription (STAT) family members and impaired response upon stimulation, indicating aberrant activation and immune suppression [14]. The expression levels of those partners in monocytes or monocyte-derived cells aforementioned may be utilized as indicators for tumor grading and prognosis [15].
Effects of tumor cells within TME on the phenotype of monocytes have been further verified ex vivo. When co-culturing patient-derived monocytes with cancer cells (MIA PaCa-2 and HPAF-II), they displayed downregulated expression of the activation marker CD86 of M1 macrophages, suggesting compromised anti-tumor features [16]. The expression level of a cancer stemness-promoting factor CD51 [17] was increased in monocytes when co-cultured with SCCHN tumor cells [7]. Conclusively, TME profoundly influences the population and phenotype of monocytes and drives monocytes to develop into the immunosuppressive phenotype within the tumor milieu.
2.2. The Effect of TME on the Differentiation of Monocytes
Factors of TME have impacts on the differentiation and ultimate fate of monocytes (Figure 1). Upon stimulation with cytokines, monocytes can differentiate into either dendritic cells (DCs) or macrophages. In most cases, DCs display anti-tumor effects since they present tumor-associated antigens (TAA) and elicit cytotoxic CD8+ T-dependent responses. However, macrophages compete with DCs to degrade the TAA, which prevents the initiation of antigen presentation and induces immune tolerance. Apart from TAA, some tumoral factors affect the balance of monocytes in differentiating into monocyte-derived DCs or monocyte-derived macrophages. Retinoic acid (RA) within TME is found to drive intra-tumoral monocytes differentiated toward TAMs but shifted away from differentiating into DCs via suppression of DC-promoting transcription factor interferon regulatory factor-4 (IRF4) [18]. A similar phenomenon is observed in a human melanoma model in vitro. The supernatant of melanoma cell culture contains a high level of IL-10 which impedes monocyte-to-DC differentiation leading to differentiation into CD163+PD-L1+ M2-like macrophages [19]. However, it remains unclear within solid tumors how monocytes preferentially differentiate into immunosuppressive TAMs rather than immunostimulatory DCs.
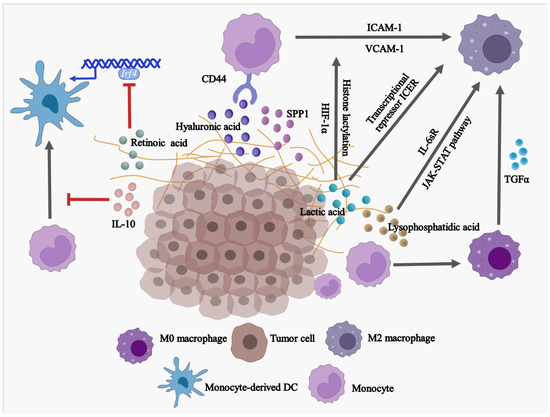
Figure 1. Factors in TME are driving the differentiation of monocytes into pro-tumoral macrophages while blocking the development of anti-tumor DCs. SPP1, secreted phosphoprotein 1/osteopontin; IL-10, Interleukin 10; TGFα, Transforming growth factor alpha; IRF4, Interferon regulatory factor 4; USP12, Ubiquitin specific peptidase 12; ICAM-1, Intercellular adhesion molecule 1; VCAM-1, Vascular cell adhesion molecule 1; HIF-1α, Hypoxia-inducible factor 1α; IL-6sR, Soluble interleukin 6 receptor.
Monocytes account for the major source of TAMs with M2-like phenotype in advanced tumors. In response to different environmental signals, macrophages are polarized toward two different subtypes: classically activated macrophages (M1) or alternatively activated macrophages (M2). M1-like macrophages are considered anti-tumor cells with the secretion of inflammatory factors, while M2-like macrophages are immunosuppressive with the association of initiation, progression, metastasis, and immune evasion of tumors [20]. Interestingly, several mechanisms have been elucidated that tumor-derived factors trigger monocyte differentiation into M2-like macrophages. In high-grade serous ovarian carcinoma (HGSOC), a high level of transforming growth factor alpha (TGFα) was directly related to the modulation of differentiation of monocytes into M2-like macrophages and, thereby, tumor transformation [21]. Hyaluronic acid (HA), a common component found in various tumor-associated extracellular matrices (ECM) [22], was found to contribute to the development of pro-tumor, immunosuppressive M2-like monocytes/macrophages. This is achieved by combining the effects of CD44 (HA receptor [23]) of THP-1 human monocytes and STAT3 [24][25]. In addition, the expression of intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in glioblastoma cells are enhanced by IL-1β stimulation. As a result, the interaction between monocytes and glioblastoma cells and regulated tumor-associated monocyte/macrophage polarization are enhanced [26]. Soluble IL-6 receptor (IL-6sR) and JAK-STAT signaling pathway have been found to promote differentiation of human monocytes into the CD14+CD163+ and CD206+ TAMs, respectively, when monocytes are co-cultured with ovarian cancer SKOV3 cells, and the differentiated TAMs acquire the ability to promote SKOV3 cell proliferation and invasion [27].
Emerging evidence suggests that TAMs are mixed cell populations with differential expression of both M1 and M2 markers, especially at an early stage of cancer. For example, both classical tissue monocytes and TAMs co-express M1/M2 markers, T cell co-inhibitory, and co-stimulatory receptors at an early stage of human lung cancer [28]. Hence, by temporally and spatially conditioning monocytes during their differentiation, monocyte-derived M1-like TAMs have the potential to reprogram to M2-like TAMs under extreme tumor conditions. In fact, tumor-derived lactic acid induces M2-like polarization of TAMs in the presence of hypoxia-inducible factor 1α (HIF-1α) [29] and histone lactylation [30]. In addition, high acidification of TME, which may be caused by lactic acid accumulation, induces G protein–coupled receptor (GPCR)-dependent expression of the transcriptional repressor ICER in TAMs. As a result, it leads to the polarization of TAMs to M2-like phenotype, promoting tumor growth [31].
2.3. The Effect of TME on the Fate of Monocytic MDSCs
MDSCs represent a group of pathologically activated neutrophils and monocytes with immunosuppressive activity [32] which include granulocytic or polymorphonuclear MDSCs (G-MDSCs or PMN-MDSCs) and monocytic MDSCs (M-MDSCs). M-MDSCs are identified as CD11b+Ly6G−Ly6Chi in mice and CD11b+CD14+HLA-DR−/loCD15− in humans [33]. Furthermore, major histocompatibility complex class II (MHC-II) is widely used for the identification of M-MDSCs from monocytes [34]. With respect to tumorigenesis, prolonged presence of myeloid growth factors and inflammatory signals, such as GM-CSF, IL-6, and IL-1β, persistently trigger pathological activation of monocytes, which leads to the development and expansion of M-MDSC [35].
Several molecules that regulate the development of MDSCs have been identified, including c-Rel [36], STAT3 [37], S100A8/9 [38], TIPE2 [39], and IRF8 [37]. The group has demonstrated c-Rel as a master effector of M-MDSC biology, which drives the expansion and immunosuppressive activity of M-MDSC [40]. As a member of the NF-κB transcription factor family [41], c-Rel is capable of promoting the transcription of immunosuppressive enzymes and other M-MDSC signature genes by directing the formation of c-Rel-C/EBPβ-pSTAT3-p65 enhanceosome [36]. Researchers have defined a subset of M-MDSC in both mice and human melanomas that are programmed by c-Rel enhanceosome, namely c-Rel-dependent monocytes (rMos). These c-Rel+IL-1βhiArg1− rMos promote tumor growth by suppressing T cell function and maintaining a suppressive TME through IL-1β-CCL2 crosstalk [42].
3. Monocytes and Monocyte-Derived Cells in Tumorigenesis
Monocytes and monocyte-derived cells that reside in TME exert dual effects by either promoting or suppressing tumor growth (Table 1). Their heterogeneity in TME may explain their functional diversity. Here, researchers focus on the connection between monocytes and their derived cells during tumor development. Monocytes and their derived cells with different functions listed in Table 1 are further discussed. With the help of single-cell technologies, researchers have been able to learn more about the functions of monocytes and their derived cells. However, most of the studies were performed using a limited number of cancer types. Whether the newly discovered monocyte subsets and their derived cells are universal across different cancer types in terms of phenotypes, signaling mechanisms, and functionality warrants further exploration.
Table 1. Pro-tumoral and anti-tumoral functions of monocyte subsets and monocyte-derived cells in human cancer.
| Subset | Cellular Origin | Function | Methods | Cancer Type | |
|---|---|---|---|---|---|
| CD66b+CD14+CD33hiCD16−/+HLA-DR+/hi monocytes | CD33hiCD14+ monocytes | Anti-tumoral | Display high phagocytic activity, matrix adhesion, and migration, and provide co-stimulation for T cell proliferation and interferon-γ (IFN-γ) secretion. | RNA-seq and flow cytometry | Breast cancer and colorectal cancer [43] |
| CXCL9+CXCL10+ CCL5+MHCII+ CD40+STAT1+ macrophages | - | Anti-tumoral | Secrete CXCL9 to facilitate recruitment of protective T cells. | scRNA-seq | Lung cancer [44][45] |
| CSFR1+CCR2−CD68+ CD163+SIGLEC1− macrophages; CSFR1+CCR2−CD68+CD163+SIGLEC1+ macrophages; CSFR1+CCR2−CD68+CD163−SIGLEC1+ macrophages | CD14++CD16−CCR2+ classical monocytes | Pro-tumoral | Engage in a tumor cell-TAM auto-stimulatory loop, increase tumor cell motility, and increase monocyte infiltration into the tumor site to generate more pro-tumoral TAMs. | RNA-seq | Breast cancer [8] |
| TREM2+FOLR2+CD163+ macrophages | S100A8+ monocytes | Pro-tumoral | Recruit suppressive regulatory T cells (Treg) and MDSCs to facilitate immunosuppressive microenvironment. | scRNA-seq | Hepatocellular carcinoma [46] |
| CD14+CD16+(FCGR3A) CD81+ITGAX+CSF1R+ monocytes/macrophages | - | Pro-tumoral | Secrete specific profibrotic, pro-metastatic growth factors involved ECM deposition and remodeling. | scRNA-seq | Small cell lung cancer [47] |
| CD11b+CCR2+IL-1βhiArg1− M-MDSCs | CD11b+CCR2+ monocytes | Pro-tumoral | Promote tumor growth, suppress T cell function, and maintain suppressive TME. | scRNA-seq | Melanoma [42][48] |
| CD84+CD11b+/CD14+ M-MDSCs | PBMC | Pro-tumoral | Exhibit T cell suppression and increase ROS production. | scRNA-seq | Breast cancer [49] |
| CD14+HLA-DRlo/− monocytes/MDSCs | CD14+HLA-DRlo/− monocytes | Pro-tumoral | Inhibit T cell responses. | Flow cytometry | Epithelial ovarian cancer [50] |
Abbreviations: ECM, extracellular matrix; ROS, reactive oxygen species; STAT1, signal transducer and activator of transcription 1; SIGLEC1, sialic acid binding Ig, such as lectin 1; TREM2, triggering receptor expressed on myeloid cells 2; FOLR2, folate receptor beta; ITGAX, integrin subunit alpha X; CSF1R, colony stimulating factor 1 receptor; PBMC, peripheral blood mononuclear cell.
References
- Ugel, S.; Canè, S.; De Sanctis, F.; Bronte, V. Monocytes in the Tumor Microenvironment. Annu. Rev. Pathol. Mech. Dis. 2021, 16, 93–122.
- Yona, S.; Kim, K.-W.; Wolf, Y.; Mildner, A.; Varol, D.; Breker, M.; Strauss-Ayali, D.; Viukov, S.; Guilliams, M.; Misharin, A.; et al. Fate Mapping Reveals Origins and Dynamics of Monocytes and Tissue Macrophages under Homeostasis. Immunity 2013, 38, 79–91.
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613.
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front. Oncol. 2020, 10, 1399.
- Kwiecień, I.; Rutkowska, E.; Polubiec-Kownacka, M.; Raniszewska, A.; Rzepecki, P.; Domagała-Kulawik, J. Blood Monocyte Subsets with Activation Markers in Relation with Macrophages in Non-Small Cell Lung Cancer. Cancers 2020, 12, 2513.
- Patysheva, M.; Larionova, I.; Stakheyeva, M.; Grigoryeva, E.; Iamshchikov, P.; Tarabanovskaya, N.; Weiss, C.; Kardashova, J.; Frolova, A.; Rakina, M.; et al. Effect of Early-Stage Human Breast Carcinoma on Monocyte Programming. Front. Oncol. 2021, 11, 800235.
- Sakakura, K.; Takahashi, H.; Motegi, S.-I.; Yokobori-Kuwabara, Y.; Oyama, T.; Chikamatsu, K. Immunological Features of Circulating Monocyte Subsets in Patients with Squamous Cell Carcinoma of the Head and Neck. Clin. Immunol. 2021, 225, 108677.
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e10.
- Martín-Sierra, C.; Martins, R.; Coucelo, M.; Abrantes, A.M.; Oliveira, R.C.; Tralhão, J.G.; Botelho, M.F.; Furtado, E.; Domingues, M.R.; Paiva, A.; et al. Elevated Soluble TNFα Levels and Upregulated TNFα MRNA Expression in Purified Peripheral Blood Monocyte Subsets Associated with High-Grade Hepatocellular Carcinoma. J. Inflamm. 2020, 17, 14.
- Edwards, C.V.; Hassan, H.; Yildirim, C.; Ferri, G.; Verma, K.P.; Murray Horwitz, M.E.; Fillmore, N.R.; Munshi, N.C. Peripheral Blood Monocyte Count Is a Dynamic Prognostic Biomarker in Multiple Myeloma. Blood Adv. 2022, 7, 482–490.
- Barclay, A.N.; Van den Berg, T.K. The Interaction between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu. Rev. Immunol. 2014, 32, 25–50.
- Chen, Y.-P.; Kim, H.J.; Wu, H.; Price-Troska, T.; Villasboas, J.C.; Jalali, S.; Feldman, A.L.; Novak, A.J.; Yang, Z.-Z.; Ansell, S.M. SIRPα Expression Delineates Subsets of Intratumoral Monocyte/Macrophages with Different Functional and Prognostic Impact in Follicular Lymphoma. Blood Cancer J. 2019, 9, 84.
- Yin, W.; Lv, J.; Yao, Y.; Zhao, Y.; He, Z.; Wang, Q.; Cui, L.; Dai, H. Elevations of Monocyte and Neutrophils, and Higher Levels of Granulocyte Colony-Stimulating Factor in Peripheral Blood in Lung Cancer Patients. Thorac. Cancer 2021, 12, 2680–2690.
- Juusola, M.; Kuuliala, K.; Kuuliala, A.; Mustonen, H.; Vähä-Koskela, M.; Puolakkainen, P.; Seppänen, H. Pancreatic Cancer Is Associated with Aberrant Monocyte Function and Successive Differentiation into Macrophages with Inferior Anti-Tumour Characteristics. Pancreatology 2021, 21, 397–405.
- Kang, S.U.; Cho, S.Y.; Jeong, H.; Han, J.; Chae, H.Y.; Yang, H.; Sung, C.O.; Choi, Y.-L.; Shin, Y.K.; Kwon, M.J. Matrix Metalloproteinase 11 (MMP11) in Macrophages Promotes the Migration of HER2-Positive Breast Cancer Cells and Monocyte Recruitment through CCL2-CCR2 Signaling. Lab. Investig. 2022, 102, 376–390.
- Cui, R.; Yue, W.; Lattime, E.C.; Stein, M.N.; Xu, Q.; Tan, X.-L. Targeting Tumor-Associated Macrophages to Combat Pancreatic Cancer. Oncotarget 2016, 7, 50735–50754.
- Zhang, B.; Ye, H.; Ren, X.; Zheng, S.; Zhou, Q.; Chen, C.; Lin, Q.; Li, G.; Wei, L.; Fu, Z.; et al. Macrophage-Expressed CD51 Promotes Cancer Stem Cell Properties via the TGF-Β1/Smad2/3 Axis in Pancreatic Cancer. Cancer Lett. 2019, 459, 204–215.
- Devalaraja, S.; To, T.K.J.; Folkert, I.W.; Natesan, R.; Alam, M.Z.; Li, M.; Tada, Y.; Budagyan, K.; Dang, M.T.; Zhai, L.; et al. Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression. Cell 2020, 180, 1098–1114.e16.
- Michielon, E.; López González, M.; Burm, J.L.A.; Waaijman, T.; Jordanova, E.S.; de Gruijl, T.D.; Gibbs, S. Micro-Environmental Cross-Talk in an Organotypic Human Melanoma-in-Skin Model Directs M2-like Monocyte Differentiation via IL-10. Cancer Immunol. Immunother. 2020, 69, 2319–2331.
- Pittet, M.J.; Michielin, O.; Migliorini, D. Clinical Relevance of Tumour-Associated Macrophages. Nat. Rev. Clin. Oncol. 2022, 19, 402–421.
- Fogg, K.C.; Miller, A.E.; Li, Y.; Flanigan, W.; Walker, A.; O’Shea, A.; Kendziorski, C.; Kreeger, P.K. Ovarian Cancer Cells Direct Monocyte Differentiation through a Non-Canonical Pathway. BMC Cancer 2020, 20, 1008.
- Kultti, A.; Li, X.; Jiang, P.; Thompson, C.B.; Frost, G.I.; Shepard, H.M. Therapeutic Targeting of Hyaluronan in the Tumor Stroma. Cancers 2012, 4, 873–903.
- Thapa, R.; Wilson, G.D. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int. 2016, 2016, 2087204.
- Lawrence, T.; Natoli, G. Transcriptional Regulation of Macrophage Polarization: Enabling Diversity with Identity. Nat. Rev. Immunol. 2011, 11, 750–761.
- Kim, H.; Cha, J.; Jang, M.; Kim, P. Hyaluronic Acid-Based Extracellular Matrix Triggers Spontaneous M2-like Polarity of Monocyte/Macrophage. Biomater. Sci. 2019, 7, 2264–2271.
- Shen, C.-K.; Huang, B.-R.; Yeh, W.-L.; Chen, C.-W.; Liu, Y.-S.; Lai, S.-W.; Tseng, W.-P.; Lu, D.-Y.; Tsai, C.-F. Regulatory Effects of IL-1β in the Interaction of GBM and Tumor-Associated Monocyte through VCAM-1 and ICAM-1. Eur. J. Pharmacol. 2021, 905, 174216.
- Feng, Y.; Xiao, M.; Cao, G.; Liu, H.; Li, Y.; Wang, S.; Zijtveld, S.; Delvoux, B.; Xanthoulea, S.; Romano, A.; et al. Human Monocytes Differentiate into Tumor-Associated Macrophages upon SKOV3 Cells Coculture and/or Lysophosphatidic Acid Stimulation. J. Inflamm. 2022, 19, 11.
- Singhal, S.; Stadanlick, J.; Annunziata, M.J.; Rao, A.S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Cantu, E.; Danet-Desnoyers, G.; Ra, H.-J.; et al. Human Tumor-Associated Monocytes/Macrophages and Their Regulation of T Cell Responses in Early-Stage Lung Cancer. Sci. Transl. Med. 2019, 11, eaat1500.
- Colegio, O.R.; Chu, N.-Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563.
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580.
- Bohn, T.; Rapp, S.; Luther, N.; Klein, M.; Bruehl, T.-J.; Kojima, N.; Aranda Lopez, P.; Hahlbrock, J.; Muth, S.; Endo, S.; et al. Tumor Immunoevasion via Acidosis-Dependent Induction of Regulatory Tumor-Associated Macrophages. Nat. Immunol. 2018, 19, 1319–1329.
- Broz, M.L.; Krummel, M.F. The emerging understanding of myeloid cells as partners and targets in tumor rejection. Cancer Immunol. Res. 2015, 3, 313–319.
- Bruger, A.M.; Dorhoi, A.; Esendagli, G.; Barczyk-Kahlert, K.; van der Bruggen, P.; Lipoldova, M.; Perecko, T.; Santibanez, J.; Saraiva, M.; Van Ginderachter, J.A.; et al. How to Measure the Immunosuppressive Activity of MDSC: Assays, Problems and Potential Solutions. Cancer Immunol. Immunother. 2019, 68, 631–644.
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells in the Era of Increasing Myeloid Cell Diversity. Nat. Rev. Immunol. 2021, 21, 485–498.
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119.
- Li, T.; Li, X.; Zamani, A.; Wang, W.; Lee, C.-N.; Li, M.; Luo, G.; Eiler, E.; Sun, H.; Ghosh, S.; et al. C-Rel Is a Myeloid Checkpoint for Cancer Immunotherapy. Nat. Cancer 2020, 1, 507–517.
- Waight, J.D.; Netherby, C.; Hensen, M.L.; Miller, A.; Hu, Q.; Liu, S.; Bogner, P.N.; Farren, M.R.; Lee, K.P.; Liu, K.; et al. Myeloid-Derived Suppressor Cell Development Is Regulated by a STAT/IRF-8 Axis. J. Clin. Investig. 2013, 123, 4464–4478.
- Sinha, P.; Okoro, C.; Foell, D.; Freeze, H.H.; Ostrand-Rosenberg, S.; Srikrishna, G. Proinflammatory S100 Proteins Regulate the Accumulation of Myeloid-Derived Suppressor Cells. J. Immunol. 2008, 181, 4666–4675.
- Yan, D.; Wang, J.; Sun, H.; Zamani, A.; Zhang, H.; Chen, W.; Tang, A.; Ruan, Q.; Yang, X.; Chen, Y.H.; et al. TIPE2 Specifies the Functional Polarization of Myeloid-Derived Suppressor Cells during Tumorigenesis. J. Exp. Med. 2020, 217, e20182005.
- Fultang, N.; Li, X.; Li, T.; Chen, Y.H. Myeloid-Derived Suppressor Cell Differentiation in Cancer: Transcriptional Regulators and Enhanceosome-Mediated Mechanisms. Front. Immunol. 2021, 11, 619253.
- Gilmore, T.D.; Gerondakis, S. The C-Rel Transcription Factor in Development and Disease. Genes Cancer 2011, 2, 695–711.
- Li, T.; Bou-Dargham, M.J.; Fultang, N.; Li, X.; Pear, W.S.; Sun, H.; Chen, Y.H. C-Rel-Dependent Monocytes Are Potent Immune Suppressor Cells in Cancer. J. Leukoc. Biol. 2022, 112, 845–859.
- Horzum, U.; Yoyen-Ermis, D.; Taskiran, E.Z.; Yilmaz, K.B.; Hamaloglu, E.; Karakoc, D.; Esendagli, G. CD66b+ Monocytes Represent a Proinflammatory Myeloid Subpopulation in Cancer. Cancer Immunol. Immunother. 2021, 70, 75–87.
- Qu, Y.; Wen, J.; Thomas, G.; Yang, W.; Prior, W.; He, W.; Sundar, P.; Wang, X.; Potluri, S.; Salek-Ardakani, S. Baseline Frequency of Inflammatory Cxcl9-Expressing Tumor-Associated Macrophages Predicts Response to Avelumab Treatment. Cell Rep. 2020, 32, 107873.
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10.
- Zhou, L.; Wang, M.; Guo, H.; Hou, J.; Zhang, Y.; Li, M.; Wu, X.; Chen, X.; Wang, L. Integrated Analysis Highlights the Immunosuppressive Role of TREM2+ Macrophages in Hepatocellular Carcinoma. Front. Immunol. 2022, 13, 848367.
- Chan, J.M.; Quintanal-Villalonga, Á.; Gao, V.R.; Xie, Y.; Allaj, V.; Chaudhary, O.; Masilionis, I.; Egger, J.; Chow, A.; Walle, T.; et al. Signatures of Plasticity, Metastasis, and Immunosuppression in an Atlas of Human Small Cell Lung Cancer. Cancer Cell 2021, 39, 1479–1496.e18.
- Li, H.; van der Leun, A.M.; Yofe, I.; Lubling, Y.; Gelbard-Solodkin, D.; van Akkooi, A.C.J.; van den Braber, M.; Rozeman, E.A.; Haanen, J.B.A.G.; Blank, C.U.; et al. Dysfunctional CD8 T Cells Form a Proliferative, Dynamically Regulated Compartment within Human Melanoma. Cell 2019, 176, 775–789.e18.
- Alshetaiwi, H.; Pervolarakis, N.; McIntyre, L.L.; Ma, D.; Nguyen, Q.; Rath, J.A.; Nee, K.; Hernandez, G.; Evans, K.; Torosian, L.; et al. Defining the Emergence of Myeloid-Derived Suppressor Cells in Breast Cancer Using Single-Cell Transcriptomics. Sci. Immunol. 2020, 5, eaay6017.
- Stenzel, A.E.; Abrams, S.I.; Joseph, J.M.; Goode, E.L.; Tario, J.D.; Wallace, P.K.; Kaur, D.; Adamson, A.-K.; Buas, M.F.; Lugade, A.A.; et al. Circulating CD14+ HLA-DRlo/− Monocytic Cells as a Biomarker for Epithelial Ovarian Cancer Progression. Am. J. Reprod. Immunol. 2021, 85, e13343.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
573
Revisions:
2 times
(View History)
Update Date:
03 Jul 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




