
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dalia Marija Kopustinskiene | -- | 3563 | 2023-06-29 16:10:44 | | | |
| 2 | Lindsay Dong | + 3 word(s) | 3566 | 2023-06-30 07:11:20 | | | | |
| 3 | Lindsay Dong | -89 word(s) | 3477 | 2023-07-05 02:29:56 | | |
Video Upload Options
Neuropathic pain, which is characterized by abnormal sensory processing due to nerve damage or dysfunction, often poses challenges in finding effective and well-tolerated therapies. Traditional analgesics, such as opioids and non-steroidal anti-inflammatory drugs (NSAIDs), may provide limited relief or be associated with significant side effects. The investigation into new drug targets and emerging pharmacotherapies in neuropathic pain could be of great interest in enhancing pain management and improving patient outcomes. In the context of neuropathic pain, repurposing drugs gained attention as a promising strategy for discovering novel treatment options. Repurposing drugs for neuropathic pain offers several advantages in the drug development process.
1. Ambroxol
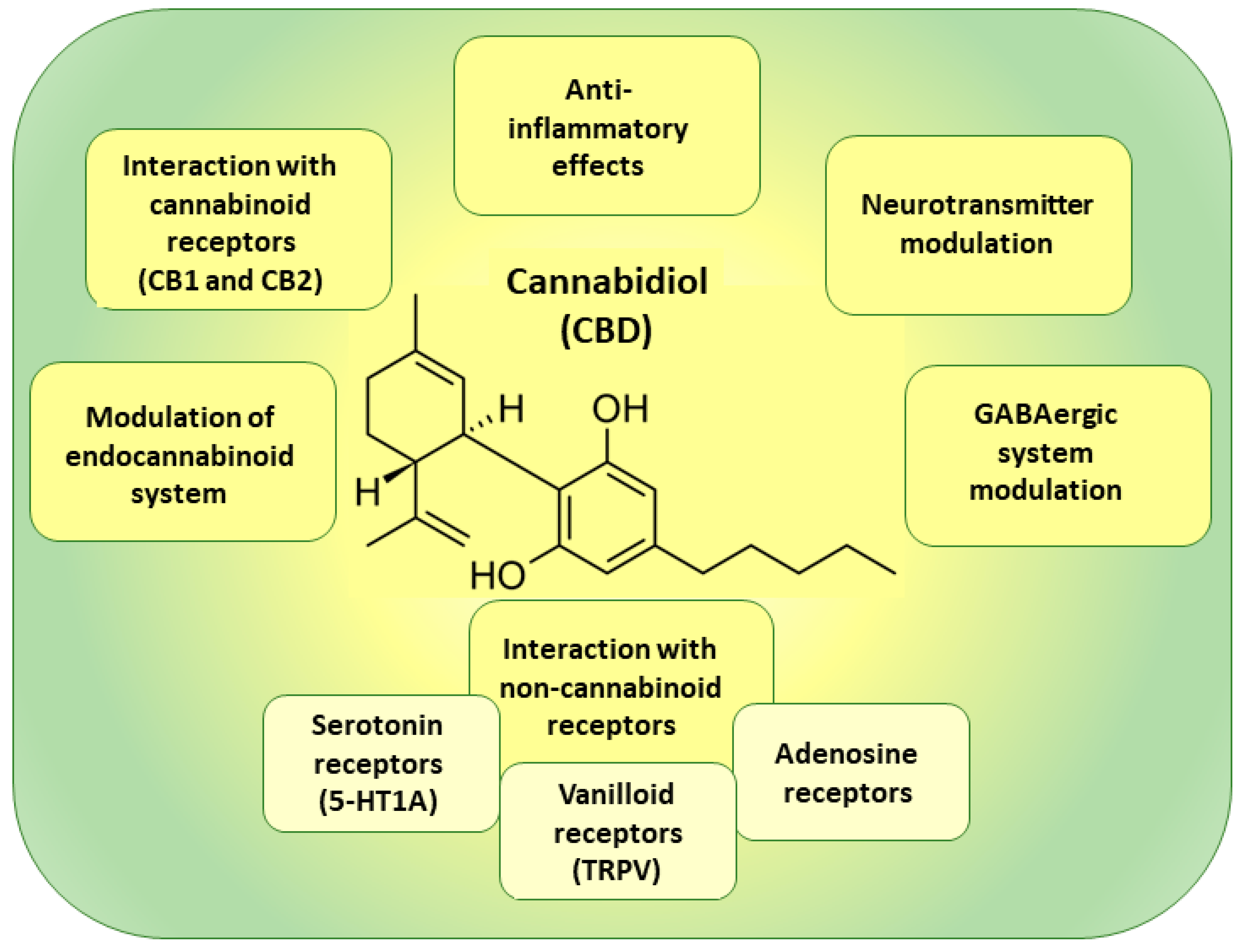
2. Cannabidiol
3. Bromelain
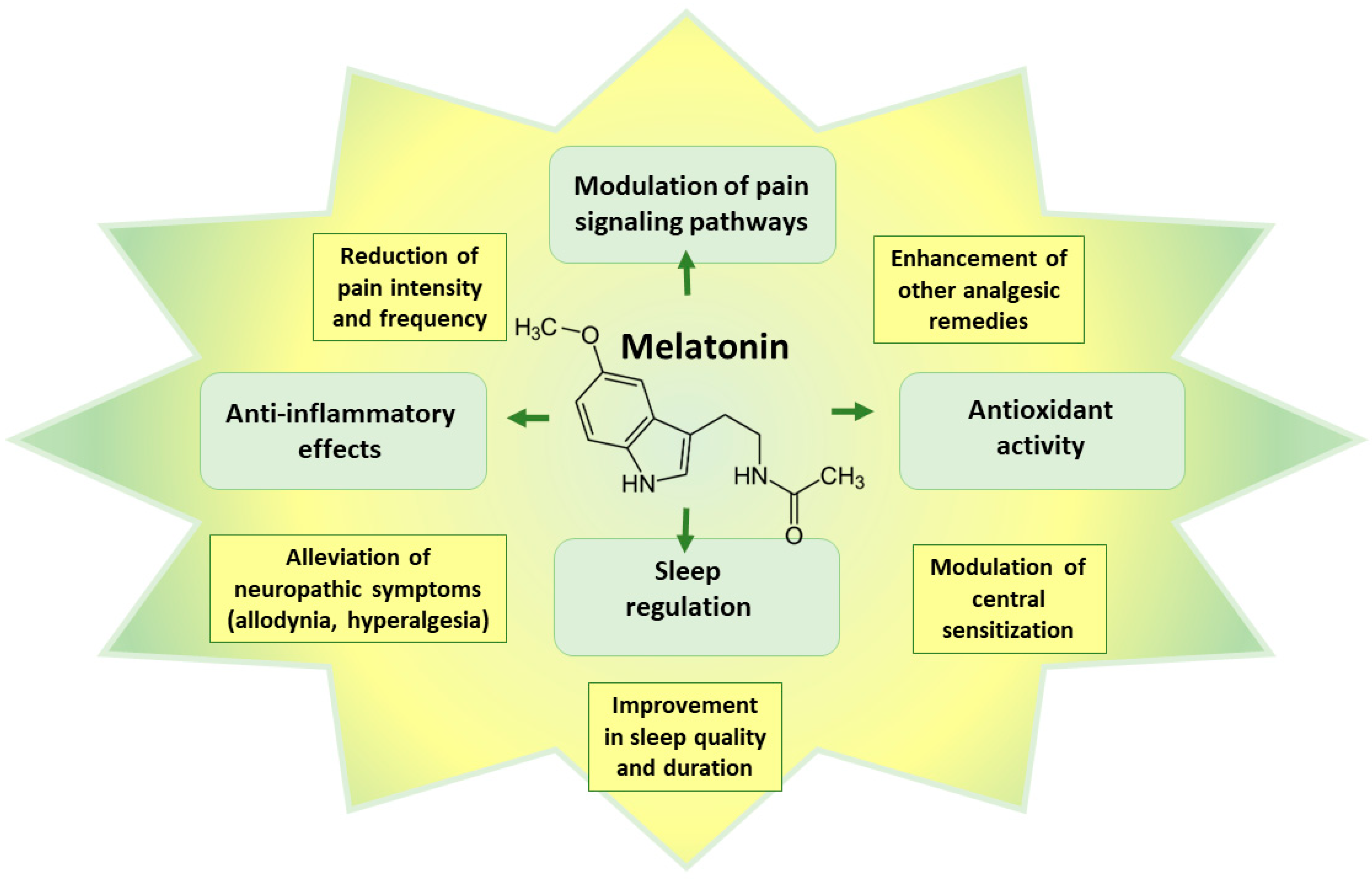
4. Melatonin
5. N-acetyl-L-cysteine
6. Other Experimental Therapies
References
- Cazan, D.; Klimek, L.; Sperl, A.; Plomer, M.; Kölsch, S. Safety of ambroxol in the treatment of airway diseases in adult patients. Expert. Opin. Drug Saf. 2018, 17, 1211–1224.
- Malerba, M.; Ragnoli, B. Ambroxol in the 21st century: Pharmacological and clinical update. Expert. Opin. Drug Metab. Toxicol. 2008, 4, 1119–1129.
- Russo, M.A.; Baron, R.; Dickenson, A.H.; Kern, K.U.; Santarelli, D.M. Ambroxol for neuropathic pain: Hiding in plain sight? Pain 2023, 164, 3–13.
- Salat, K.; Gryzlo, B.; Kulig, K. Experimental Drugs for Neuropathic Pain. Curr. Neuropharmacol. 2018, 16, 1193–1209.
- Gaida, W.; Klinder, K.; Arndt, K.; Weiser, T. Ambroxol, a Nav1.8-preferring Na+ channel blocker, effectively suppresses pain symptoms in animal models of chronic, neuropathic and inflammatory pain. Neuropharmacology 2005, 49, 1220–1227.
- Furgała, A.; Fijałkowski, Ł.; Nowaczyk, A.; Sałat, R.; Sałat, K. Time-shifted co-administration of sub-analgesic doses of ambroxol and pregabalin attenuates oxaliplatin-induced cold allodynia in mice. Biomed. Pharmacother. 2018, 106, 930–940.
- Hama, A.T.; Plum, A.W.; Sagen, J. Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol. Biochem. Behav. 2010, 97, 249–255.
- Kern, K.U.; Weiser, T. Topical ambroxol for the treatment of neuropathic pain. An initial clinical observation. Schmerz 2015, 29 (Suppl. S3), S89–S96.
- Maihöfner, C.; Schneider, S.; Bialas, P.; Gockel, H.; Beer, K.G.; Bartels, M.; Kern, K.U. Successful treatment of complex regional pain syndrome with topical ambroxol: A case series. Pain Manag. 2018, 8, 427–436.
- Kern, K.U.; Schwickert-Nieswandt, M.; Maihöfner, C.; Gaul, C. Topical Ambroxol 20% for the Treatment of Classical Trigeminal Neuralgia—A New Option? Initial Clinical Case Observations. Headache 2019, 59, 418–429.
- McCarberg, B.H.; Barkin, R.L. The future of cannabinoids as analgesic agents: A pharmacologic, pharmacokinetic, and pharmacodynamic overview. Am. J. Ther. 2007, 14, 475–483.
- Karst, M.; Salim, K.; Burstein, S.; Conrad, I.; Hoy, L.; Schneider, U. Analgesic effect of the synthetic cannabinoid CT-3 on chronic neuropathic pain: A randomized controlled trial. JAMA 2003, 290, 1757–1762.
- Atakan, Z. Cannabis, a complex plant: Different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254.
- Kogan, N.M.; Mechoulam, R. Cannabinoids in health and disease. Dialogues Clin. Neurosci. 2007, 9, 413–430.
- Malfait, A.M.; Gallily, R.; Sumariwalla, P.F.; Malik, A.S.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566.
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273.
- Rock, E.M.; Bolognini, D.; Limebeer, C.L.; Cascio, M.G.; Anavi-Goffer, S.; Fletcher, P.J.; Mechoulam, R.; Pertwee, R.G.; Parker, L.A. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 2012, 165, 2620–2634.
- Baker, D.; Pryce, G.; Croxford, J.L.; Brown, P.; Pertwee, R.G.; Huffman, J.W.; Layward, L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 2000, 404, 84–87.
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448.
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301.
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215.
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805.
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Perchuk, A.; Meozzi, P.A.; Myers, L.; Mora, Z.; Tagliaferro, P.; Gardner, E.; et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann. N. Y. Acad. Sci. 2006, 1074, 514–536.
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332.
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting Cannabinoid Signaling in the Immune System: “High”-ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 1487.
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorganic Med. Chem. 2015, 23, 1377–1385.
- Elmes, M.W.; Kaczocha, M.; Berger, W.T.; Leung, K.; Ralph, B.P.; Wang, L.; Sweeney, J.M.; Miyauchi, J.T.; Tsirka, S.E.; Ojima, I.; et al. Fatty acid-binding proteins (FABPs) are intracellular carriers for Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD). J. Biol. Chem. 2015, 290, 8711–8721.
- Petzke, F.; Tölle, T.; Fitzcharles, M.A.; Häuser, W. Cannabis-Based Medicines and Medical Cannabis for Chronic Neuropathic Pain. CNS Drugs 2022, 36, 31–44.
- Campos, R.M.P.; Aguiar, A.F.L.; Paes-Colli, Y.; Trindade, P.M.P.; Ferreira, B.K.; de Melo Reis, R.A.; Sampaio, L.S. Cannabinoid Therapeutics in Chronic Neuropathic Pain: From Animal Research to Human Treatment. Front. Physiol. 2021, 12, 785176.
- Kocot-Kępska, M.; Zajączkowska, R.; Mika, J.; Kopsky, D.J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszyńska, A. Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative Review. Pharmaceutics 2021, 13, 450.
- Baron-Flores, V.; Diaz-Ruiz, A.; Manzanares, J.; Rios, C.; Burelo, M.; Jardon-Guadarrama, G.; Martínez-Cárdenas, M.; Mata-Bermudez, A. Cannabidiol attenuates hypersensitivity and oxidative stress after traumatic spinal cord injury in rats. Neurosci. Lett. 2022, 788, 136855.
- Dos Santos, R.; Veras, F.; Netto, G.; Elisei, L.; Sorgi, C.; Faccioli, L.; Galdino, G. Cannabidiol prevents chemotherapy-induced neuropathic pain by modulating spinal TLR4 via endocannabinoid system activation. J. Pharm. Pharmacol. 2023, 75, 655–665.
- Eeswara, A.; Pacheco-Spiewak, A.; Jergova, S.; Sagen, J. Combined non-psychoactive Cannabis components cannabidiol and β-caryophyllene reduce chronic pain via CB1 interaction in a rat spinal cord injury model. PLoS ONE 2023, 18, e0282920.
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.P.; McGuire, P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799–1806.
- Ford, T.C.; Hayley, A.C.; Downey, L.A.; Parrott, A.C. Cannabis: An Overview of its Adverse Acute and Chronic Effects and its Implications. Curr. Drug Abus. Rev. 2017, 10, 6–18.
- Huestis, M.A.; Solimini, R.; Pichini, S.; Pacifici, R.; Carlier, J.; Busardò, F.P. Cannabidiol Adverse Effects and Toxicity. Curr. Neuropharmacol. 2019, 17, 974–989.
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482.
- Huestis, M.A. Human cannabinoid pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804.
- Bakare, A.O.; Owoyele, B.V. Antinociceptive and neuroprotective effects of bromelain in chronic constriction injury-induced neuropathic pain in Wistar rats. Korean J. Pain 2020, 33, 13–22.
- Bakare, A.O.; Owoyele, B.V. Bromelain reversed electrolyte imbalance in the chronically constricted sciatic nerve of Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 457–467.
- Bakare, A.O.; Owoyele, B.V. Bromelain reduced pro-inflammatory mediators as a common pathway that mediate antinociceptive and anti-anxiety effects in sciatic nerve ligated Wistar rats. Sci. Rep. 2021, 11, 289.
- Desideri, I.; Francolini, G.; Becherini, C.; Terziani, F.; Delli Paoli, C.; Olmetto, E.; Loi, M.; Perna, M.; Meattini, I.; Scotti, V.; et al. Use of an alpha lipoic, methylsulfonylmethane and bromelain dietary supplement (Opera(®)) for chemotherapy-induced peripheral neuropathy management, a prospective study. Med. Oncol. 2017, 34, 46.
- Carpentieri, A.; Díaz de Barboza, G.; Areco, V.; Peralta López, M.; Tolosa de Talamoni, N. New perspectives in melatonin uses. Pharmacol. Res. 2012, 65, 437–444.
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384.
- Acuña Castroviejo, D.; López, L.C.; Escames, G.; López, A.; García, J.A.; Reiter, R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011, 11, 221–240.
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278.
- López, A.; García, J.A.; Escames, G.; Venegas, C.; Ortiz, F.; López, L.C.; Acuña-Castroviejo, D. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J. Pineal Res. 2009, 46, 188–198.
- Leon, J.; Acuña-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.X.; Reiter, R.J. Melatonin and mitochondrial function. Life Sci. 2004, 75, 765–790.
- Acuña-Castroviejo, D.; Martín, M.; Macías, M.; Escames, G.; León, J.; Khaldy, H.; Reiter, R.J. Melatonin, mitochondria, and cellular bioenergetics. J. Pineal Res. 2001, 30, 65–74.
- Liu, L.; Labani, N.; Cecon, E.; Jockers, R. Melatonin Target Proteins: Too Many or Not Enough? Front. Endocrinol. 2019, 10, 791.
- Legros, C.; Chesneau, D.; Boutin, J.A.; Barc, C.; Malpaux, B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 2014, 26, 151–163.
- Kuthati, Y.; Lin, S.H.; Chen, I.J.; Wong, C.S. Melatonin and their analogs as a potential use in the management of Neuropathic pain. J. Formos. Med. Assoc. 2019, 118, 1177–1186.
- Srinivasan, V.; Lauterbach, E.C.; Ho, K.Y.; Acuña-Castroviejo, D.; Zakaria, R.; Brzezinski, A. Melatonin in antinociception: Its therapeutic applications. Curr. Neuropharmacol. 2012, 10, 167–178.
- Ambriz-Tututi, M.; Rocha-González, H.I.; Cruz, S.L.; Granados-Soto, V. Melatonin: A hormone that modulates pain. Life Sci. 2009, 84, 489–498.
- Srinivasan, V.; Zakaria, R.; Jeet Singh, H.; Acuna-Castroviejo, D. Melatonin and its agonists in pain modulation and its clinical application. Arch. Ital. Biol. 2012, 150, 274–289.
- Posa, L.; De Gregorio, D.; Gobbi, G.; Comai, S. Targeting Melatonin MT2 Receptors: A Novel Pharmacological Avenue for Inflammatory and Neuropathic Pain. Curr. Med. Chem. 2018, 25, 3866–3882.
- Al-Omary, F.A. Melatonin: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2013, 38, 159–226.
- Dai, C.Q.; Guo, Y.; Chu, X.Y. Neuropathic Pain: The Dysfunction of Drp1, Mitochondria, and ROS Homeostasis. Neurotox. Res. 2020, 38, 553–563.
- Landis, C.A. Is melatonin the next “new” therapy to improve sleep and reduce pain? Sleep 2014, 37, 1405–1406.
- Borsani, E.; Buffoli, B.; Bonazza, V.; Reiter, R.J.; Rezzani, R.; Rodella, L.F. Single Administration of Melatonin Modulates the Nitroxidergic System at the Peripheral Level and Reduces Thermal Nociceptive Hypersensitivity in Neuropathic Rats. Int. J. Mol. Sci. 2017, 18, 2143.
- Huang, C.T.; Chen, S.H.; Chang, C.F.; Lin, S.C.; Lue, J.H.; Tsai, Y.J. Melatonin reduces neuropathic pain behavior and glial activation through MT(2) melatonin receptor modulation in a rat model of lysophosphatidylcholine-induced demyelination neuropathy. Neurochem. Int. 2020, 140, 104827.
- Fakhri, S.; Kiani, A.; Jalili, C.; Abbaszadeh, F.; Piri, S.; Farzaei, M.H.; Rastegari-Pouyani, M.; Mohammadi-Noori, E.; Khan, H. Intrathecal Administration of Melatonin Ameliorates the Neuroinflammation- Mediated Sensory and Motor Dysfunction in A Rat Model of Compression Spinal Cord Injury. Curr. Mol. Pharmacol. 2021, 14, 646–657.
- Mokhtari, T.; Yue, L.P.; Hu, L. Exogenous melatonin alleviates neuropathic pain-induced affective disorders by suppressing NF-κB/ NLRP3 pathway and apoptosis. Sci. Rep. 2023, 13, 2111.
- Marchesi, N.; Govoni, S.; Allegri, M. Non-drug pain relievers active on non-opioid pain mechanisms. Pain Pract. 2022, 22, 255–275.
- Raghu, G.; Berk, M.; Campochiaro, P.A.; Jaeschke, H.; Marenzi, G.; Richeldi, L.; Wen, F.Q.; Nicoletti, F.; Calverley, P.M.A. The Multifaceted Therapeutic Role of N-Acetylcysteine (NAC) in Disorders Characterized by Oxidative Stress. Curr. Neuropharmacol. 2021, 19, 1202–1224.
- Horst, A.; de Souza, J.A.; Santos, M.C.Q.; Riffel, A.P.K.; Kolberg, C.; Partata, W.A. Effects of N-acetylcysteine on spinal cord oxidative stress biomarkers in rats with neuropathic pain. Braz. J. Med. Biol. Res. 2017, 50, e6533.
- Horst, A.; Kolberg, C.; Moraes, M.S.; Riffel, A.P.; Finamor, I.A.; Belló-Klein, A.; Pavanato, M.A.; Partata, W.A. Effect of N-acetylcysteine on the spinal-cord glutathione system and nitric-oxide metabolites in rats with neuropathic pain. Neurosci. Lett. 2014, 569, 163–168.
- Bernabucci, M.; Notartomaso, S.; Zappulla, C.; Fazio, F.; Cannella, M.; Motolese, M.; Battaglia, G.; Bruno, V.; Gradini, R.; Nicoletti, F. N-Acetyl-cysteine causes analgesia by reinforcing the endogenous activation of type-2 metabotropic glutamate receptors. Mol. Pain 2012, 8, 77.
- Özgül, C.; Nazıroğlu, M. TRPM2 channel protective properties of N-acetylcysteine on cytosolic glutathione depletion dependent oxidative stress and Ca2+ influx in rat dorsal root ganglion. Physiol. Behav. 2012, 106, 122–128.
- Sözbir, E.; Nazıroğlu, M. Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab. Brain Dis. 2016, 31, 385–393.
- Notartomaso, S.; Scarselli, P.; Mascio, G.; Liberatore, F.; Mazzon, E.; Mammana, S.; Gugliandolo, A.; Cruccu, G.; Bruno, V.; Nicoletti, F.; et al. N-Acetylcysteine causes analgesia in a mouse model of painful diabetic neuropathy. Mol. Pain 2020, 16, 1744806920904292.
- Li, J.; Xu, L.; Deng, X.; Jiang, C.; Pan, C.; Chen, L.; Han, Y.; Dai, W.; Hu, L.; Zhang, G.; et al. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain 2016, 157, 1711–1723.
- Zhu, D.; Fan, T.; Chen, Y.; Huo, X.; Li, Y.; Liu, D.; Cai, Y.; Cheung, C.W.; Tang, J.; Cui, J.; et al. CXCR4/CX43 Regulate Diabetic Neuropathic Pain via Intercellular Interactions between Activated Neurons and Dysfunctional Astrocytes during Late Phase of Diabetes in Rats and the Effects of Antioxidant N-Acetyl-L-Cysteine. Oxid. Med. Cell Longev. 2022, 2022, 8547563.
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane Database Syst. Rev. 2019, 4, Cd010528.
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251.
- Santos, W.; Guimarães, J.O.; Pina, L.T.S.; Serafini, M.R.; Guimarães, A.G. Antinociceptive effect of plant-based natural products in chemotherapy-induced peripheral neuropathies: A systematic review. Front. Pharmacol. 2022, 13, 1001276.
- Freo, U.; Brugnatelli, V.; Turco, F.; Zanette, G. Analgesic and Antidepressant Effects of the Clinical Glutamate Modulators Acetyl-L-Carnitine and Ketamine. Front. Neurosci. 2021, 15, 584649.
- Sarzi-Puttini, P.; Giorgi, V.; Di Lascio, S.; Fornasari, D. Acetyl-L-carnitine in chronic pain: A narrative review. Pharmacol. Res. 2021, 173, 105874.
- Rolim, L.C.; da Silva, E.M.; Flumignan, R.L.; Abreu, M.M.; Dib, S.A. Acetyl-L-carnitine for the treatment of diabetic peripheral neuropathy. Cochrane Database Syst. Rev. 2019, 6, Cd011265.
- Rowin, J. Integrative neuromuscular medicine: Neuropathy and neuropathic pain: Consider the alternatives. Muscle Nerve 2019, 60, 124–136.
- Viana, M.D.M.; Lauria, P.S.S.; Lima, A.A.; Opretzka, L.C.F.; Marcelino, H.R.; Villarreal, C.F. Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. Antioxidants 2022, 11, 2420.
- Lang-Illievich, K.; Klivinyi, C.; Lasser, C.; Brenna, C.T.A.; Szilagyi, I.S.; Bornemann-Cimenti, H. Palmitoylethanolamide in the Treatment of Chronic Pain: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials. Nutrients 2023, 15, 1350.
- Yousefi-Manesh, H.; Shirooie, S.; Noori, T.; Sheibani, M.; Tavangar, S.M.; Hemmati, S.; Sadeghi, M.A.; Akbarniakhaky, H.; Mohammadi, Z.; Foroutani, L.; et al. Spermidine reduced neuropathic pain in chronic constriction injury-induced peripheral neuropathy in rats. Fundam. Clin. Pharmacol. 2023.
- Miguel, C.A.; Noya-Riobó, M.V.; Mazzone, G.L.; Villar, M.J.; Coronel, M.F. Antioxidant, anti-inflammatory and neuroprotective actions of resveratrol after experimental nervous system insults. Special focus on the molecular mechanisms involved. Neurochem. Int. 2021, 150, 105188.
- Shen, C.L.; Castro, L.; Fang, C.Y.; Castro, M.; Sherali, S.; White, S.; Wang, R.; Neugebauer, V. Bioactive compounds for neuropathic pain: An update on preclinical studies and future perspectives. J. Nutr. Biochem. 2022, 104, 108979.
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.Q.; Rittner, H.; Tian, Y.K.; Cai, X.Y.; Ye, D.W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140.
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135.
- Roganović, J.; Petrović, N. Clinical Perspectives of Non-Coding RNA in Oral Inflammatory Diseases and Neuropathic Pain: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 8278.




