Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christopher Mwanza | -- | 4488 | 2023-06-28 08:21:38 | | | |
| 2 | Sirius Huang | -4 word(s) | 4484 | 2023-06-29 02:52:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mwanza, C.; Ding, S. Principles of Bipolar Electrochemistry. Encyclopedia. Available online: https://encyclopedia.pub/entry/46158 (accessed on 08 February 2026).
Mwanza C, Ding S. Principles of Bipolar Electrochemistry. Encyclopedia. Available at: https://encyclopedia.pub/entry/46158. Accessed February 08, 2026.
Mwanza, Christopher, Shou-Nian Ding. "Principles of Bipolar Electrochemistry" Encyclopedia, https://encyclopedia.pub/entry/46158 (accessed February 08, 2026).
Mwanza, C., & Ding, S. (2023, June 28). Principles of Bipolar Electrochemistry. In Encyclopedia. https://encyclopedia.pub/entry/46158
Mwanza, Christopher and Shou-Nian Ding. "Principles of Bipolar Electrochemistry." Encyclopedia. Web. 28 June, 2023.
Copy Citation
Analytical techniques based on electrochemiluminescence (ECL) and bipolar electrochemistry (BPE) [ECL-BPE] have emerged as indispensable tools in various fields, and of particular interest, in biosensing. BPE has been a robust and reliable technique for some time now, and it has been used since the early 1970s for designing electrochemical reactors and batteries.
bipolar electrodes
electrochemiluminescence
biosensors
1. An Overview of Bipolar Electrochemistry
Electrochemistry in general is a vibrant and versatile field that has seen increased attention due to its applications in various areas, including biosensors, chemical sensors, and electroanalytical chemistry. One of the most exciting aspects of electrochemistry is the existence of BPE designs that offers unique advantages in terms of sensitivity and selectivity. BPE exploits a working electrode being a bipolar electrode (BE, Figure 1) which is a single conductive object/substrate that drives redox reactions at its two ends [1]. The BE is remotely positioned in the cell where relevant analytes are collected [2]. In this technique, there are two distinct Faradaic reactions, that is, redox reactions, occurring at two ends of this conductive substrate (the BE) which has been polarized in an adequately high electric field [3].
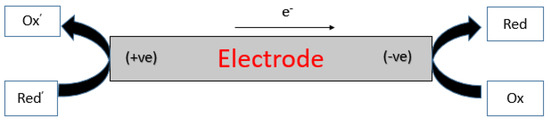
Figure 1. Schematic diagram of a substrate made of conducting.
As will be noted later, the BE can essentially be regarded as the heart of BPE. Worth noting is that BPE and traditional electrolysis are different but only slightly so. For example, the former is also a well-established “wireless technique” [4] that uses a conducting material immersed in an electrolyte solution and generates or induces asymmetric, electrochemical reactions on surfaces of two poles of a conductive object when a sufficient voltage bias is applied to the driving electrodes [5][6]. In BPE, the BE assumes both roles of an anode and a cathode and provides a physical medium for redox reactions to take place simultaneously [2]. The BE is by definition equipotential, however, the potential gradient that exists in the solution surrounding the BE will result in an inhomogeneous potential difference between the electrolyte and the BE. This potential difference varies along the BE’s main axis parallel to the electric field lines; this results in the polarization of the BE. When this is the case, one extremity of the BE will be the site of oxidation processes, whereas the opposite extremity will provide a surface for the reduction reaction [7][8]. The foregoing description of BPE is dissimilar to that of traditional electrochemistry where the two electrodes to wit, anode, and cathode are separated physically.
In the classical three-electrode electrochemistry system, the working electrode is the electrode of interest; depending on its polarization with regard to the electrolyte, either a reduction or an oxidation reaction takes place. On the contrary, this is not the case with BPE because as earlier stated, both reduction and oxidation take place simultaneously on the BE [7]. Moreover, BPE is also different from the traditional three-electrode system in the sense that the BPE set-up is much simpler [9] qualifying it for easier operation when also compared to other common analytical techniques. Nonetheless, in as much as at first glance, BPE would appear a bit different from normal electrochemical methods; but a thorough grasp of the underlying principles of the two techniques reveals that they are more similar than they are different. To be specific, in both BPE and normal electrochemistry, it is the interfacial potential difference that drives electrochemical reactions. The difference between the two is basically which side of the interface is being controlled, i.e., the solution or the electrode [10].
BPE has been a robust and reliable technique for some time now, and it has been used since the early 1970s for designing electrochemical reactors and batteries [11]. This was, of course, preceded by the groundbreaking research conducted by Fleischmann and co-workers who in 1969 described the concept of fluidized bed electrodes, where when a sufficient voltage is applied between two driving electrodes enables electrochemical reactions at conductive particles [12], i.e., BEs. There has been no other published work regarding this subject matter prior to the aforementioned, thus the findings emanating from Fleischmann and others’ investigations could be regarded as the foundation under which modern-day BPE is premised. Prior to the past two decades or so, BEs were largely referred to as fluidized bed electrodes and were used for designing industrial reactors meant for applications in processes such as electrosynthesis, and water splitting, and also for the purposes of increasing fuel cell performances [13]. Before being widely adopted for electroanalytical purposes, BPE had attracted great attention and had been used for many years in a variety of fields including battery technologies [14] concentration enrichment [15], separation [16] wastewater treatment [17], etc. In their research, Laws and others also investigated BPEs as a means of enriching and separating charged species electrokinetically where a single Au BPE was used to locally enrich several different charged markers in a single microchannel [16].
Whereas BPE can be applied for analytical and biosensing purposes, it still requires appropriate instrumentation and methods of detection to interpret the electrochemical signal that is produced at the BE. So in many cases for the purposes of providing an optical signal readout, BPE is usually used in combination with ECL, fluorescence, and anodic dissolution [18]. However, ECL possesses advantages over other forms of luminescence such as fluorescence because it does not require a source of light for photo-excitation; as a consequence, the instrumentation is quite simple thus, it is usually associated with low or zero background signal and allows optical detectors to be used at their maximum sensitivity [19][20][21]. In addition, there is also no backscattering [22]. The principles of BPE and ECL techniques will be discussed in detail in the forthcoming passages. Furthermore, what makes BPE when working in tandem with other detection techniques for multiplex assaying is because of its wireless capabilities which allow arbitrarily large arrays of BEs to be powered simultaneously in a very simple setup [23].
When BPE works in synergy with ECL, the sufficiently high voltage applied to the driving electrodes generates ECL at the anodic pole of each BE [24] which in turn facilitates a redox reaction where signal reporting is based on optical probes such as ECL. BPE, even though it is debatable, has also received tremendous attention in recent times due to the ease of miniaturization [25] as argued by advocates of BPE miniaturization. Because BPE has exceptionally unique properties coupled with the fact that it can be used for sensing, separations, and concentration enrichment in microelectrochemical systems [14]. Therefore, the technique is worth further exploring particularly for multiplex assaying of analytes including many different types of biomarkers in various sample matrices not only in the widely employed blood samples but also in non-invasive, yet complex samples such as hair. Overall, recent uses of bipolar electrochemistry concentrate on areas such as sensing, electrografting, electrodissolution, and electrodeposition, as applied to various fields including chemistry, biology, materials science, and device fabrication [26].
2. Fundamentals of Bipolar Electrodes
BPE, as aforesaid is essentially composed of a conductor (a BE) submerged in an electrolyte solution without direct connection to the external power source. A BE is an object made of a conductive material, such as a metal strip or bead, that facilitates electrically coupled Faradaic reactions at its opposing poles [27]. In terms of the experimental setup, the critical components of BPE include; two driving electrodes (feeder electrodes), an aqueous and inert dilute electrolytic solution, and an external power supply of uniform current applied across the solution [10][11]. The electrolytic solution can be a simple aqueous electrolyte or more exotic electrolytes such as ionic liquids [7]. The poles of a BE are oriented in the opposite polarity of the feeder electrodes [24]. Basically, an electric field is induced across the electrolyte by an external bias of the feeder electrodes; as a result of the interaction between the solution and the BE, the BE develops what is known as floating potential and the potential gradient varies along its length. With this sufficient external voltage, a substantial potential difference may also be established between the ends of the BE. This potential that develops on the BE drives redox reactions where oxidation occurs at the anode end whereas reduction occurs on the other cathode end [25][28]. In essence, it is these Faradaic reactions taking place at the ends of the BEs that most BPE studies focus on [29].
The potential of the electrode (∆EBPE) floats to a value intermediate to that of the electrolyte it is in contact with. In a BPE cell, there is the development of interfacial potential differences with opposite signs at the ends of the BE. These interfacial potential differences are the anodic and cathodic overpotentials [10][27]. To maintain electroneutrality at the bipolar electrode, the electron production and consumption must be equal on both sides [14][30]. To estimate the potential across the BPE given as (∆EBPE), the following Equation (1) below can be used [31].
where Etot is the voltage applied on the driving electrodes, lBPE is the length of BPE, l0 is the distance between two driving electrodes. Basically, the parameters that control BPE processes include Etot, lBPE, and l0. Ultimately, ∆EBPE, being the driver of the redox reactions at the poles of the BE, is the main parameter taken into consideration for analyzing electrochemical processes in BEs [4].
3. Configurations of Bipolar Electrochemistry
There are two types of BEs configurations that can be employed in BPE studies; these are open BPE (o-BPE) and closed BPE (c-BPE) systems [32] and a comparative schematic of the two systems is illustrated in Figure 2. These two systems differ in their design and operational characteristics. Both configurations have unique strengths and limitations that border on sensitivity, selectivity, reproducibility, cost-effectiveness, robustness, etc. Between the two, reports suggest that the o-BPE system was the first to be researched before c-BPE, which only appeared later [9].
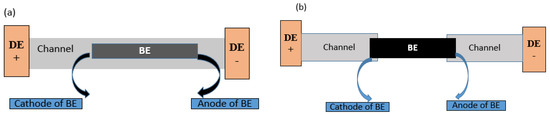
Figure 2. An illustration of an o-BPE (a) and c-BPE (b) systems. DE = driving electrode. Inspired by Ref. [33].
In c-BPE, the bipolar electrodes’ two poles concurrently engage with two electrolyte solutions, each containing a driving electrode, which are physically segregated within separate chambers [28], and the BE provides the current path (electronic pathway without the presence of the ionic current path) between the two half cells [34] that is, the current flows in the two half cells through the BE only. This design ensures that the two poles on the BE are located in two separate solutions [35]. In c-BPE, the anode and cathode of the same BE are placed into different microchannels containing unalike solution electrolytes and so they are fluidically and chemically isolated [33]. In contrast, in the o-BPE configuration, the BE is located in one channel, i.e., located within a single electrolyte solution [28]. Basically, the microchannel itself acts as a conductor. In o-BPE as opposed to c-BPE, the electrochemical current passage through the BE is facilitated by the flow of electrons (electronic pathway) or may result from the mobility of ions in the electrolyte solution (ionic pathway) [11]. Generally, in electrochemistry, the application of a suitable external driving voltage between the feeder electrodes generates a current that flows either via ionic or electronic pathways [32][36]; the latter current is the one that is relevant to BPE and to exploit this current efficiently, a BE with a conductivity higher than that of the electrolyte solution is used [11].
4. Closed vs. Open Bipolar Electrodes: A Comprehensive Review of Their Limitations and Advantages
Two different configurations of BPE, c-BPE and o-BPE possess different strengths and limitations. To start with, it is argued that c-BPE possesses some exclusive merits, notably in the area of detection. For instance, it has been reported that the c-BPE configuration has the distinctive ability to separate the reagents for generating detection signals such as ECL [37] and fluorescence from the targets of detection, which is actually conducive to reducing the background signals and interferences [32]. c-BPE also requires less driving potential to drive the Faraday reaction and is characterized by higher electron transfer efficiency (which is 100% in theory) in comparison to o-BPE [38]. Faraday reactions basically are electrolytic reactions that play a pivotal role in BPE and involve the oxidation or reduction at surfaces of a BE. Typically, they comprise the movement of electrons from one electrode to the other. Oppositely directed Faradaic reactions (reduction/oxidation) may be caused at the cathodic and anodic sides of the BE due to the potential difference between it and the electrolyte [39]. Furthermore, the c-BPE system offers somewhat a novel level of suppleness in respect of experimental design because a variety of conducting solutions with a diverse chemical constitution can be implemented in the very two independent compartments. The experimental conditions (pH, temperature, and light exposure) can also be manipulated independently to favor desired reactions [28]. These distinguished qualities of c-BPE have seen interest spike in its applications in the past decade as evidenced by the enormous numbers of scientists using c–BPE in their research works. For example, it was utilized in the investigation of electrical coupling between the BE photosystems 1 and 2 [40] and is of great interest; c-BPE was utilized in the development of an electrochromic sensor for multiplex detection of a variety of metabolites [41]. The ability to perform multiplex detection in this research was achieved by using a c-BPE electrochromic detector which was modified by integrating three sets of detection chemistries into one sensing device and detection of the analytes viz. glucose, lactate, and uric acid simultaneously demonstrated. In addition, another research group [42] demonstrated side-by-side the difference between o-BPE and c-BPE. In this investigation, it was deduced that excellent spatial resolution and specific surface functionalization of the c-BPE-ECL approach made it suitable for constructing multiplex imaging platforms with improved sensitivity. In addition, an ECL based on c-BPE was also used in the visual screening of Salmonella typhimurium with a very low limit of detection [43]. So, modifying c-BPE may also enhance the detection sensitivity, and selectivity accuracy of multiplex assaying.
Some studies suggest that in the o-BPE system, the detection relies on ECL or visual metal electro-dissolution [19] and this is because of the difficulty in measuring the direct current that flows through the BE [37][44]. On the contrary, in the c-BPE configuration where the two poles of the BE are in two individual compartments, it is contended that measuring the electric current is somewhat easier [45]. c-BPEs have several other advantages, including a high selectivity of the reaction taking place, low background current, and the ability to eliminate interference from other electroactive species. However, other limitations of c-BPE also include their relatively low sensitivity and ability to detect only specific ions or analytes [46]. To add on, based on recent research publications, o-BEs are generally preferable over c-BEs in biosensor applications due to their high sensitivity and versatility in detecting multiple analytes. c-BEs, on the other hand, are usually useful in ion selective electrode applications [47], potentiometric sensors [48], and detecting low levels of heavy metal ions in the environment [49]. What is also worth noting is that o-BPE systems are advantageous because of their capability to incorporate with comparable ease, a large number of BEs [50]. However, the shortcoming is that in this o-BPE system, the output signal could easily be influenced by the electroactive chemical species since they are present in the same solution. On the other hand, this limitation is avoided in c-BPE because the anodic and cathodic poles are immersed in different solutions in separate compartments. Nonetheless, as a consequence of the presence of the separating wall between the two compartments, some studies that have employed the c-BPE configuration for multiplexing have only been able to incorporate a limited number of BEs in the system [51][52][53]. In the o-BPE configuration, however, this may not be the case because the incorporation of a limitless number of BEs is possible.
For the most part, however, recent research has predominantly focused on the use of c-BPE in the development of ECL-based multiplex biosensors. The c-BPE configuration possesses several advantages over o-BPE, including improved reliability and reproducibility, reduced electrode fouling, and enhanced kinetic reversibility. c-BPE is especially compatible with fabricating sensors based on ECL. ECL is a process that involves the conversion of electrical energy into luminescent output. By separating the sensing and reporting reactions at opposite ends of the BPE, the electroactive substances generated in the biosensing system can be isolated from the ECL reaction, thereby enhancing the flexibility of the biosensing approach used [53]. These advantageous features make closed BEs ideal for use in the development of high-performance electrochemical biosensors. Over the last ten years or so, researchers have placed more emphasis on the use of c-BPE within the analytical field as compared to o-BPE [38]. Overall, the use of c-BPEs in ECL-based biosensors has enabled the accurate and sensitive detection of multiple analytes in a single assay, making it a highly promising technique for multiplex biosensing applications. As will be observed later, several studies have demonstrated the effectiveness of c-BPE in the development of multiplex biosensors. Nonetheless, researchers may still carefully evaluate the advantages and limitations of both systems in terms of targeted application requirements. Further research is required to enhance and optimize the performance of bipolar electrodes for different biosensing applications.
5. Bipolar Electrode Miniaturization: Uncovering the Opportunities and Challenges
As noted in the preceding sections, one of the potential advantages associated with BPE is its ability to be miniaturized: miniaturized BPE has been used in various devices for instance in microelectronic devices and point-of-care devices [2][33][54], and it also has the potential to be incorporated in environmental monitoring systems, among other applications. Miniaturized devices also go by the following names; microfluidic devices, lab-on-a-chip, and micro total analysis systems [55]. Generally, miniaturization is associated with cost-effectiveness in terms of materials needed for electrode fabrication along with small volumes of sample, fast response, precision, multiplex operation, and rapid results acquisition [56][57]. The reduction in sample volume and cost, accompanied by the possibility of automation, can provide significant benefits in the point-of-care and clinical settings, particularly in developing countries where both human and financial resources may be limited. The development of microfluidic chips (miniature devices) has also greatly contributed to the simplification of the operation of BPE. Microfluidic devices offer cost, reagent, and time efficiencies in comparison to traditional devices, exhibiting their strength in biological sample detection and the development of portable detection tools [53]. It is no doubt that the miniaturization of bipolar electrodes is a viable concept.
However, despite the potential benefits, miniaturizing BPE presents numerous challenges. Some research scientists have highlighted a couple of limitations regarding BPE miniaturization. For instance, it is argued that despite BPE being a well-established technique, its inherent inadequacy is generally related to the length of the BE: the shorter it is, the larger the applied potential required to have polarized and consequently induce redox reactions at its two poles [11][24]. This limitation constitutes a very important drawback for nanometric and micrometric BEs as high potentials in the order of tens of kV or more is required to successfully achieve their polarization [58]. Therefore as observed by another independent researcher [3], it would be problematic to apply an electric field to attain polarization of a micro- or nonmetric metallic particle acting as a BE in a living cell to conduct wireless biosensing, for example. Other challenges include issues to do with scalability and sensitivity. Miniaturization comes with increasing complexity and additional effects, showing deviations from macroscopic models, have been reported. These effects can occur due to some unexpected behaviors at the nanoscale, which can be summarized as “nano-effects” or because of additional interfaces causing confinement that affects the electrochemical process, such as “confinement effects” [59]. To address these challenges, researchers have proposed various strategies, such as optimizing the interelectrode distance and selecting appropriate materials for the electrodes to enhance the sensitivity [60]. These approaches, alongside further research and development, provide promising opportunities to overcome the challenges associated with the miniaturization of BEs. The microfluidic format has also been reported to face two major obstacles that is, limited sensitivity attributed to the tiny sample volumes employed and the cost of microchip production [55]. However, the complex microchip system can be replaced with a cost-effective textile substrate featuring inbuilt microchannels built in the mesh of its fibers. Additionally, integrating this textile-based microfluidics with electrophoresis and BPE can lead to considerable enhancements in solute detection, as the analytes of interest are concentrated and focused [61][62].
All in all, two interesting properties of BPE that have justified the attention it has garnered in the past years of research are due to two noteworthy characteristics: (a) its wireless nature that eases electrochemical sensing and supports high throughput analysis; (b) the gradient potential distribution on the surface of the electrode is a useful tool for preparing gradient surfaces and materials. These important characteristics make BPE very suitable for further modification and analytical applications even on a micro/nanoscale surface [6]. In conclusion, the miniaturization of ECL based on BPE is a promising and rapidly growing field. Despite the challenges, the technique offers great opportunities for developing analytical devices for point-of-care applications, and the efficient monitoring of biological and bioanalytical systems.
6. Synthesis and Functionalization Strategies of BEs for Biosensing
The fabrication of BEs should be adapted according to their intended application in different fields by utilizing an assortment of materials and dimensions. Polyethylene, glass, and polymethyl methacrylate are commonly utilized in their preparation due to their transparency [4]. The exact design and composition of the BE substrate will be dependent on the specific application and desired properties. Various materials can be employed as driving electrodes in BPE, such as metallic substances such as Pt, Ag (or Ag/AgCl), Au, and stainless steel [63][64][65]. The synthesis and functionalization strategies of BE play a critical role in determining their corresponding performance in biosensing. In terms of synthesis techniques, electrodeposition is a common method used for the synthesis of both carbon-based materials and metal-based nanoparticles. Electrodeposition allows the control of size, shape, and chemical reactivity of the nanoparticles formed [66][67]. For example, carbon nanotubes can be synthesized by chemical vapor deposition (CVD) using a variety of catalysts [68]. Thin films of polyimide can be deposited using spin coating [69]. BE fabrication can also be achieved using several materials such as glassy carbon [70]. It is well-recognized that carbon-based materials such as glassy carbon, graphite, and carbon nanotubes are desirable due to their distinct advantages, such as electrical conductivity, biocompatibility, low cost, and straightforward fabrication [71][72][73]. As an example, one research group [74] also fabricated an inexpensive and disposable sensing platform for detecting H2O2 using the pencil core as the BE. The design of BEs for ECL-based biosensing requires a careful choice of materials used in its fabrication, synthesis techniques, and functionalization strategies.
Functionalization of the BE is one way in which the range of different analyte detection using the BPE technique can be improved. Functionalization of materials involves modifying their surface chemistry to add novel features, capabilities, or properties [75]. In another study, BE was fabricated by microchip manufacturing technology; here, it was observed that the BE mainly exhibited the electrochemical behaviors of one pole in a one-half cell, but was influenced by the redox reaction in the other half-cell [32] As observed so far, it is very possible to fabricate hybrid conducting materials with a range of functionalities both at the macro- and nanoscale [13] and this is quite interesting from the multiplex biosensing viewpoint. Another study [33] was conducted to create an ECL system that is both sensitive and portable, using laser-induced graphene-BE for biosensing vitamin B-12. The device was made using polyimide (PI) material and the BE system was fabricated by subjecting the PI sheet to a carbon dioxide laser. PI offers several benefits such as high dependability, greater stability, a one-step manufacturing process, and swift prototyping [76]. Several biofunctionalization strategies can be used to enhance BPEs’ properties and allow for the detection of specific analytes. Biofunctionalization strategies using antibodies, DNA/aptamers, and redox enzyme immobilization have been widely used to promote analyte recognition [77] for multiplex biosensing applications, for example. The surface of BEs can be modified to promote analyte recognition and improve their electrochemical properties by biofunctionalizing them with biocompatible and biorecognition molecules. As a significant example, nano-bovine serum albumin (nano-BSA) has shown great potential as a biofunctionalization agent due to its unique structural and chemical properties. The high surface area-to-volume ratio of the nano-BSA enables more binding sites for biomolecules [78][79] while its stability in various buffer solutions allows for long-term use in biosensing applications, Additionally, its biocompatibility and low immunogenicity make it an excellent choice for use in various biological applications, including biosensors. For instance, in one research, nano-bovine serum albumin was utilized to biofunctionalize a graphene interface to create a highly sensitive multiplex assay for detecting cancer biomarkers [80]. In another study, a glassy carbon electrode’s surface was fabricated with high luminescent polydopamine nanospheres and chitosan using the layer-by-layer assembly method for detecting Mucin-1 [81]. Stimulatingly, however, one study [82] developed a single electrode electrochemical system (SEES) for multiplex assaying and they were successful. The SEES which utilizes only one electrode has a microelectrochemical cell built with a hole in an insulating self-adhesive plastic film for a single experiment and multiple holes for multiplex experiments. When an external voltage is applied to both ends of the single electrode, it generates a potential difference between the two ends of the microelectrochemical cell and triggers Faradaic reactions. The system generates ECL reactions through a potential difference induced by the electrode’s resistance [83]. In comparison to traditional BPE, this SEES system possesses a couple of inherent advantages such as being further simplified and more cost-effective especially for high throughput analysis since only a single electrode is in use. The system is comparatively economical because it does not involve complicated and costly fabrication procedures of the electrode arrays. Further, the system is also devoid of ECL background problems from the driving electrode in the BPE system. However, this system is yet to be extensively explored.
In general, the use of a substrate made of conducting material as a BE offers significant advantages for electrochemical applications. The BE design further improves the efficiency and versatility of BE by allowing for the simultaneous use of both sides of the electrode. Further research and development in this area will likely lead to new and innovative uses for this technology. In summary, BPE is a promising technique that has a wide range of applications in various fields. In the field of biosensing, BPE has the potential to improve the sensitivity and selectivity of electrochemical sensors by generating more stable, reproducible, and controllable signals. Because of being wireless and lack any connection to an external device, the electrochemical reactions occurring at one pole related to detection are usually reported through ECL on the other BE [84]. Currently, ECL is the most prominent reporting strategy for analytical BPE. BPE allows for the spatial segregation of sensing and reporting electrodes, and ECL offers an easy-to-read, sensitive visual display [32]. Hence, by incorporating BPE with other techniques such as ECL, the sensitivity and detection limit of biosensors can be further enhanced. As it shall be noted later, the combination of BPE with ECL offers exciting new opportunities for enhancing the sensitivity and specificity of multiplex assaying. The synergy between these two techniques is indubitably essential for developing highly sensitive, real-time, and reliable biosensing systems.
References
- Qin, X.; Gao, J.; Jin, H.-J.; Li, Z.-Q.; Xia, X.-H. Closed Bipolar Electrode Array for Optical Reporting Reaction-Coupled Electrochemical Sensing and Imaging. Chem. A Eur. J. 2023, 29, e202202687.
- Cauteruccio, S.; Pelliccioli, V.; Grecchi, S.; Cirilli, R.; Licandro, E.; Arnaboldi, S. Bipolar Electrochemical Analysis of Chirality in Complex Media through Miniaturized Stereoselective Light-Emitting Systems. Chemosensors 2023, 11, 131.
- Wang, Y.; Jin, R.; Sojic, N.; Jiang, D.; Chen, H.Y. Intracellular Wireless Analysis of Single Cells by Bipolar Electrochemiluminescence Confined in a Nanopipette. Angew. Chem. 2020, 132, 10502–10506.
- Karimian, N.; Hashemi, P.; Afkhami, A.; Bagheri, H. The principles of bipolar electrochemistry and its electroanalysis applications. Curr. Opin. Electrochem. 2019, 17, 30–37.
- Hu, S.; Gao, J. Materials and Physics of Light-Emitting Electrochemical Cells (LECs), 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 727–757.
- Wang, Y.L.; Cao, J.T.; Liu, Y.M. Bipolar Electrochemistr—A Powerful Tool for Micro/Nano-Electrochemistry. ChemistryOpen 2022, 11, e202200163.
- Bouffier, L.; Zigah, D.; Sojic, N.; Kuhn, A.; Bouffier, L.; Zigah, D.; Sojic, N.; Kuhn, A.; Bouffier, L.; Zigah, D.; et al. Bipolar (bio) electroanalysis. Hal Open Sci. 2021, 14, 65–86.
- Baflnes, D.W.; Khrushchov, N.G. Fluidized Bed Electrodes-Fundamental Measurements and Implications. Dokl. Akad. Nauk TJSSII 1968, 2, 50.
- Zhang, X.; Zhai, Q.; Xing, H.; Li, J.; Wang, E. Bipolar Electrodes with 100% Current Efficiency for Sensors. ACS Sens. 2017, 2, 320–326.
- Crooks, R.M. Principles of Bipolar Electrochemistry. ChemElectroChem 2016, 3, 357–359.
- Loget, G.; Zigah, D.; Bouffier, L.; Sojic, N.; Kuhn, A. Bipolar Electrochemistry: Science to Motion and Beyond. Acc. Chem. Res. 2013, 46, 2513–2523.
- Backhurst, J.R.; Coulson, J.M.; Goodridge, F.; Plimley, R.E.; Fleischmann, M. A Preliminary Investigation of Fluidized Bed Electrodes. J. Electrochem. Soc. 1969, 116, 1600.
- Loget, G.; Kuhn, A. Shaping and exploring the micro- and nanoworld using bipolar electrochemistry. Anal. Bioanal. Chem. 2011, 400, 1691–1704.
- Mavré, F.; Anand, R.K.; Laws, D.R.; Chow, K.F.; Chang, B.Y.; Crooks, J.A.; Crooks, R.M. Bipolar electrodes: A useful tool for concentration, separation, and detection of analytes in microelectrochemical systems. Anal. Chem. 2010, 82, 8766–8774.
- Perdue, R.K.; Laws, D.R.; Hlushkou, D.; Tallarek, U.; Crooks, R.M. Bipolar electrode focusing: The effect of current and electric field on concentration enrichment. Anal. Chem. 2009, 81, 10149–10155.
- Laws, D.R.; Hlushkou, D.; Perdue, R.K.; Tallarek, U.; Crooks, R.M. Bipolar electrode focusing: Simultaneous concentration enrichment and separation in a microfluidic channel containing a bipolar electrode. Anal. Chem. 2009, 81, 8923–8929.
- Qi, Z.; You, S.; Liu, R.; Chuah, C.J. Performance and mechanistic study on electrocoagulation process for municipal wastewater treatment based on horizontal bipolar electrodes. Front. Environ. Sci. Eng. 2020, 14, 40.
- Zhang, X.; Ding, S.N. Graphite paper-based bipolar electrode electrochemiluminescence sensing platform. Biosens. Bioelectron. 2017, 94, 47–55.
- Arora, A.; Eijkel, J.C.T.; Morf, W.E.; Manz, A. A wireless electrochemiluminescence detector applied to direct and indirect detection for electrophoresis on a microfabricated glass device. Anal. Chem. 2001, 73, 3282–3288.
- Qi, H.; Zhang, C. Electrogenerated chemiluminescence biosensing. Anal. Chem. 2020, 92, 524–534.
- Knezevic, S.; Bouffier, L.; Liu, B.; Jiang, D.; Sojic, N. Electrochemiluminescence microscopy: From single objects to living cells. Curr. Opin. Electrochem. 2022, 35, 101096.
- Spehar-Délèze, A.M.; Gransee, R.; Martinez-Montequin, S.; Bejarano-Nosas, D.; Dulay, S.; Julich, S.; Tomaso, H.; O’Sullivan, C.K. Electrochemiluminescence dna sensor array for multiplex detection of biowarfare agents. Anal. Bioanal. Chem. 2015, 407, 6657–6667.
- Zhang, H.-R.; Wang, Y.-Z.; Zhao, W.; Xu, J.-J.; Chen, H.-Y. Visual Color-Switch Electrochemiluminescence Biosensing of Cancer Cell Based on Multichannel Bipolar Electrode Chip. Anal. Chem. 2016, 88, 2884–2890.
- Fosdick, S.E.; Knust, K.N.; Scida, K.; Crooks, R.M. Bipolar electrochemistry. Angew. Chem. Int. Ed. 2013, 52, 10438–10456.
- Li, M.; Liu, S.; Jiang, Y.; Wang, W. Visualizing the Zero-Potential Line of Bipolar Electrodes with Arbitrary Geometry. Anal. Chem. 2018, 90, 6390–6396.
- Mele, C.; Lionetto, F.; Bozzini, B. An Erosion-Corrosion Investigation of Coated Steel for Applications in the Oil and Gas Field, Based on Bipolar Electrochemistry. Coatings 2020, 10, 92.
- Rahn, K.L.; Anand, R.K. Recent Advancements in Bipolar Electrochemical Methods of Analysis. Anal. Chem. 2021, 93, 103–123.
- Gamero-Quijano, A.; Molina-Osorio, A.F.; Peljo, P.; Scanlon, M.D. Closed bipolar electrochemistry in a four-electrode configuration. Phys. Chem. Chem. Phys. 2019, 21, 9627–9640.
- Wu, M.S.; Qian, G.S.; Xu, J.J.; Chen, H.Y. Sensitive electrochemiluminescence detection of c-Myc mRNA in breast cancer cells on a wireless bipolar electrode. Anal. Chem. 2012, 84, 5407–5414.
- Goodridge, F. Some recent developments of monopolar and bipolar fluidized bed electrodes. Electrochim. Acta 1977, 22, 929–933.
- Mavré, F.; Chow, K.F.; Sheridan, E.; Chang, B.Y.; Crooks, J.A.; Crooks, R.M. A theoretical and experimental framework for understanding electrogenerated chemiluminescence (ECL) emission at bipolar electrodes. Anal. Chem. 2009, 81, 6218–6225.
- Zhang, J.D.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Electrochemical behaviors in closed bipolar system with three-electrode driving mode. J. Electroanal. Chem. 2016, 781, 56–61.
- Bhaiyya, M.; Pattnaik, P.K.; Goel, S. Simultaneous detection of Vitamin B12 and Vitamin C from real samples using miniaturized laser-induced graphene based electrochemiluminescence device with closed bipolar electrode. Sens. Actuators A Phys. 2021, 331, 112831.
- Liu, R.; Zhang, C.; Liu, M. Open bipolar electrode-electrochemiluminescence imaging sensing using paper-based microfluidics. Sens. Actuators B Chem. 2015, 216, 255–262.
- Ma, C.; Pei, S.; You, S. Closed bipolar electrode for decoupled electrochemical water decontamination and hydrogen recovery. Electrochem. Commun. 2019, 109, 106611.
- Ongaro, M.; Gambirasi, A.; Ugo, P. Closed Bipolar Electrochemistry for the Low-Potential Asymmetrical Functionalization of Micro-and Nanowires. ChemElectroChem 2016, 3, 450–456.
- Wu, S.; Zhou, Z.; Xu, L.; Su, B.; Fang, Q. Integrating bipolar electrochemistry and electrochemiluminescence imaging with microdroplets for chemical analysis. Biosens. Bioelectron. 2014, 53, 148–153.
- Che, Z.Y.; Wang, X.Y.; Ma, X.; Ding, S.N. Bipolar electrochemiluminescence sensors: From signal amplification strategies to sensing formats. Coord. Chem. Rev. 2021, 446, 214116.
- Koefoed, L.; Pedersen, S.U.; Daasbjerg, K. Bipolar electrochemistry—A wireless approach for electrode reactions. Curr. Opin. Electrochem. 2017, 2, 13–17.
- Eßmann, V.; Zhao, F.; Hartmann, V.; Nowaczyk, M.M.; Schuhmann, W.; Conzuelo, F. In Operando Investigation of Electrical Coupling of Photosystem 1 and Photosystem 2 by Means of Bipolar Electrochemistry. Anal. Chem. 2017, 89, 7160–7165.
- Xu, W.; Fu, K.; Bohn, P.W. Electrochromic sensor for multiplex detection of metabolites enabled by closed bipolar electrode coupling. ACS Sens. 2017, 2, 1020–1026.
- Wu, M.S.; Liu, Z.; Shi, H.W.; Chen, H.Y.; Xu, J.J. Visual electrochemiluminescence detection of cancer biomarkers on a closed bipolar electrode array chip. Anal. Chem. 2015, 87, 530–537.
- Luo, Y.; Lv, F.; Wang, M.; Lu, L.; Liu, Y.; Xiong, X. A multicolor electrochemiluminescence device based on closed bipolar electrode for rapid visual screening of Salmonella typhimurium. Sens. Actuators B Chem. 2021, 349, 130761.
- Guerrette, J.P.; Percival, S.J.; Zhang, B. Fluorescence coupling for direct imaging of electrocatalytic heterogeneity. J. Am. Chem. Soc. 2013, 135, 855–861.
- Dhopeshwarkar, R.; Hlushkou, D.; Nguyen, M.; Tallarek, U.; Crooks, R.M. Electrokinetics in microfluidic channels containing a floating electrode. J. Am. Chem. Soc. 2008, 130, 10480–10481.
- Curulli, A. Electrochemical biosensors in food safety: Challenges and perspectives. Molecules 2021, 26, 2940.
- Jansod, S.; Bakker, E. Tunable Optical Sensing with PVC-Membrane-Based Ion-Selective Bipolar Electrodes. ACS Sens. 2019, 4, 1008–1016.
- Jaworska, E.; Michalska, A.; Maksymiuk, K. Implementation of a Chloride-selective Electrode Into a Closed Bipolar Electrode System with Fluorimetric Readout. Electroanalysis 2020, 32, 812–819.
- Moghaddam, M.R.; Carrara, S.; Hogan, C.F. Multi-colour bipolar electrochemiluminescence for heavy metal ion detection. Chem. Commun. 2019, 55, 1024–1027.
- Chow, K.F.; Mavré, F.; Crooks, J.A.; Chang, B.Y.; Crooks, R.M. A large-scale, wireless electrochemical bipolar electrode microarray. J. Am. Chem. Soc. 2009, 131, 8364–8365.
- Wang, F.; Liu, Y.; Fu, C.; Li, N.; Du, M.; Zhang, L.; Ge, S.; Yu, J. Paper-based bipolar electrode electrochemiluminescence platform for detection of multiple miRNAs. Anal. Chem. 2021, 93, 1702–1708.
- Oh, C.; Park, B.; Sundaresan, V.; Schaefer, J.L.; Bohn, P.W. Closed Bipolar Electrode-Enabled Electrochromic Sensing of Multiple Metabolites in Whole Blood. ACS Sens. 2023, 8, 270–279.
- Liu, Y.; Zhang, N.; Pan, J.-B.; Song, J.; Zhao, W.; Chen, H.-Y.; Xu, J.-J. Bipolar Electrode Array for Multiplexed Detection of Prostate Cancer Biomarkers. Anal. Chem. 2022, 94, 3005–3012.
- Liang, Y.; Xue, K.; Shi, Y.; Zhan, T.; Lai, W.; Zhang, C. Dry Chemistry-Based Bipolar Electrochemiluminescence Immunoassay Device for Point-of-Care Testing of Alzheimer-Associated Neuronal Thread Protein. Anal. Chem. 2023, 95, 3434–3441.
- Khan, J.U.; Ruland, A.; Sayyar, S.; Paull, B.; Chen, J.; Innis, P.C. Wireless bipolar electrode-based textile electrofluidics: Towards novel micro-total-analysis systems. Lab Chip 2021, 21, 3979–3990.
- Puneeth, S.B.; Kulkarni, M.B.; Goel, S. Microfluidic viscometers for biochemical and biomedical applications: A review. Eng. Res. Express 2021, 3, 022003.
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advances in Microfluidics-Based Electrochemical Sensors for Foodborne Pathogen Detection. Biosensors 2023, 13, 246.
- Warakulwit, C.; Nguyen, T.; Majimel, J.; Delville, M.H.; Lapeyre, V.; Garrigue, P.; Ravaine, V.; Limtrakul, J.; Kuhn, A. Dissymmetric carbon nanotubes by bipolar electrochemistry. Nano Lett. 2008, 8, 500–504.
- Huang, X.; Li, B.; Lu, Y.; Liu, Y.; Wang, S.; Sojic, N.; Jiang, D.; Liu, B. Direct Visualization of Nanoconfinement Effect on Nanoreactor via Electrochemiluminescence Microscopy. Angew. Chem. Int. Ed. 2023, 62, e202215078.
- Borchers, J.S.; Campbell, C.R.; Van Scoy, S.B.; Clark, M.J.; Anand, R.K. Redox Cycling at an Array of Interdigitated Bipolar Electrodes for Enhanced Sensitivity in Biosensing. ChemElectroChem 2021, 8, 3482–3491.
- Farajikhah, S.; Cabot, J.M.; Innis, P.C.; Paull, B.; Wallace, G. Life-Saving Threads: Advances in Textile-Based Analytical Devices. ACS Comb. Sci. 2019, 21, 229–240.
- Weng, X.; Kang, Y.; Guo, Q.; Peng, B.; Jiang, H. Recent advances in thread-based microfluidics for diagnostic applications. Biosens. Bioelectron. 2019, 132, 171–185.
- Liang, Y.; Lai, W.; Su, Y.; Zhang, C. A novel cloth-based multiway closed bipolar electrochemiluminescence biosensor for accurate detection of uric acid. Microchem. J. 2022, 179, 107657.
- Motaghi, H.; Ziyaee, S.; Mehrgardi, M.A.; Kajani, A.A.; Bordbar, A.-K. Electrochemiluminescence detection of human breast cancer cells using aptamer modified bipolar electrode mounted into 3D printed microchannel. Biosens. Bioelectron. 2018, 118, 217–223.
- Wang, D.; Liang, Y.; Su, Y.; Shang, Q.; Zhang, C. Sensitivity enhancement of cloth-based closed bipolar electrochemiluminescence glucose sensor via electrode decoration with chitosan/multi-walled carbon nanotubes/graphene quantum dots-gold nanoparticles. Biosens. Bioelectron. 2019, 130, 55–64.
- Jaugstetter, M.; Blanc, N.; Kratz, M.; Tschulik, K. Electrochemistry under confinement. Chem. Soc. Rev. 2022, 51, 2491–2543.
- Wang, Y.; Liu, R.; Ma, Y.; Shen, X.; Wang, D. Electrodeposition of Metal Nanoparticles inside Carbon Nanopipettes for Sensing Applications. Anal. Chem. 2022, 94, 16987–16991.
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822.
- Zhou, Z.; Zhou, S.; Cheng, X.; Liu, W.; Wu, R.; Wang, J.; Liu, B.; Zhu, J.; Van der Bruggen, B.; Zhang, Y. Ultrathin polyamide membranes enabled by spin-coating assisted interfacial polymerization for high-flux nanofiltration. Sep. Purif. Technol. 2022, 288, 120648.
- Yuan, F.; Qi, L.; Fereja, T.H.; Snizhko, D.V.; Liu, Z.; Zhang, W.; Xu, G. Regenerable bipolar electrochemiluminescence device using glassy carbon bipolar electrode, stainless steel driving electrode and cold patch. Electrochim. Acta 2018, 262, 182–186.
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon nanotube—A review on Synthesis, Properties and plethora of applications in the field of biomedical science. Sens. Int. 2020, 1, 100003.
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319.
- Silva, R.M.; da Silva, A.D.; Camargo, J.R.; de Castro, B.S.; Meireles, L.M.; Silva, P.S.; Janegitz, B.C.; Silva, T.A. Carbon Nanomaterials-Based Screen-Printed Electrodes for Sensing Applications. Biosensors 2023, 13, 453.
- Lu, W.X.; Bao, N.; Ding, S.N. A bipolar electrochemiluminescence sensing platform based on pencil core and paper reservoirs. RSC Adv. 2016, 6, 25388–25392.
- Ma, Y.; Qu, X.; Liu, C.; Xu, Q.; Tu, K. Metal-Organic Frameworks and Their Composites Towards Biomedical Applications. Front. Mol. Biosci. 2021, 8, 805228.
- Jayapiriya, U.S.; Rewatkar, P.; Goel, S. Miniaturized polymeric enzymatic biofuel cell with integrated microfluidic device and enhanced laser ablated bioelectrodes. Int. J. Hydrog. Energy 2021, 46, 3183–3192.
- Antiochia, R.; Tortolini, C.; Tasca, F.; Gorton, L.; Bollella, P. Chapter 1—Graphene and 2D-Like Nanomaterials: Different Biofunctionalization Pathways for Electrochemical Biosensor Development. In Advanced Nanomaterials; Tiwari, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–35.
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Al Zoubi, M.S.; Al-Trad, B.; Nasef, M.M.; Satija, S.; Mehta, M.; et al. Albumin Nano-Encapsulation of Piceatannol Enhances Its Anticancer Potential in Colon Cancer Via Downregulation of Nuclear p65 and HIF-1α. Cancers 2020, 12, 113.
- Shen, X.; Liu, X.; Li, T.; Chen, Y.; Chen, Y.; Wang, P.; Zheng, L.; Yang, H.; Wu, C.; Deng, S.; et al. Recent Advancements in Serum Albumin-Based Nanovehicles Toward Potential Cancer Diagnosis and Therapy. Front. Chem. 2021, 9, 746646.
- Zhou, L.; Wang, K.; Sun, H.; Zhao, S.; Chen, X.; Qian, D.; Mao, H.; Zhao, J. Novel Graphene Biosensor Based on the Functionalization of Multifunctional Nano-bovine Serum Albumin for the Highly Sensitive Detection of Cancer Biomarkers. Nano-Micro Lett. 2019, 11, 20.
- Liang, Z.; Liu, Y.; Zhang, Q.; Guo, Y.; Ma, Q. The high luminescent polydopamine nanosphere-based ECL biosensor with steric effect for MUC1 detection. Chem. Eng. J. 2020, 385, 123825.
- Gao, W.; Muzyka, K.; Ma, X.; Lou, B.; Xu, G. A single-electrode electrochemical system for multiplex electrochemiluminescence analysis based on a resistance induced potential difference. Chem. Sci. 2018, 9, 3911–3916.
- Du, F.; Dong, Z.; Liu, F.; Anjum, S.; Hosseini, M.; Xu, G. Single-electrode electrochemical system based on tris(1,10-phenanthroline)ruthenium modified carbon nanotube/graphene film electrode for visual electrochemiluminescence analysis. Electrochim. Acta 2022, 420, 140431.
- Hsueh, A.-J.; Mutalib, N.A.A.; Shirato, Y.; Suzuki, H. Bipolar Electrode Arrays for Chemical Imaging and Multiplexed Sensing. ACS Omega 2022, 7, 20298–20305.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
909
Revisions:
2 times
(View History)
Update Date:
29 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




