Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Denis G. Alekseev | -- | 2447 | 2023-06-27 09:26:28 | | | |
| 2 | Camila Xu | Meta information modification | 2447 | 2023-06-27 10:02:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Volova, L.T.; Kotelnikov, G.P.; Shishkovsky, I.; Volov, D.B.; Ossina, N.; Ryabov, N.A.; Komyagin, A.V.; Kim, Y.H.; Alekseev, D.G. 3D Bioprinting of Hyaline Articular Cartilage. Encyclopedia. Available online: https://encyclopedia.pub/entry/46097 (accessed on 07 February 2026).
Volova LT, Kotelnikov GP, Shishkovsky I, Volov DB, Ossina N, Ryabov NA, et al. 3D Bioprinting of Hyaline Articular Cartilage. Encyclopedia. Available at: https://encyclopedia.pub/entry/46097. Accessed February 07, 2026.
Volova, Larisa T., Gennadiy P. Kotelnikov, Igor Shishkovsky, Dmitriy B. Volov, Natalya Ossina, Nikolay A. Ryabov, Aleksey V. Komyagin, Yeon Ho Kim, Denis G. Alekseev. "3D Bioprinting of Hyaline Articular Cartilage" Encyclopedia, https://encyclopedia.pub/entry/46097 (accessed February 07, 2026).
Volova, L.T., Kotelnikov, G.P., Shishkovsky, I., Volov, D.B., Ossina, N., Ryabov, N.A., Komyagin, A.V., Kim, Y.H., & Alekseev, D.G. (2023, June 27). 3D Bioprinting of Hyaline Articular Cartilage. In Encyclopedia. https://encyclopedia.pub/entry/46097
Volova, Larisa T., et al. "3D Bioprinting of Hyaline Articular Cartilage." Encyclopedia. Web. 27 June, 2023.
Copy Citation
Hyaline articular cartilage (HAC) is a smooth, wear-resistant, highly specialized hyaline cartilage covering the epiphyses and certain anatomical areas of the bone within the synovial joint capsule. HAC reduces friction, allowing smooth joint movement. The emergence of biofabrication technologies, including three-dimensional (3D) bioprinting, at the end of the 20th century, allowed reconstructive interventions to get a second wind. Three-dimensional bioprinting creates volume constraints that mimic the structure and function of natural tissue due to the combinations of biomaterials, living cells, and signal molecules to create.
hyaline articular cartilage
traumatology and orthopaedics
regenerative medicine
tissue engineering
1. Hyaline Articular Cartilage Damages, Regeneration Process, and Current Treatment Approaches
Hyaline articular cartilage (HAC) is a smooth, wear-resistant, highly specialized hyaline cartilage covering the epiphyses and certain anatomical areas of the bone within the synovial joint capsule (Figure 1). HAC reduces friction, allowing smooth joint movement [1][2]. The HAC lacks nerve endings as well as blood vessels, so its nutrition depends on articular (synovial) fluid, the underlying (subchondral) bone, and mechanical loading [3][4]. Through the porous upper layer of the cartilage matrix, nutrients soluble in synovial fluid enter, and metabolism products are removed from HAC. With the underlying subchondral bone, metabolism is realised by diffusion from numerous blood capillaries [5][6].
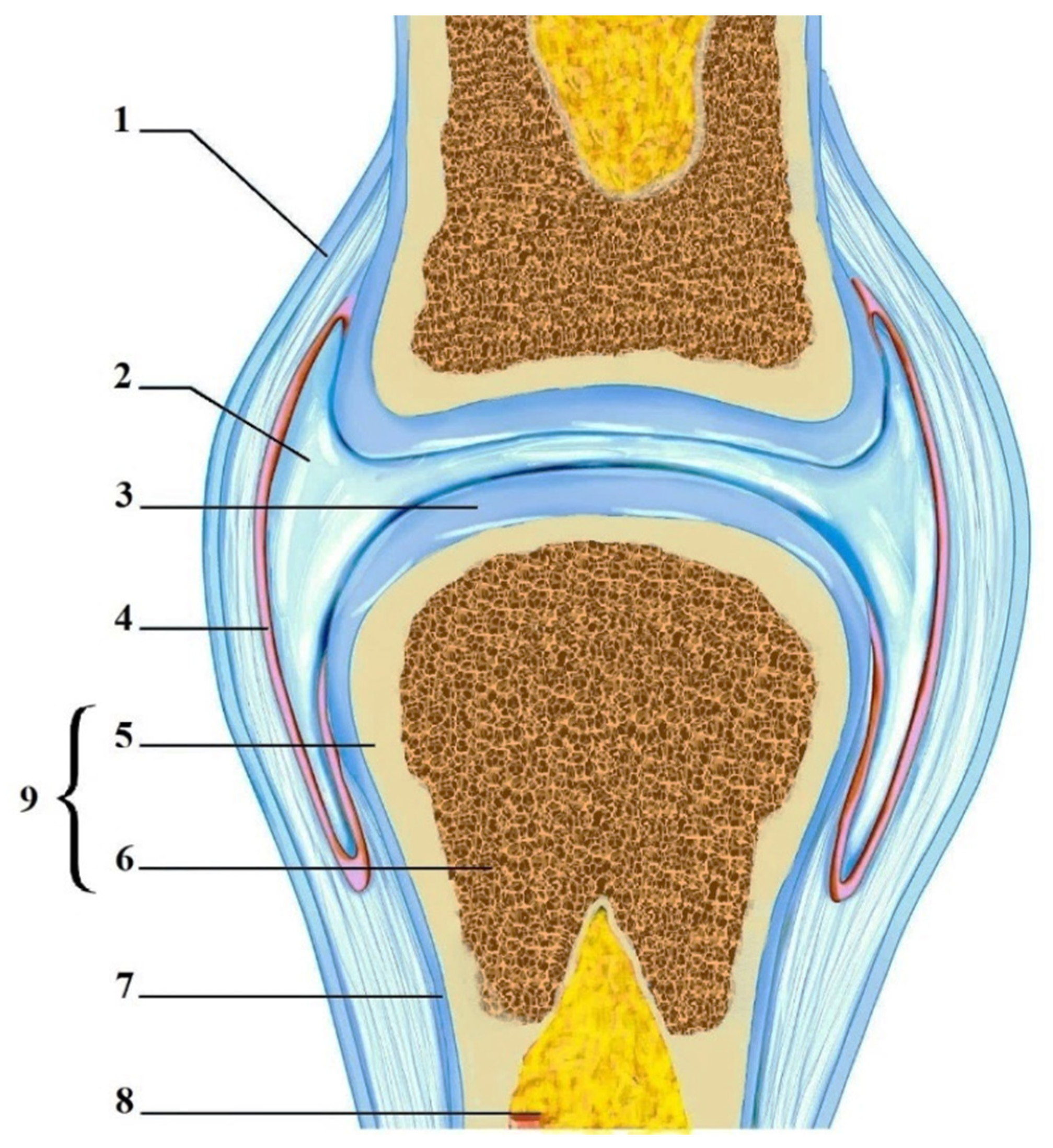
Figure 1. Articular cartilage. 1—Articular capsule; 2—Joint cavity with synovial fluid; 3—Hyaline articular cartilage; 4—Synovial membrane; 5—Compact bone; 6—Spongy bone; 7—Periosteum; 8—Bone marrow; and 9—Bone.
HAC is laid in the foetal period in which its regeneration is possible. After birth, there is moderate regenerative activity in the HAC in childhood. In adults, the HAC is not regenerated, except if injuries affect the subchondral bone, when regeneration is possible due to the proliferation of poorly differentiated osteogenic cells located in the zone of the blood capillaries of bone tissue, and their subsequent differentiation in two directions: into bone and cartilage cells. In such cases, the regeneration process is not comprehensive because fibrous cartilage tissue is formed, the mechanical properties of which are worse than the hyaline one [7][8][9].
Macroscopically, HAC has a homogeneous, opalescent appearance with a bluish tint. Morphologically, HAC is a special connective tissue consisting of cells (chondroblasts and chondrocytes) and an extracellular matrix (ECM), which they produce. The ECM is formed by glycosaminoglycans, glycoproteins, collagen and elastin fibres, and water [3][10][11].
In HAC, chondrocytes are located in the lacunas and completely fill them. Chondrocytes synthesize and secrete all components of the surrounding ECM. In the adult organism, chondrocytes do not divide because they are long-lived and age with the body. Chondroblasts, or perichondrial cells, are mesenchymal progenitor cells that form chondrocytes in the growing ECM as a result of endochondral ossification. Chondroblasts are the youngest cells in the HAC and they are capable of mitosis [12][13][14].
The predominant biopolymer of ECM is collagen type II. Collagens of types IX, X, and XI are identified in small amounts. The tensile strength of cartilage is conditioned by collagen. As we age, the water content of cartilage and the number of bonds between collagen molecules gradually decrease. As a result, cartilage tissue becomes less elastic and has less resistance to stretching, twisting, and compression loads. In other words, as we age, the cartilage becomes more vulnerable to damage [15][16][17][18].
Glycosaminoglycans are also identified in significant amounts in the cartilage ECM. They form macromolecular aggregates that bind water. Cartilage owes its resistance to pressure to the ability of ECM glycosaminoglycans to attract and retain water. The most characteristic glycosaminoglycans for HAC are chondroitin sulfate and keratan sulfate. The core of the structure of glycosaminoglycans is a giant molecule of such biopolymer as hyaluronic acid. Large molecules of the glycoprotein chondronektin also control the consistency of the surrounding ECM [19][20][21][22].
The ECM of living cartilage contains macromolecule-bound water, which provides elasticity to cartilage tissue and diffusion. The latter is the only way for nutrient and hormone intake into the chondrocytes, as well as metabolite removing and gas exchanging [10][23].
In general, the HAC is a highly organized striated structure in which cells (chondrocytes and chondroblasts) and ECM components, such as collagen fibres and glycosaminoglycan molecules, are arranged in a strict order, depending on the depth of the cartilage zone. Anatomically, four such zones are distinguished:
-
Superficial;
-
Transitional;
-
Deep;
-
Calcified.
Each zone has a different composition of the ECM, structural organization, and cell density. Such a unique anatomical structure of the HAC determines the gradient of its physical, mechanical, and biological properties. Additionally, the synergistic functionality of the HAC and subchondral bone is very important. The bone–cartilage interface in joints is formed by different layers of bone and cartilage cells with a gradient of mechanical properties and cell organization [7][9][10][24].
The complex striated structure of the HAC, and the peculiarities of its trophic (absence of supracartilage as well as feeding vessels and nerves), cause constrained self-regeneration of the HAC [14][25][26][27]. Therefore, its destruction due to traumatic injuries or pathological processes, such as osteoarthritis and rheumatoid arthritis, is an actual problem, complemented by the wide prevalence of the pathology in the population [28][29][30][31][32].
Minor defects of the HAC after the slight injuries or in the early stages of a disease can be treated with physical therapy and medication. In the latter variant, along with anti-inflammatory and metabolic systemic therapy, local treatment is also important. An example is viscosupplementation or injection of hyaluronic acid preparations into the joint cavity for normalizing the intra-articular environment, as well as to restore such properties of synovial fluid as elasticity and viscosity. It has analgesic, anti-inflammatory, anabolic, and chondroprotective effects and stalls the progression of the disease. The effectiveness of viscosupplementation depends largely on the type of medication, its origin, production technology, and physical and chemical properties [33]. Cross-linked hyaluronic acid hydrogels have the most acceptable characteristics today [34][35][36]. However, as the defects progress, significant cartilage destruction occurs, which worsens the patient’s quality of life due to severe pain, stiffness, and swelling in the affected joints [31][37][38][39][40].
2. Regenerative Medicine and Tissue Engineering in the Treatment of Hyaline Articular Cartilage Injuries
New perspectives in solving the problem of HAC injuries for scientists and doctors turned out to be related to biomedicine and its advanced direction such as regenerative medicine and corresponding to its molecular–biological, cellular, tissue-engineering, and other closely related and interrelated fields of scientific research. At the heart of this field is the process of regeneration, which is the ability of living organisms to renew (physiological regeneration) or, over time, to repair damaged tissues and sometimes entire lost organs (reparative regeneration) [41][42][43][44].
Learning how to manage these capabilities means improving the quality of human life, prolonging working age, and reducing the cost of long-term treatment of patients with chronic diseases. The main exploratory directions of regenerative medicine are:
-
Stimulation of regeneration with bioactive factors accelerating cell reproduction, growth, and differentiation;
-
Cell therapy using stem cells;
-
Tissue engineering.
Thus, the main areas of research in regenerative medicine are related to the use of factors affecting cell growth and maturation; cell and tissue engineering, which is a new biomedical discipline involving the use of a combination of cells; and biomaterials and suitable biochemical and physicochemical factors, as well as engineering approaches, to restore, maintain, improve, or replace various types of biological tissue [45][46][47][48].
3. 3D Tissue Bioprinting
One of the promising areas of tissue engineering is biofabrication, which specializes in the research, development, and implementation of biologically modified processes and automation in the production of functional tissue and organ analogues. At the same time, the creation of bioanalogues occurs in vitro, through bioassembly, three-dimensional (3D) bioprinting (TDB), and several other methods, such as directed assembly, enzymatic assembly, and self-assembly and the subsequent processes of functionalization (“maturation”) of tissues [49][50][51][52][53][54][55]. A wide range of sources, including biomaterials of various origins and their derivatives, signalling molecules, and cells and their aggregates, are used to create biobased products [56][57][58][59].
Many approaches of automated assembly of tissue-engineered constructs by TDB methods were a qualitatively new step in biofabrication and a separate direction in 3D printing technology, whose emergence in the late 20th century paved the way for mainstream innovations in many areas, such as engineering, industry, art, education, and medicine [54][55][60][61][62][63]. In modern 3D printers, cartridges with print heads can move in three dimensions during the volume printing process and distribute various materials—polymers, metals, ceramics, and even chocolate—in space, forming, layer-by-layer, 3D objects [64][65]. The combination of technologies that create a 3D object by adding material in a layer-by-layer manner is called additive manufacturing [66][67][68][69].
In medicine, additive manufacturing technologies are used to manufacture disposable sterile instruments, including personalized instruments for a specific patient; to create individual dental structures, prostheses, and crowns in dentistry; for hearing aids and implants in otorhinolaryngology; in traumatology and orthopaedics—for printing prostheses, endoprostheses, and orthoses, taking into account anatomical features of patients; in 3D printing of dummies (phantoms) and organ models for the educational process; and for microfabrication, which allows for printing medical devices and parts of micron-sized instruments [61][66][70][71][72]. A qualitative breakthrough in 3D printing technology, which occurred at the beginning of the 21st century, allowed scientists to print volume constructs using biomaterials, living cells, and auxiliary components, and to further create on their basis fully functional analogues of living tissues and organs [73][74].
Compared to conventional 3D printing, there are some factors that complicate the production process in TDB. These include the proper selection and combination of biomaterials, cells, and signal molecules, as well as consideration of the technical complexities associated with the equipment used. Such issues require the interaction of technologies at the intersection of engineering, physics, and biomedicine. From a technical point of view, the TDB process involves three sequential steps: pre-bioprinting, bioprinting, and post-bioprinting [63][74][75].
-
Pre-bioprinting, or the “preparation” stage, includes computer modelling of the future 3D object; isolation and cultivation of cell cultures; and biomaterial adjustment.
-
Bioprinting, or the “production” stage, involves the creation of a volume tissue-engineered construct in a 3D bioprinter by “layer-by-layer” deposition of biomaterials, auxiliary components, and living cells on a substrate.
-
Post-bioprinting, or the “functionalization” stage, is necessary for the stabilization of the bioprinted construct and “maturation” of its cells; this stage is implemented in bioreactors, where basic structural and functional characteristics of a bioprinted construct such as mechanical strength, structural integrity, and others are formed.
Thus, preparing and implementing the TDB process and capturing printed products requires, at a minimum, the following critical components [74][76][77]:
-
Equipment: 3D scanner (appropriate medical diagnostic equipment—MSCT, MRI, and 3D X-ray), personal computer (specialist workplace), 3D bioprinter, cell and tissue bioreactors, and equipment for input and output certification.
-
3D printing job preparation software, CAD software for product design, and special utilities for converting from the DICOM format (MSCT and MRI data storage format) to 3D printing data format (STL files).
-
Cell culture, as well as biomaterials of a natural or synthetic origin (including their combinations), as the basis of the volume matrix (analogue of ECM), in which cells will be placed.
4. Vectors for the Development of Tridimensional Bioprinting of Hyaline Articular Cartilage
Today, TDB is one of the promising methods of additive manufacturing and biofabrication of complex volume constructs with given rheology and increased structural, mechanical, and biological properties for organ and tissue regeneration [78]. One of the advantages of additive manufacturing technology is the ability to produce personalized implantable individual constructs considering the anatomy, pathology, and biomechanical properties of the patient’s tissues [78][79].
Biofabrication and TDB, including the creation of HAC biosimilars and articular cartilage defect replacements, have made an impressive step forward in their development over the last quarter century since their emergence. It appeals to all aspects of the TDB technology—equipment, appropriate software, biomaterials, and their compounding, as well as methods of printing and functionalization of bioprinted volume constructs. In experiments involving laboratory animals and, in some countries, in clinical trials, products of the required scale are already being used, capable of replacing damage to HAC and the underlying subchondral bone with subsequent resorption and replacement of fibrous and even full-fledged HAC within the process of reparative chondrosteogenesis [80]. However, these approaches are still far from being implemented in clinical orthopaedics.
Further development and improvement of TMB approaches of HAC biosimilars are associated with the usage of biomaterials capable, according to the results of 3D printing, of reproducing the structure of ECM and its composition (internal structure, pore size, stiffness, and protein composition, including morphogenetic proteins—BMP-2, BMP-3, BMP-4, BMP-6, BMP-7, and the growth factors TGF-β, PDGF, IGF I, IGF II, bFGF, and aFGF) with maximum precision. The optimal sources for the fabrication of such tissue-engineered constructs are biomaterials of an allogeneic origin. They are more compatible with the tissue environment than other biomaterials, have inductive and conductive activity, are not toxic, and meet the criteria of reparative regeneration in terms of resorption with substitution by the newly formed original tissue. Moreover, during the first stages of tissue-engineered construct existence, its matrix made of allogenic materials excellently plays a nutrient medium role for the cell spheroids contained therein, ensuring their proliferation and differentiation. Considering the influence of mechanical loading factors and oxygen concentration on the indicated processes in HAC, the stage of post-bioprinting with the tissue bioreactor’s usage gains particular interest to researchers.
A significant breakthrough in tissue biofabrication was the development of the technology of obtaining and creating decellularized ECM (dECM). However, a personalized approach to its use, as applied to the elimination of defects in the area of HAC and subchondral bone, turned out to be difficult. The way out was the transformation of dECM into the form of the hydrogel, using the latter to build volume constructs by the TDB method. However, until now, hydrogels made of dECM and 3D bioprinted constructs based on them cannot fully reproduce the complex structure and composition of native ECM of HAC, since the specific spatial position of each unique protein and even their composition are violated in the process of transformation of dECM into a hydrogel. Accordingly, improving the available physical, mechanical, and biochemical methods of creating hydrogels from allogenic biomaterials should also become a point of research efforts.
The hydrogels obtained from dECM are represented prevalently by collagen, the main protein of ECM of HAC, which, unfortunately, is characterized by low chondrogenic activity, weak mechanical strength, and significant shrinkage after TDB and implantation. To improve the mechanical properties and biological activity of the collagen-based volume matrix, its properties could be modified by mixing with other biopolymer molecules (e.g., chitosan or synthetic polymers). However, the possibilities of using additional materials are limited due to collagen easily denaturing under the influence of various factors. In this aspect, the concept of a hybrid TDB using temporary sacrificial structures, internal reinforcement, and modular assembly has good prospects. The configuration of hybrid scaffolds may be the optimal solution to improve the mechanical and structural components of 3D collagen-based dECM constructs without compromising their bioactivity and compatibility with cells.
References
- Firner, S.; Zaucke, F.; Michael, J.; Dargel, J.; Schiwy-Bochat, K.H.; Heilig, J.; Rothschild, M.A.; Eysel, P.; Brüggemann, G.P.; Niehoff, A. Extracellular Distribution of Collagen II and Perifibrillar Adapter Proteins in Healthy and Osteoarthritic Human Knee Joint Cartilage. J. Histochem. Cytochem. 2017, 65, 593–606.
- Kheir, E.; Shaw, D. Hyaline Articular Cartilage. Orthop. Trauma 2009, 23, 450–455.
- Beddoes, C.M.; Whitehouse, M.R.; Briscoe, W.H.; Su, B. Hydrogels as a Replacement Material for Damaged Articular Hyaline Cartilage. Materials 2016, 9, 443.
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nature Rev. Dis. Prim. 2016, 2, 16072.
- Lories, R.J.; Luyten, F.P. The Bone-Cartilage Unit in Osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 43–49.
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The Basic Science of the Subchondral Bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433.
- Lepage, S.I.M.; Robson, N.; Gilmore, H.; Davis, O.; Hooper, A.; St John, S.; Kamesan, V.; Gelis, P.; Carvajal, D.; Hurtig, M.; et al. Beyond Cartilage Repair: The Role of the Osteochondral Unit in Joint Health and Disease. Tissue Eng. Part B Rev. 2019, 25, 114–125.
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular Fibrocartilage—Why Does Hyaline Cartilage Fail to Repair? Adv. Drug Deliv. Rev. 2019, 146, 289–305.
- Karuppal, R. Current Concepts in the Articular Cartilage Repair and Regeneration. J. Orthop. 2017, 14, A1–A3.
- Bergholt, M.S.; Serio, A.; Albro, M.B. Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front. Bioeng. Biotechnol. 2019, 7, 303.
- Manou, D.; Caon, I.; Bouris, P.; Triantaphyllidou, I.E.; Giaroni, C.; Passi, A.; Karamanos, N.K.; Vigetti, D.; Theocharis, A.D. The Complex Interplay between Extracellular Matrix and Cells in Tissues. Methods Mol. Biol. 2019, 1952, 1–20.
- Poole, C.A. Articular Cartilage Chondrons: Form, Function and Failure. J. Anat. 1997, 191, 1–13.
- Kvist, A.J.; Nyström, A.; Hultenby, K.; Sasaki, T.; Talts, J.F.; Aspberg, A. The Major Basement Membrane Components Localize to the Chondrocyte Pericellular Matrix--a Cartilage Basement Membrane Equivalent? Matrix Biol. 2008, 27, 22–33.
- Carballo, C.B.; Nakagawa, Y.; Sekiya, I.; Rodeo, S.A. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425.
- Poole, A.R.; Kobayashi, M.; Yasuda, T.; Laverty, S.; Mwale, F.; Kojima, T.; Sakai, T.; Wahl, C.; El-Maadawy, S.; Webb, G.; et al. Type II Collagen Degradation and Its Regulation in Articular Cartilage in Osteoarthritis. Ann. Rheum. Dis. 2002, 61 (Suppl. 2), ii78–ii81.
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958.
- Sharma, U.; Carrique, L.; Vadon-Le Goff, S.; Mariano, N.; Georges, R.N.; Delolme, F.; Koivunen, P.; Myllyharju, J.; Moali, C.; Aghajari, N.; et al. Structural Basis of Homo- and Heterotrimerization of Collagen I. Nat. Commun. 2017, 8, 14671.
- Stoilov, I.; Starcher, B.C.; Mecham, R.P.; Broekelmann, T.J. Measurement of Elastin, Collagen, and Total Protein Levels in Tissues. Methods Cell Biol. 2018, 143, 133–146.
- Pfeiffer, E.; Vickers, S.M.; Frank, E.; Grodzinsky, A.J.; Spector, M. The Effects of Glycosaminoglycan Content on the Compressive Modulus of Cartilage Engineered in Type II Collagen Scaffolds. Osteoarthr. Cartil. 2008, 16, 1237–1244.
- Deshmukh, A.S.; Murgia, M.; Nagaraj, N.; Treebak, J.T.; Cox, J.; Mann, M. Deep Proteomics of Mouse Skeletal Muscle Enables Quantitation of Protein Isoforms, Metabolic Pathways, and Transcription Factors. Mol. Cell. Proteom. 2015, 14, 841–853.
- Hosseininia, S.; Önnerfjord, P.; Dahlberg, L.E. Targeted Proteomics of Hip Articular Cartilage in OA and Fracture Patients. J. Orthop. Res. 2019, 37, 131–135.
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug. Deliv. Rev. 2016, 97, 4–27.
- Garantziotis, S.; Savani, R.C. Hyaluronan Biology: A Complex Balancing Act of Structure, Function, Location and Context. Matrix Biol. 2019, 78–79, 1–10.
- Shim, J.H.; Jang, K.M.; Hahn, S.K.; Park, J.Y.; Jung, H.; Oh, K.; Park, K.M.; Yeom, J.; Park, S.H.; Kim, S.W.; et al. Three-Dimensional Bioprinting of Multilayered Constructs Containing Human Mesenchymal Stromal Cells for Osteochondral Tissue Regeneration in the Rabbit Knee Joint. Biofabrication 2016, 8, 014102.
- Medvedeva, E.V.; Grebenik, E.A.; Gornostaeva, S.N.; Telpuhov, V.I.; Lychagin, A.V.; Timashev, P.S.; Chagin, A.S. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366.
- Zhang, S.; Hu, B.; Liu, W.; Wang, P.; Lv, X.; Chen, S.; Liu, H.; Shao, Z. Articular Cartilage Regeneration: The Role of Endogenous Mesenchymal Stem/Progenitor Cell Recruitment and Migration. Semin. Arthritis Rheum. 2020, 50, 198–208.
- Guettler, J.H.; Demetropoulos, C.K.; Yang, K.H.; Jurist, K.A. Osteochondral defects in the human knee: Influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am. J. Sports Med. 2004, 32, 1451–1458.
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Polymers (Basel) 2019, 27, 1578–1589.
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020, 72, 220–233.
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The Global Burden of Hip and Knee Osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1323–1330.
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee Osteoarthritis Has Doubled in Prevalence since the Mid-20th Century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336.
- Peck, J.; Slovek, A.; Miro, P.; Vij, N.; Traube, B.; Lee, C.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Sherman, W.F.; et al. A Comprehensive Review of Viscosupplementation in Osteoarthritis of the Knee. Orthop. Rev. 2021, 13, 25549.
- Burdick, J.A.; Chung, C. Influence of Three-Dimensional Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254.
- Zheng, Z.; Patel, M.; Patel, R. Hyaluronic Acid-Based Materials for Bone Regeneration: A Review. React. Funct. Polym. 2022, 171, 105151.
- Felson, D.T. Clinical practice. Osteoarthritis of the knee. N. Engl. J. Med. 2006, 354, 841–848.
- Collins, J.E.; Losina, E.; Nevitt, M.C.; Roemer, F.W.; Guermazi, A.; Lynch, J.A.; Katz, J.N.; Kent Kwoh, C.; Kraus, V.B.; Hunter, D.J. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016, 68, 2422–2431.
- Rim, Y.A.; Ju, J.H. The Role of Fibrosis in Osteoarthritis Progression. Life 2021, 11, 3.
- Remst, D.F.G.; Davidson, E.N.B.; Van Der Kraan, P.M. Unravelling Osteoarthritis-Related Synovial Fibrosis: A Step Closer to Solving Joint Stiffness. Rheumatology (Oxford) 2015, 54, 1954–1963.
- Li, D.; Wang, H.; He, J.Y.; Wang, C.L.; Feng, W.J.; Shen, C.; Zhu, J.F.; Wang, D.L.; Chen, X.D. Inflammatory and Fibrosis Infiltration in Synovium Associated with the Progression in Developmental Dysplasia of the Hip. Mol. Med. Rep. 2019, 19, 2808–2816.
- Ashammakhi, N.; Ahadian, S.; Pountos, I.; Hu, S.K.; Tellisi, N.; Bandaru, P.; Ostrovidov, S.; Dokmeci, M.R.; Khademhosseini, A. In Situ Three-Dimensional Printing for Reparative and Regenerative Therapy. Biomed. Microdevices 2019, 21, 42.
- Cohen, D.L.; Lipton, J.I.; Bonassar, L.J.; Lipson, H. Additive Manufacturing for in Situ Repair of Osteochondral Defects. Biofabrication 2010, 2, 035004.
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal Stem Cell Therapy in the Treatment of Osteoarthritis: Reparative Pathways, Safety and Efficacy—A Review. BMC Musculoskelet. Disord. 2016, 17, 230.
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430.
- Madeira, C.; Santhagunam, A.; Salgueiro, J.B.; Cabral, J.M.S. Advanced Cell Therapies for Articular Cartilage Regeneration. Trends Biotechnol. 2015, 33, 35–42.
- Facchin, F.; Bianconi, E.; Canaider, S.; Basoli, V.; Biava, P.M.; Ventura, C. Tissue Regeneration without Stem Cell Transplantation: Self-Healing Potential from Ancestral Chemistry and Physical Energies. Stem Cells Int. 2018, 3, 7412035.
- Yang, Z.; Li, H.; Yuan, Z.; Fu, L.; Jiang, S.; Gao, C.; Wang, F.; Zha, K.; Tian, G.; Sun, Z.; et al. Endogenous Cell Recruitment Strategy for Articular Cartilage Regeneration. Acta Biomater. 2020, 114, 31–52.
- Moraes, V.Y.; Lenza, M.; Tamaoki, M.J.; Faloppa, F.; Belloti, J.C. Platelet-Rich Therapies for Musculoskeletal Soft Tissue Injuries. Cochrane Database Syst. Rev. 2013, 12, CD010071.
- Lee, J.K.; Link, J.M.; Hu, J.C.Y.; Athanasiou, K.A. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb. Perspect. Med. 2017, 7, a025668.
- Schon, B.S.; Hooper, G.J.; Woodfield, T.B.F. Modular Tissue Assembly Strategies for Biofabrication of Engineered Cartilage. Ann. Biomed. Eng. 2016, 45, 100–114.
- Jakab, K.; Norotte, C.; Marga, F.; Murphy, K.; Vunjak-Novakovic, G.; Forgacs, G. Tissue Engineering by Self-Assembly and Bio-Printing of Living Cells. Biofabrication 2010, 2, 022001.
- Mekhileri, N.V.; Lim, K.S.; Brown, G.C.J.; Mutreja, I.; Schon, B.S.; Hooper, G.J.; Woodfield, T.B.F. Automated 3D Bioassembly of Micro-Tissues for Biofabrication of Hybrid Tissue Engineered Constructs. Biofabrication 2018, 10, 024103.
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the Definition of an Evolving Field. Biofabrication 2016, 8, 013001.
- Shishkovsky, I.; Volchkov, S.; Scherbakov, V.; Volova, L. Biocompatible Features of Magnetic Nano-Oxide Core/ PCL Shell 3D Composites Fabricated via SLS/M Process. Adv. Mater. Lett. 2018, 9, 31–35.
- Shishkovsky, I.V. Fundamentals of Additive Technologies of High Resolutions; Peter Publish: Sankt-Peterburg, Russia, 2016; p. 400. Available online: http://fian.smr.ru/old-rp/presentations/Basis%20of%20Additive%20Manufacturing-RU-Shishkovsky2016.pdf (accessed on 7 June 2023).
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E. Advanced Strategies for Tissue Engineering in Regenerative Medicine: A Biofabrication and Biopolymer Perspective. Molecules 2021, 26, 2518.
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407.
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D Printing of Functional Biomaterials for Tissue Engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112.
- Vining, K.H.; Mooney, D.J. Mechanical Forces Direct Stem Cell Behaviour in Development and Regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742.
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404.
- Ford, S.; Minshall, T. Invited Review Article: Where and How 3D Printing Is Used in Teaching and Education. Addit. Manuf. 2019, 25, 131–150.
- Campbell, I.; Diegel, O.; Kowen, J.; Wohlers, T. Wohlers Report 2018: 3D Printing and Additive Manufacturing State of the Industry: Annual Worldwide Progress Report, 23rd ed.; Wohlers: Hamburg, Germany, 2018; p. 343. Available online: https://researchspace.auckland.ac.nz/docs/uoa-docs/rights.htm (accessed on 7 June 2023).
- Michalski, M.H.; Ross, J.S. The Shape of Things to Come: 3D Printing in Medicine. JAMA 2014, 312, 2213–2214.
- Mendes, L.; Kangas, A.; Kukko, K.; Mølgaard, B.; Säämänen, A.; Kanerva, T.; Flores Ituarte, I.; Huhtiniemi, M.; Stockmann-Juvala, H.; Partanen, J.; et al. Characterization of Emissions from a Desktop 3D Printer. J. Ind. Ecol. 2017, 21, S94–S106.
- Shahrubudina, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296.
- Huang, S.H.; Liu, P.; Mokasdar, A.; Hou, L. Additive Manufacturing and Its Societal Impact: A Literature Review. Int. J. Adv. Manuf. Technol. 2013, 67, 1191–1203.
- Gupta, N.; Weber, C.; Newsome, S. Additive Manufacturing: Status and Opportunities; Science and Technology Policy Institute: Washington, DC, USA, 2012; Available online: https://www.researchgate.net/publication/312153354_Additive_Manufacturing_Status_and_Opportunities (accessed on 7 June 2023).
- Goh, G.D.; Sing, S.L.; Yeong, W.Y. A Review on Machine Learning in 3D Printing: Applications, Potential, and Challenges. Artif. Intell. Rev. 2021, 54, 63–94.
- Urhal, P.; Weightman, A.; Diver, C.; Bartolo, P. Robot Assisted Additive Manufacturing: A Review. Robot Comput. Integr. Manuf. 2019, 59, 335–345.
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D Printing in Dentistry. Br. Dent. J. 2015, 219, 521–529.
- Rahman, Z.; Charoo, N.A.; Kuttolamadom, M.; Asadi, A.; Khan, M.A. Printing of Personalized Medication Using Binder Jetting 3D Printer. Precis. Med. Investig. Pract. Provid. 2020, 3, 473–481.
- Ho, C.M.B.; Ng, S.H.; Yoon, Y.J. A Review on 3D Printed Bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046.
- Naveau, A.; Smirani, R.; Catros, S.; de Oliveira, H.; Fricain, J.C.; Devillard, R. A Bibliometric Study to Assess Bioprinting Evolution. Appl. Sci. 2017, 7, 1331.
- Duarte Campos, D.F.; Blaeser, A. 3D-Bioprinting. In Basic Concepts on 3D Cell Culture; Springer International Publishing: Cham, Switzerland, 2021; pp. 201–232.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2016, 34, 422–434.
- Ahn, S.H.; Lee, J.; Park, S.A.; Kim, W.D. Three-Dimensional Bio-Printing Equipment Technologies for Tissue Engineering and Regenerative Medicine. Tissue Eng. Regen. Med. 2016, 13, 663–676.
- Gu, B.K.; Choi, D.J.; Park, S.J.; Kim, Y.J.; Kim, C.H. 3D Bioprinting Technologies for Tissue Engineering Applications. Adv. Exp. Med. Biol. 2018, 1078, 15–28.
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-Based Bioinks for Hard Tissue Engineering Applications: A Comprehensive Review. J. Mater. Sci. Mater. Med. 2019, 30, 32.
- Nagarajan, N.; Dupret-Bories, A.; Karabulut, E.; Zorlutuna, P.; Vrana, N.E. Enabling Personalized Implant and Controllable Biosystem Development through 3D Printing. Biotechnol. Adv. 2018, 36, 521–533.
- Irawan, V.; Sung, T.C.; Higuchi, A.; Ikoma, T. Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng. Regen. Med. 2018, 15, 673–697.
More
Information
Subjects:
Cell & Tissue Engineering
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
726
Revisions:
2 times
(View History)
Update Date:
27 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




