
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Grazia Maria Liuzzi | -- | 1466 | 2023-06-26 18:48:00 | | | |
| 2 | Lindsay Dong | Meta information modification | 1466 | 2023-06-27 02:54:22 | | |
Video Upload Options
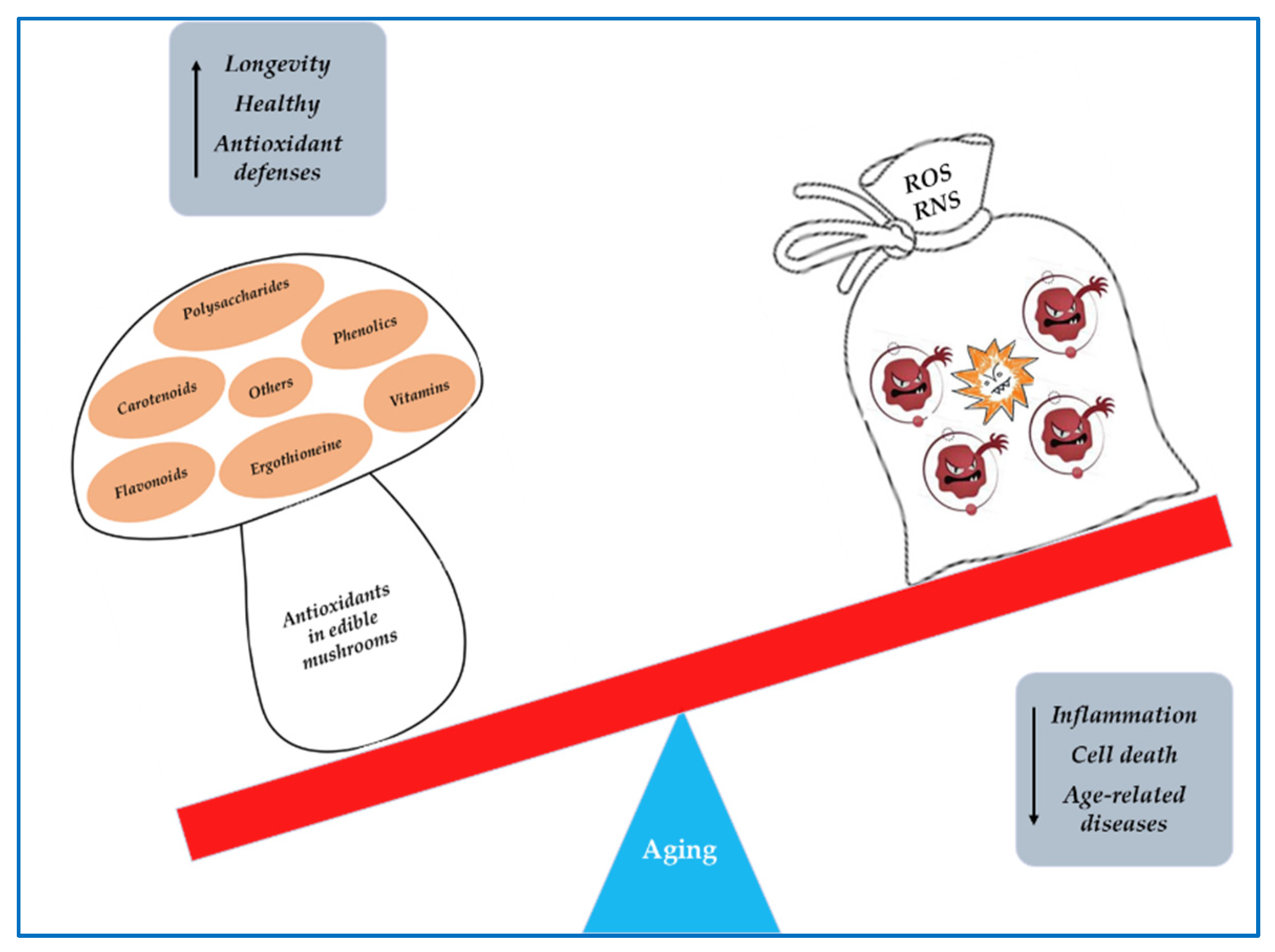
Mushrooms have been used for their nutritional value and medicinal properties. They therefore represent not only a food but also a precious source of biologically active compounds that act as nutraceuticals. Numerous studies have shown that edible mushrooms possess anticancer, anti-atherosclerotic, hypocholesterolemic, hypolipidemic, antiviral, antimicrobial, immunostimulant, anti-inflammatory, antioxidant, and anti-aging effects. The antioxidant properties of edible mushrooms are mainly related to their content in phenolic compounds and polysaccharides. Among polyphenol groups, phenolic acids are the main antioxidants, whereas the major antioxidant effects of polysaccharides are attributed to beta-glycans. These compounds show significant reactive oxygen species (ROS) scavenging activity and are also able to stimulate the activity of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) enzymes.
1. Mushrooms as Sources of Antioxidant Compounds
2. Oxidative stress in aging and age-related neurodegenerative diseases
Alzheimer’s Disease
Parkinson’s Disease
3. Antioxidant Compounds of Mushrooms as Neuroprotective Agents
References
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A comprehensive review on bioactive compounds with health benefits and processing aspects. Foods 2021, 10, 2996.
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible mushrooms as functional ingredients for development of healthier and more sustainable muscle foods: A flexitarian approach. Molecules 2021, 26, 2463.
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Gimenez, A. Supplementation in mushroom crops and its impact on yield and quality. AMB Express 2018, 8, 146.
- Li, H.; Tian, Y.; Menolli, N.; Ye, L.; Karunarathna, S.C.; Perez-Moreno, J.; Rahman, M.M.; Rashid, M.H.; Phengsintham, P.; Rizal, L.; et al. Reviewing the world’s edible mushroom species: A new evidence-based classification system. Comprehensive Reviews. Food Sci. Food Saf. 2021, 20, 1982–2014.
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599.
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. Pharma Nutr. 2017, 5, 35–46.
- Gaoxing, M.; Wenjian, Y.; Liyan, Z.; Fei, P.; Donglu, F.; Qiuhui, H. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness. 2018, 7, 125–133.
- You, S.W.; Hoskin, R.T.; Komarnytsky, S.; Moncada, M. Mushrooms as functional and nutritious food ingredients for multiple applications. ACS Food Sci. Technol. 2022, 2, 1184–1195.
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trend Food Sci. Technol. 2017, 66, 48–62.
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525.
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134.
- Lindequist, U.; Niedermayer, T.H.J.; Julich, W.D. The pharmacological potential of mushrooms. Evid. Based Complement. Alternat. Med. 2005, 2, 285–299.
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274.
- Feeney, M.J.; Dwyer, J.; Hasler-Lewis, C.M.; Milner, J.A.; Noakes, M.; Rowe, S.; Wach, M.; Beelman, R.B.; Caldwell, J.; Cantorna, M.T. Mushrooms and health summit proceedings. J. Nutr. 2014, 144, 1128S–1136S.
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173.
- Islam, T.; Ganesan, K.; Xu, B. New insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A Review. Int. J. Med. Mushrooms 2019, 21, 237–251.
- Petraglia, T.; Latronico, T.; Liuzzi, G.M.; Fanigliulo, A.; Crescenzi, A.; Rossano, R. Edible mushrooms as source of fibrin (ogen) olytic enzymes: Comparison between four cultivated species. Molecules 2022, 27, 8145.
- Slusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A Review. Nutrients 2021, 13, 3178.
- Dubost, N.J.; Ou, B.; Beelman, R.B. Quantification of polyphenols and ergothioneine in cultivated mushrooms and correlation to total antioxidant capacity. Food Chem. 2007, 105, 727–735.
- Sanchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22.
- Sganzerla, W.G.; Todorov, S.D.; da Silva, A.P.G. Research trends in the study of edible mushrooms: Nutritional properties and health benefits. Int. J. Med. Mushrooms 2022, 24, 1–18.
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344.
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639.
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer’s disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172.
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641.
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53.
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325.
- Obulesu, M.; Venu, R.; Somashekhar, R. Lipid peroxidation in Alzheimer’s disease: Emphasis on metal-mediated neurotoxicity. Acta Neurol. Scand. 2011, 124, 295–301.
- Mantyh, P.W.; Ghilardi, J.R.; Rogers, S.; DeMaster, E.; Allen, C.J.; Stimson, E.R.; Maggio, J.E. Aluminum, Iron, and Zinc Ions Promote Aggregation of Physiological Concentrations of Β-amyloid Peptide. J. Neurochem. 1993, 61, 1171–1174.
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121.
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772.
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol. Neurobiol. 2016, 53, 648–661.
- Forno, L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp Neurol. 1996, 55, 259–272.
- Spillantini, M.G.; Goedert, M. The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann. N. Y. Acad. Sci. 2000, 920, 16–27.
- Danielson, S.R.; Andersen, J.K. Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radic. Biol. Med. 2008, 44, 1787–1794.
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174.
- Abe, T.; Isobe, C.; Murata, T.; Sato, C.; Tohgi, H. Alteration of 8-hydroxyguanosine concentrations in the cerebrospinal fluid and serum from patients with Parkinson’s disease. Neurosci. Lett. 2003, 336, 105–108.
- Zhang, J.; Perry, G.; Smith, M.A.; Robertson, D.; Olson, S.J.; Graham, D.G.; Montine, T.J. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999, 154, 1423–1429.
- Burai, R.; Ait-Bouziad, N.; Chiki, A.; Lashuel, H.A. Elucidating the role of site-specific nitration of α-synuclein in the pathogenesis of Parkinson’s disease via protein semisynthesis and mutagenesis. J. Am. Chem. Soc. 2015, 137, 5041–5052.
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell. 2019, 18, e13031.
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative sress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583.
- Dalfó, E.; Ferrer, I. Early alpha-synuclein lipoxidation in neocortex in Lewy body diseases. Neurobiol. Aging 2008, 29, 408–417.
- Castellani, R.; Smith, M.A.; Richey, P.L.; Perry, G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996, 737, 195–200.
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A Mechanistic Review on Medicinal Mushrooms-Derived Bioactive Compounds: Potential Mycotherapy Candidates for Alleviating Neurological Disorders. Planta Med. 2020, 86, 1161–1175.
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621.
- Wei, S.; Van Griensven, L.J.L.D. Pro-and antioxidative properties of medicinal mushroom extracts. Int. J. Med. Mushrooms 2008, 10, 315–324.
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560.
- Cheng, J.H.; Tsai, C.L.; Lien, Y.Y.; Lee, M.S.; Sheu, S.C. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement. Altern. Med. 2016, 16, 170.
- Zhang, J.; An, S.; Hu, W.; Teng, M.; Wang, X.; Qu, Y.; Liu, Y.; Yuan, Y.; Wang, D. The Neuroprotective Properties of Hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer’s disease mouse model. Int. J. Mol. Sci. 2016, 17, 1810.
- Kushairi, N.; Phan, C.W.; Sabaratnam, V.; David, P.; Naidu, M. Lion’s Mane Mushroom, Hericium erinaceus (Bull.: Fr.) Pers. Suppresses H2O2-induced oxidative damage and LPS-induced inflammation in HT22 hippocampal neurons and BV2 microglia. Antioxidants 2019, 8, 261.
- An, S.; Lu, W.; Zhang, Y.; Yuan, Q.; Wang, D. Pharmacological basis for use of Armillaria mellea polysaccharides in Alzheimer’s Disease: Antiapoptosis and antioxidation. Oxid. Med. Cell. Longev. 2017, 2017, 4184562.
- Zhang, Y.; Yang, X.; Jin, G.; Yang, X.; Zhang, Y. Polysaccharides from Pleurotus ostreatus alleviate cognitive impairment in a rat model of Alzheimer’s disease. Int. J. Biol. Macromol. 2016, 92, 935–941.
- Chen, Z.; Tang, Y.; Liu, A.; Jin, X.; Zhu, J.; Lu, X. Oral administration of Grifola frondosa polysaccharides improves memory impairment in aged rats via antioxidant action. Mol. Nutr. Food Res. 2017, 61, 1700313.
- Tripodi, F.; Falletta, E.; Leri, M.; Angeloni, C.; Beghelli, D.; Giusti, L.; Milanesi, R.; Sampaio-Marques, B.; Ludovico, P.; Goppa, L.; et al. Anti-aging and neuroprotective properties of Grifola frondosa and Hericium erinaceus extracts. Nutrients 2022, 14, 4368.




