1. Nanoemulsions for Neurological Disorders
Neurological disorders include a broad range of disabilities that progressively impair the nervous system associated with vital functions of the body. i.e., mobility, sensation, coordination, reasoning, and learning
[1]. Epilepsy, autism, Parkinson’s, Alzheimer’s and other neuromuscular disorders are just a few to be named in this category. These specific ailments require precise delivery of therapeutic agents inside the brain for effective results. BBB presents a formidable hurdle that limits the efficacy of conventional formulations
[2]. For instance, acetylcholinesterase inhibitors such as rivastigmine, memantine and galantamine have the potential to manage Alzheimer’s disease but possess poor brain targeting owing to theirerratic pharmacokinetic and pharmacodynamic profiles
[3]. Nanosized nanoemulsion and its structural architecture facilitate site-specific targeted drug release with minimum adverse effects.
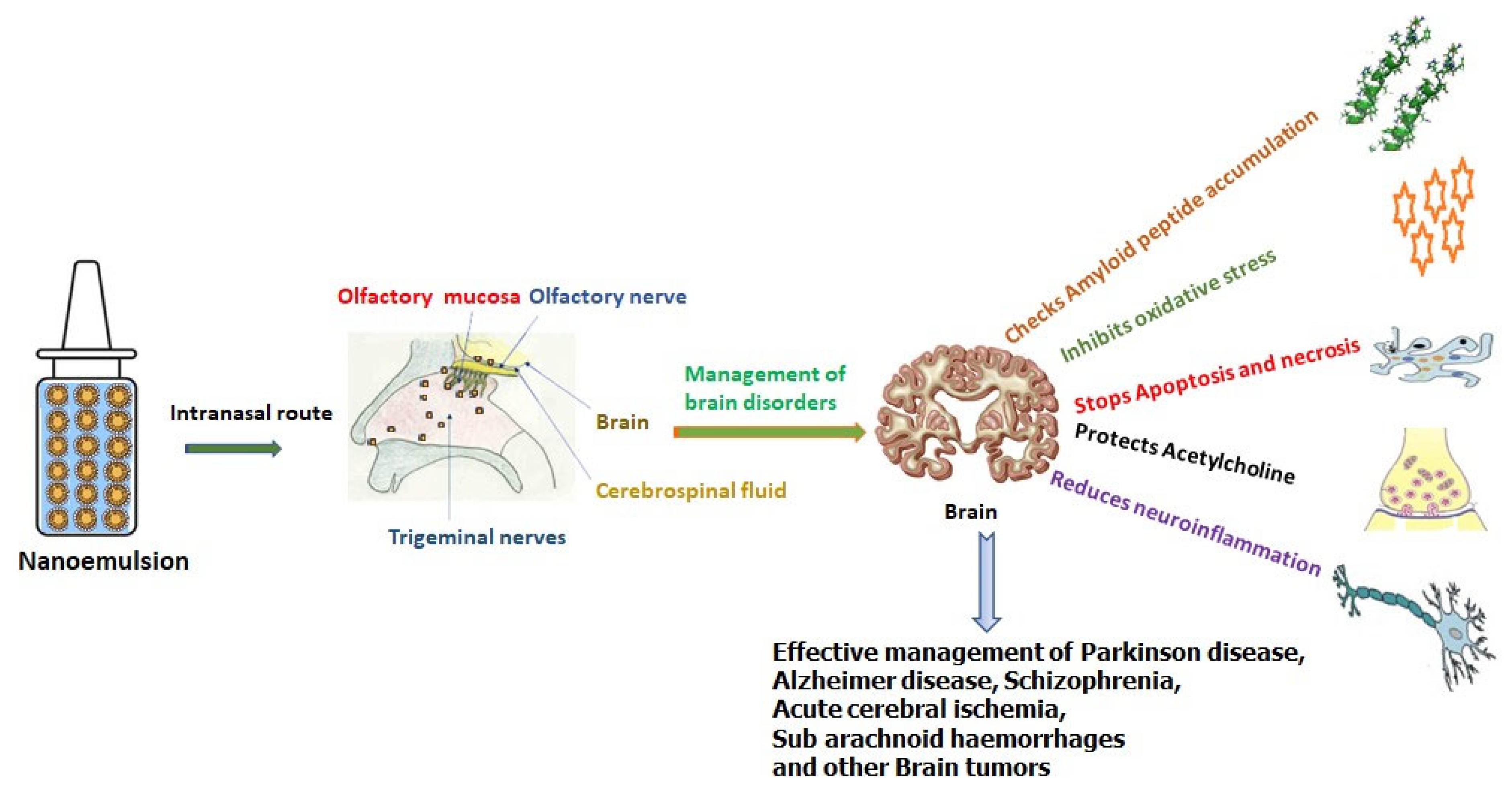
Figure 1 summarizes the role of nanoemulsions in the management of brain disorders.
Figure 1. Intra nasal delivered nanoemulsion and management of brain disorders.
Jiang et al. (2022) explored herbal huperzine for the management of the neurological issue. i.e., Alzheimer’s disorder. Huperzine is obtained from
Huperzia serrata, a Chinese club moss, and has poor brain transportation. Performed pharmacokinetic studies displayed greater mean residence time of modified Lf-hup-NE (4.07 h) compared to non-modified nanoemulsion (3.03 h). Similarly, Cmax and AUC
0–t of Lf-hup-NE were also higher. i.e., ~52.29 ng/mL and ~245.09 ng/mL X h compared to developed non-modified nanoemulsions ~50.54 ng/mL and 123.63 ng/mL Xh, respectively. A rat model olfactory nerve transaction was designed to observe nanoemulsion transportation via the nose-to-brain route. Of the nanoemulsion, 50µLwas intranasal administered both in olfactory nerve transacted rats and normal rats, and in vivo fluorescence images of treated rats were collected that depicted retention of the drug in brain regions. The results confirmed successful drug absorption and transportation of hup-NE and modified Lf-hup-NE via blood circulation that implicated both direct and indirect drug delivery. P2 signals intensity of modified Lf-hup-NE was high owing to its greater efficiency of translocation in brain areas through intranasal delivery. Further, the developed modified Lf-hup-NE had the potential to inhibit P-gp efflux protein and enhanced drug concentration there. This novel formulation can be exploited for better transportation and accumulation of herbal huperzine in brain cells and can achieve better therapeutic responses
[4].
One of the progressive neurodegenerative motor neuron disorders is amyotrophic lateral sclerosis. It is characterized by symptoms such as the depletion of upper and lower motor neurons. A BCS class II drug, riluzole, has limited bioavailability (60%) due to poor penetration across BBB. This drug is indicated for the management of amyotrophic lateral sclerosis (ALS). Parikh et al. (2016) formulated riluzole containing o/w nanoemulsion by phase titration method. Sefsol 218 and tween 80/carbitol (1:1) were employed as oil substitutes and surfactants, respectively. The developed nanoemulsion was thermodynamically stable with a drop size of ~23 nm. Further, the formulation was free of nasal ciliotoxicity and significantly increased brain uptake of riluzole (
p < 4.1 × 10
−6) via intranasal delivery on comparing with oral nanoemulsion to the Wistar albino rats. This novel nanoemulsion has displayed a promising alternative approach for the treatment of people living with ALS
[5].
Recently, nose-to-brain delivery of bromocriptine mesylate- and glutathione-embedded nanodimension emulsion was designed by Ashhar et al. (2022) for the effective treatment of another distinguished neurodegenerative Parkinson’s disease. This fatal disorder is provoked by the generation of free radicals in neurons (dopaminergic); consequently, oxidative stress-induced neuron degradation occurs. The researchers designed intranasal dosage for containing bromocriptine mesylate and glutathione-embedded nanoemulsion to relieve oxidative stress. Performed DPPH radical scavenging analysis revealed enhanced antioxidant action due to the combined effect of both bromocriptine mesylate and glutathione present in nanoemulsion. The formulated nanoemulsion was estimated for depth of permeation with confocal laser scanning microscopy after intranasal administration, which resulted in superior penetration to the brain cells. Further, the pharmacokinetic study estimated AUC
0–8 of nanoemulsion that revealed a higher concentration of both compounds in brain regions of the Wistar rat model after intranasal administration. The nasal ciliotoxicity study in Wistar rats explained the biocompatibility of the formulated nanoemulsion. Thereafter, the biochemical study displayed a reduced level of interleukin-6, alpha tumour necrosis factor and thiobarbituric acid, which are reactive substances, and concluded that bromocriptine mesylate- and glutathione-loaded nanoemulsion has the potential to overcome oxidative stress level in persons living with Parkinson’s disease
[6].
Another chronic CNS complaint: ‘epilepsy’ is frequently characterized by instant senseless seizures due to neurons’ electric instabilities in the brain region. BCS class II topiramate(anticonvulsant) has been employed for the clinical management of partial and generalized seizures. Its poor bioavailability on oral administration is due to poor entry across BBB. An o/w nanoemulsion was prepared utilizing Capmul MCM C8 (2%
w/
w) by Patel and coworkers (2020) for the improvement of the brain delivery of topiramate. A blend of 32% surfactant and co-surfactant Tween 20 and carbitol at a ratio of 2:1 was included to customize the globular size, PDI, zeta potential and viscosity. The resulting nanoemulsion possessed a ~4.73 nm mean particle size with a stability of six months. The brain uptake efficiency of the intranasally delivered piramate contained a nanoemulsion that was quite higher (
p < 1.8 × 10
−8) compared to the orally delivered nanoemulsion. The pharmacokinetic studies in Wistar albino rats depicted enhanced bioavailability with minimum adverse effects after delivering the nanoemulsion through the intranasal route
[7].
Mucoadhesive buspirone-loaded nanoemulsion was formulated for direct delivery to the brain and modification of bioavailability after administering through an intranasal route. A total of 5% of
w/
v hydroxypropyl beta-cyclodextrin and chitosan hydrochloride (1%
w/
v) were selected for the preparation of mucoadhesive nanoemulsion. The assay resulted in a 61% improvement in bioavailability, which exhibited peak plasma concentration in rats’ brains at 30 min lesser compared to bare buspirone nanoemulsion (60 min). Further, after nasal administration, buspirone–chitosan nanoemulsion exhibited 2.5 times higher AUC
0–480 in the brain (~711 ng/g) compared to I/V administration (~282 ng/g) and bare buspirone nasal formulation (~354 ng/g). Mucoadhesive buspirone–chitosan nanoemulsion revealed a high percentage of drug transport (75.77%) and targeting efficiency in the brain region
[8]. Recently, the BCS class II drug ‘melatonin’ was aimed to develop a mucoadhesive nanoemulsion to enhance brain bioavailability and alleviatedepression. The formulated nanoemulsion improved the poor aqueous solubility of melatonin, and added chitosan provided a mucoadhesive property that exhibited prolonged retention time (0.641 min) in the brain region. The locomotor activity of model rats exhibited improved behavioural responses
[9].
2. Nanoemulsions for Brain Tumour
Managing and treating brain tumours has always remained the most crucial and challenging task, as approximately 0.2 million clinical diagnoses of the brain and other associated CNS malignancies are reported worldwide. One of the WHO surveys stated that nearly 80%of primary brain tumours are concerned with the origin of gliomas (glial cells)
[10]. Glioblastoma multiforme, or GBM, is the most prominent and aggressive grade IV glioma that affects nearly8%of individuals in a population of 100,000. This malignant brain tumour is highly fatal, has a very low survival rate (1 year), and requires immediate surgery and other treatments, including neuroimaging and photodynamic/radiotherapy
[11]. Scientific reports confirmed that CD73 is accountable for the development of adenosine that is overexpressed in glioblastoma cells and hence targeted to treat this tumour in the brain region. Originally, surface enzyme CD73 was a biomarker and described as a lymphocyte differentiation antigen, and its inhibition checks glioblastoma pathogenesis
[12]. Failure in chemotherapy may be due to impaired therapeutic action via poor permeation across BBB. i.e., microvasculature environment of the CNS.
FDA-approved first-line antineoplastic therapeutic ‘temozolomide or temodar
®’ is widely employed for the management of glioblastoma multiforme or GBM owing to permeation efficacy across BBB. It is a DNA alkylating drug administered orally and intravenously due to its very short half-life. However, administering high doses leads to severe adverse toxicities, including cardiomyopathy, oral ulceration, myelo-suppression and hematological issues
[13]. To avoid these critical issues at present novel strategies such as topical implants, convection-enhanced delivery and nanotechnological-based formulations are clinically required for the management of brain gliomas. The last decade was the era of successful brain-targeted drug delivery approaches following a peculiar nasal path where trigeminal and olfactory nerves facilitate precise drug transportation due to the sole connection between the central nervous system and brain region. This non-invasive and safe route represents the quick onset of action with minimum systemic toxicity and adverse risks that comply with brain tumour sufferers. Moreover, low therapeutic doses and bypassing hepatic first-pass metabolism offer extramerits to the intranasal antineoplastic drug-delivery system.
However, half-life clearance (15–20 min) and limited volume of the formulation (25–200 µL) in a single dose pose strains and must be considered while formulating nose-to-brain delivery
[14].
Biocompatible lipidic nanoemulsions are a highly preferred delivery system for intranasal delivery and brain targeting. The extended residing time in the nasal cavity releases the adequate therapeutic agent from the nanostructured emulsion. Bayanati et al. (2021) designed in situ emulsion-based gel of antineoplastic drug temozolomide through low energy technique for the chemotherapy of glioblastoma. The formulation bypassed BBB after delivering through the intranasal route. A suitable pseudoternary phase diagram with various quantities of triacetin, labrasol (surfactant) and transcutol
®P (permeation enhancer) was established and was added for the preparation of the nanoemulsion. The nanoemulsion contained a range of particle sizes from 19–23 nm with PDI from 0.18–0.25. A slight positive zeta potential. i.e., ~1.6 might be due to the non-ionic nature of the selected surfactant/co-surfactant. By contrast, the developed in situ gel with a blend of amphiphilic poloxamer 407 and 188 exhibited a slightly increased mean particle size (16.25 nm) and PDI (~0.35) with an acceptable pH (~6.5) that showed a compatibility with nasal mucosa without irritation. The augmented viscosity (113.57 cp) of the formulation also advocated prolonged nasal retention due to reduced mucociliary clearance. Further, in vitro release efficiencies of both nanoemulsion and its in situ gel were nearly 90% and 87%, respectively, and appeared to be sustained compared to the nasal solution after 6 h. Noticeable risen in percentage mucoadhesion was observed in situ gel (37.03%) compared to nanoemulsion (20.35%) owing to the gel-forming amphiphilic poloxamer that might have formed a noncovalent entanglement with nasal mucosa. Further, the developed in situ nanoemulsion exhibited a 1.52 times higher permeation compared to the control solution. Added labrasol and transcutol
®P efficiently enhanced the solubility of temozolomide and worked as permeation enhancers across nasal olfactory–trigeminal pathways. Labrasol inhibited P-gp and acted as an efflux transporter. Both temozolomide nanoemulsion and gel with poloxamer 407 were stable during freeze-thaw cycles and performed centrifugation. Gamma scintigraphy revealed an accumulation of radio-labelled temozolomide in the brain after intranasal delivery and concluded efficient uptake to the affected site of the brain
[15].
An amalgamation of antineoplastic paclitaxel and C (6)-ceramide-loaded oil in water nanoemulsion was developed, including a high concentration of polyunsaturated fatty acid (pinenut oil) for enhanced therapeutic action against human U-118 glioblastoma cells. The average particle size of the nanoemulsion was observed at 200 nm. Epi-fluorescent microscopy was employed for the uptake and biodistribution of rhodamine-labelled paclitaxel and nitro-benzofurazone-labelled C (6)-ceramide nanoemulsion in glioblastoma cells. On intranasal administration, the developed nanoemulsion exhibited prominently high cytotoxicity and apoptosis within the malignant cells, thus suggesting a promising approach for the therapy of aggressive glioblastoma tumours
[16]. Disulfiram, an alcohol withdrawal drug, is an FDA-approved antineoplastic drug that has been clinically proven in various cancerous cases, including glioblastoma. Its inclusion complex with copper ions has already gained success in the management of adenosine over-expressive human brain tumour. Qu et al. (2021) designed a novel inclusion complex of disulfiram and hydroxypropyl –β-cyclodextrin with a copper ion that augmented drug solubility and antitumour activity with increased safety in vitro.
Disulfiram is considered an active antitumour agent while complexed with copper ions. Here, disufiram is entrapped within the structure of hydroxyl propyl β-cyclodextrin; thus, the solubility and safety profile of the drug were amplified. An intense fluorescence signal was observed in the Wistar male rat brain model that indicated extreme brain targeting via the intranasal delivery of the drug. Developed inclusion complex embedded nanoemulsion promoted apoptosis after intranasal administration, thus inhibiting cell proliferation and tumour growth. Furthermore, histopathological outcomes displayed nonobvious damage to normal cells
[17].
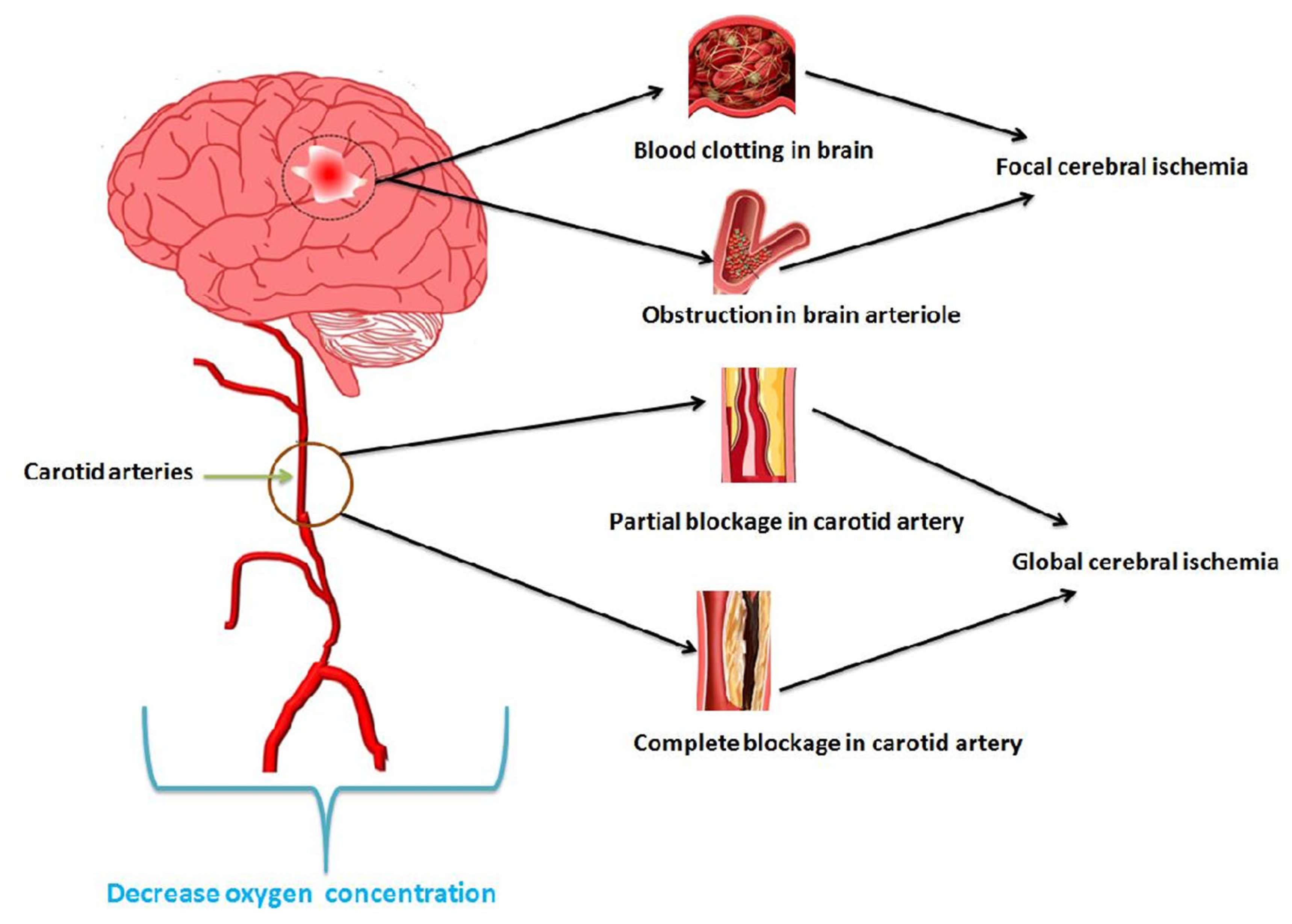
3. Nanoemulsions in Cerebral Ischemia
Cerebral ischemia is a condition aroused by acute brain injury that results in inadequate blood flow in the brain region. It is also a medical emergency that, if ignored, may lead to cerebral infarction and, ultimately, permanent brain disability. Broad cerebral ischemia is categorized as global and focal. The former happens due to shock and systemic hypotension.
Structural and functional cardiac issues, including arrhythmia, mediate global ischemia. At the same time, the obstruction of arterial blood flow (thrombosis or embolism) to the brain and the irreversible neuronal loss leads to focal ischemia. Nearly 60–70% of cerebral ischemia clinically reported is due to embolism (formation of a clot) in the heart or in a large artery
[18].
Figure 2 portrays focal and global cerebral ischemic conditions that arise due to oxygen deficiency in the brain.
Figure 2. Cerebral ischemia developed due to decreased oxygen concentration in carotid arteries.
Vitamin D3 entrapped nanoemulsion was formulated using a blend of Tween 20, PEG400 and oleic acid for the improvement in cerebral ischemia. The targeting potential of formulated nanoemulsion was analysed through gamma scintigraphy in a rat model. The developed nanoemulsion possessed an average globular size of ~49.29 nm with a positive 13.7 mV zeta potential. The stable thermodynamic preparation was found to have a permeation coefficient of 7.8 cm/h after 3 h in sheep nasal mucosa. Further, analysed radiometry and gamma scintigraphy displayed an efficient percentage deposition of 99
mTc-vitamin D3 nanoemulsion across nasal mucosa compared to IV-administered solution (0.8%). The magnetic resonance imaging on the ischemic rat model confirmed the promising antioxidant action of developed nanoemulsion through the intranasal pathway
[19].
The neuroprotective property of antioxidant safranal was explored in a focal ischemic model that contained major issues, including neurobehavioral loss, hippocampal cell loss and release of oxidative stress markers. Sadeghnia et al. (2017) developed a safranal-loaded nanoemulsion for intranasal delivery to overcome the above-said issues related to the cerebral ischemic rat model. The study significantly demonstrated a reduction in neurological, hippocampal cell loss and thiobarbituric acid reactive substances (TBARS). Moreover, marked increases in antioxidant capacity and SH content were also observed, suggesting a potential role of antioxidant herbal safranal in neuroprotection, free radical suppression and the treatment of cerebral ischemic reperfusion
[20]. Antioxidant thymoquinone has poor aqueous solubility and bioavailability. Its neuroprotective action and potential to ameliorate cerebral ischemia have attracted researchers to explore the design of nanoemulsion. Mucoadhesive thymoquinone-loaded nanoemulsion was prepared via the ionic–gelation method. The formulated system comprised small globules (average size ~94.8 nm) with negative zeta potential (−13.5 mV). Viscosity and percentage drug content were reported as~110 cp and 99.86%, respectively.
The developed bioanalytical method displayed comparatively enhanced biodistribution and brain bioavailability after nasal route delivery than the intravenous pathway. Thymoquinone brain targeting potential was observed up to 89.97% after post-intranasal delivery of nanoemulsion. A performed neurobehavioral activity on the middle cerebral artery occlusion-persuaded ischemic rat model revealed the potential for antioxidant thymoquinone-embedded nanoemulsion to treat cerebral ischemia
[21].
4. Nanoemulsions in Brain Infections
Brain infections may occur in the regions of the cerebrum, cerebellum, spinal cord and associated nerves. In this context, encephalitis, meningitis and abscess are declared as medical emergencies and their long-term sequelae may result in substantial mortality. Bloodborne pathogens, head trauma and skull fractures can mediate the opening of tight junctions between the CNS and other nerves. Further, neurosurgical procedures and medical device implantation (exterior drainage tube, shunt) may cause infection due to microbial colonization and thus behaveas the foci of infection
[22]. Rinaldi et al. (2020) suggested a successful nose-to-brain intranasal delivery route for the management of fatal meningitis and encephalitis. Essential oils composed of nanoemulsions (mean average diameter 100 nm with PDI 0.2) were chosen to transport at the infected site of the central nervous system. In this context, antibacterial oils extracted from
Thymus vulgaris and
Syzygium aromaticum were individually incorporated in the chitosan-coated nanoemulsions (C-TV-NEs and C-SA-NEs) and investigated against multidrug-resistant methicillin-susceptible
S. aureus and carbapenem-resistant
A. baumannii and
K.pneumonia. Nanoemulsions, i.e., TV-NEs and SA-Nes, exhibited negative zeta potentials as −40 mV and −30 mV, respectively, that were converted into positive charges after chitosan coating by virtue of electrostatic interaction. However, the mean droplet size and PDI were amplified after the coating of the mucoadhesive chitosan polymer.
Intranasally administered nanoemulsions appeared more suitable for the potential curing of brain infections caused by Gram-negative bacteria compared to the intravenously delivered high dose of the formulation, presenting an efficient alternative therapy for the cure of serious meningitis and encephalitis
[23]. A blend of essential oils is widely used to manage different virus infections caused by human rhinovirus, bovine rotavirus, herpes virus, H5N1 and HIV. Essential oils, such as thyme oil, eucalyptol, borneol, alpha terpineol and sage oil, are reported to be included in the formulation of nanoemulsion. Their mixture initiates nucleoprotein trafficking abnormalities in the surface protein of the virus, hence interfering or masking the virion envelope and blocking virus internalization
[24]. A myriad of viruses, including poliovirus, rabies, herpes simplex and HIV, can reach the CNS through an intraneural path, causing encephalitis and brain abscesses, whereas meningitis is spread through bacterial pathogen at the subarachnoid space. Any breaches or damages (necrosis, microhemorrhage) of the BBB mechanical obstruction (infected RBCs, WBCs or platelets) and excessive production of cytokines disturb the structural architecture (tight junction) of BBB that led to the brain infection. Several reported pathogens, including a wide range of bacteria, fungi, viruses, cerebral malaria and spirochetes, are extensive causes of CNS or brain infections
[25].
The immunologically privileged central nervous system (CNS) remains the residing site for human immunodeficiency virus 1. Poor permeability across the tight junctions of BBB leads to inadequate and limited delivery of most of the anti-HIV therapeutics. Therefore, nanotechnology-based formulations have attracted medical scientists and researchers to design novel strategies for the management of neuro-AIDS. Saquinavir mesylate, a protease inhibitor, has been explored as an antiretroviral drug, but due to poor solubility and bioavailability (4%), its use is limited. O/W intranasal nanoemulsion containing saquinavir mesylate was formulated via a spontaneous emulsification method to enhance CNS bioavailability. The developed nanoemulsion was thermodynamically stable on analysing through freeze–thaw and heating–cooling cycles. The obtained low PDI (0.078) and smaller globular diameter (176.3 nm) indicated the development of monodispersed nanoemulsion that would be suitable for brain targeting via intranasal delivery. Estimated optimum zeta potential (−10.3 mV), pH (5.8) and refractive index (1.412) depicted good stability, non-irritancy and the compatibility of nanoemulsion. Higher-percentage drug permeation and permeability coefficients after 4 h on intranasal administration were observed as 76.96% and 0.51 cm/h, respectively, compared to the pure drug (26.73%, 0.17 cm/h).
The in vivo study in sheep nasal mucosa displayed higher drug permeation and biodistribution rate of nanoemulsion on comparing with its suspension. Further, the cilia-toxicity study depicted no prominent adverse action on the sheep nasal mucosa. Gamma scintigraphy images demonstrated higher drug transportation region in the rat brain that concluded its efficiency for treating neuro-AIDS by reducing the devastating viral load from reservoir sites
[26].
5. Nanoemulsions in Migraine and Cerebral Vasospasm
Another neuron-disabling disorder, ‘migraine’, is characterized by intense throbbing headaches in one of the halves of the brain. One of the surveys demonstrated that migraine is the sixth most prevailing brain disorder and affects approximately 15% of sufferers worldwide
[27].
Recurrent episodes of pain in unilateral headaches are allied with other visual, auditory and autonomic nervous disorders. Mostly, females are more affected due to their poor lifestyle. The pain is so intense and unbearable that the patient needs quick relief from it
[28]. Poor solubility, erratic absorption, inadequate permeation and fewerpenetration properties of most of the conventional therapies across BBB led to poor efficacy in the brain region. Delayed gastric emptying, first-pass metabolism, slow onset of action, nausea and vomiting and the intake of high doses are undesirable issues associated with anti-migraine drugs if given orally.
The intranasal route is more effective for the delivery of anti-migraine therapeutics owing to the involvement of olfactory and trigeminal nerves. These anatomical features are potentially involved in the greater distribution of drugs without facing issues of first-pass metabolism
[29].
Rizatriptan, a serotonin 5HT 1B/1D receptor agonist, exhibits only a 40% bioavailability upon oral delivery. An intranasal preparation of rizatriptan benzoate nanoemulsion improved brain tissue deposition and offered a non-invasive alternative approach for brain targeting. Biodistribution through the olfactory pathway directly delivers the drug to the central nervous system
[30]. Another well-tolerated anti-migraine drug, zolmitriptan, also possesses low bioavailability after oral administration. Ahigh-dose prescription in conventional preparation causes serious side effects, including irregular heart rhythm, stroke and Raynaud’s disease. Ebtsam et al., 2017 formulated a mucoadhesive nanoemulsion for the direct nose-to-brain transportation of zolmitriptan. The improved and quick onset of action is desired to alleviate acute migraine fulfilled with this formulation. An added 0.3% of chitosan acted as a mucoadhesive agent in the preparation and increased the residing time and drug permeation across associated nasal mucosa, hence assisting the transport of the drug to the brain tissues. Performed in vivo studies displayed shorter Tmax and higher AUC
0–8 of intranasally administered nanoemulsion compared to intravenous or nasal zolmitriptan solution
[31]. Sumatriptan has been widely employed for the management of severe and painful migraine for decades. High hydrophilicity and less mucoadhesive features limit its application through the nasal route. Ribeiro et al. 2020 have developed a novel nanoemulsion containing sumatriptan, copaiba oil and organic biopolymers. i.e., alginate, pullulan, xanthan and pectin. Mean particle size, PDI and zeta potential of nanoemulsion were observed as approximately 120 nm, 0.2 and −25 mV, respectively.
The developed alginate-based nanoemulsion exhibited long term storage stability (1 year) due to optimised zeta potential. In vitro study depicted extended release from sumatriptan-loaded alginate nanoemulsion upto 24 h. The in vivo toxicity in the zebrafish model revealed no evidence of mortality and other cardiac toxicity and did not disturb spontaneous changes in zebrafish larvae, hence showing a promising alternative approach for the alleviation of brain diseases, including migraine
[32].
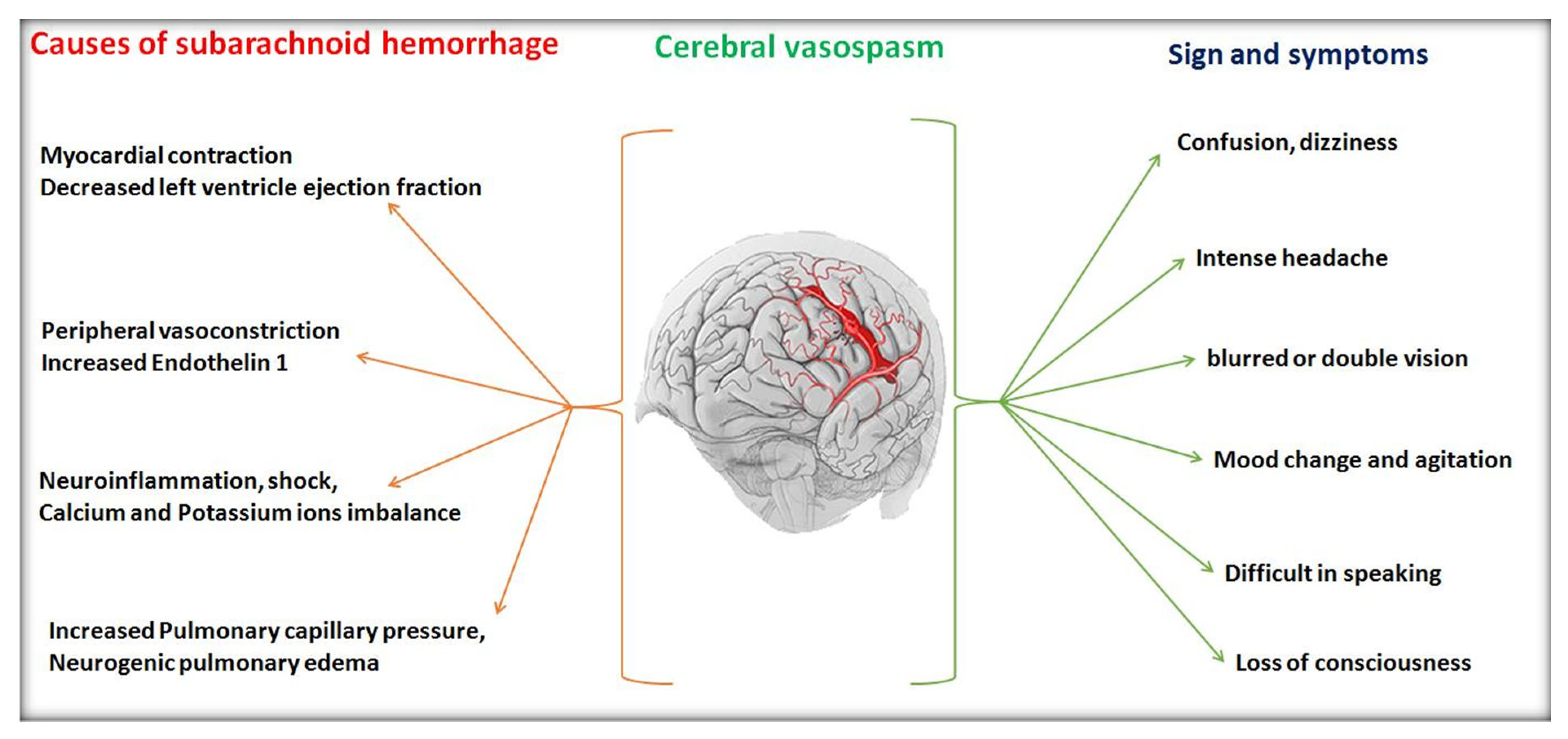
Cerebral vasospasm is defined as the temporary narrowing or thinning of cerebral arteries. More often, subarachnoid haemorrhage and traumatic brain injuries result in cerebral vasospasm.
The appearance of new focal neurological signs, inflammation, microcirculatory failure, bipolar disorders and loss of consciousness re-associated aspects are addressed with subarachnoid haemorrhage
[33]. Cerebral vasospasm is the leading cause of mortality if not identified and treated immediately. It typically affects concerned blood vessels at the skull base that influences arterial contraction, blood pressure and blood viscosity.
Figure 3 defines different causes of cerebral vasospasm and its different signs and symptoms that have appeared in the sufferer.
Figure 3. Various causes and symptoms of cerebral vasospasm.
Endovascular therapies are recommended for the management of severe vasospasm cases. However, the radiological and computed tomography evidence suggested that approximately 20% of cases of vasospasm can be treated via performing angiograms within a week of aneurysmal rupture. Some clinical reports also described that the level of nitric oxide was found to be decreased due to vasoconstrictor endothelin and extra vascular oxyhaemoglobin
[34].
Cerebral vasospasm is often evident after aneurysmal subarachnoid haemorrhage. However, in some instances, it is observed after skull surgery associated with intractable epilepsy and amygdala-hippocampectomy. Carbamazepine 400 mg is recommended to overcome developed issues in this case. Dhobale et al., 2018 formulated carbamazepine nanoemulsion for efficient brain targeting via the intranasal route. A spontaneous emulsification method was employed for the formulation of nanoemulsion utilizing Capmul MCM (oil) and TWEEN 80/PEG-600 (3:1) as surfactant and co-surfactant, respectively. The developed nanoemulsion contained a smaller globular size of ~71.7 nm with a 0.256 ± 0.002 polydispersity index. The in vivo study depicted efficient drug distribution in 5 h
[35]. Survival from cerebral vasospasm or aneurysmal subrachanoid haemorrhage was improved. FDA-approved nimodine is a calcium channel blocker and is widely prescribed clinically to treat subarachnoid haemorrhage. In this series, fasudil (Rho-kinase inhibitor), nicardipine (calcium channel blocker), statins (hypolipidemic), clazosentan (endothelin receptor antagonist), cilostazol (platelet aggregation factor and heparin (anticoagulant) has also been added as effective therapeutics in clinical practice
[36].
Ahmad et al. have discussed a novel bioimaging tool. i.e., targeting probe that is based on an on–off signal and significantly traces the translocation of the therapeutic agent throughout the nose-to-brain pathway. A cargo was developed containing environmentally responsive dye P2 and P4, caumarin 6 and DiR conventional probes. Translocation of nanoemulsion (prepared with Labrafac®CC/ WL1349 and solutol®HS15) after intranasal administration was evident either via bioimaging or through histopathological examination in rats. Outcomes concluded that nanoemulsions (coated with chitosan and naked) with a mean particle size of 100 nm exhibited higher retention duration in the nasal cavity with slower clearance compared to larger particles. Weak P2 and P4 signals were also observed in the region of the olfactory bulb for coated nanoemulsions of particle size 100 nm. Moreover, nanoemulsion particle sizes of more than 900 nm were unable to reach the olfactory bulb. Signals obtained from caumarin 6 and DiR represented significant transportation of nanoemulsion in the brain region.
The importance of particle size and their cytoprotective efficiency under the circumstances of oxygen–glucose deprivation and reoxygenation were also signified by Varlamova and coworkers in 2022. It was reported that the average particle size of selenium (50 nm) could induce calcium ion responses and inhibit apoptosis in the brain cortex cells. At the same time, upon increasing particle size, i.e., 100 nm and 400 nm, exhibited induction of calcium ion oscillation and mixed pattern of calcium signals, respectively. Hence, cryoprotective action under oxygen–glucose deprivation and reoxygenation conditions can be provided at the size range 50–400 nm that is required to protect brain tissues from ischemic conditions through modulation of the calcium ions signal system of astrocytes
[37][38].