
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tiziana Ciarambino | -- | 2780 | 2023-06-23 13:38:44 | | | |
| 2 | Dean Liu | -1 word(s) | 2779 | 2023-06-25 04:38:38 | | |
Video Upload Options
Cerebral sinus venous thrombosis (CSVT) is a relatively rare acute disorder of cerebral circulation, but it can potentially be associated with serious sequelae and a poor prognosis. The neurological manifestations associated with it are often not adequately taken into consideration given the extreme variability and nuances of its clinical presentation and given the need for radiological methods suitable for this type of diagnosis. CSVT is usually more common in women, but so far there are little data available in the literature on sex-specific characteristics regarding this pathology. CSVT is the result of multiple conditions and is therefore to be considered a multifactorial disease where at least one risk factor is present in over 80% of cases.
1. Introduction
2. Myeloproliferative Neoplasms and CSVT
3. Cerebral Vein Thrombosis and COVID-19 Infection
-
Retrograde cerebral infection starting from the nasal mucosa colonized by the virus and subsequent inflammatory and neuropathic damage of the olfactory nerve [33].
-
Indirect action mediated by the inflammatory hyperactivity triggered by the virulence factors of COVID-19, which would be associated with a real storm of cytokines, dysregulation of the immune system up to the establishment of disseminated vascular coagulation [34].
-
The finding of some autoantibodies circulating during infection would lead to the suspicion of a mechanism of autoimmune genesis which is already the basis of cases of CSVT in the population affected by autoimmune pathologies [35].
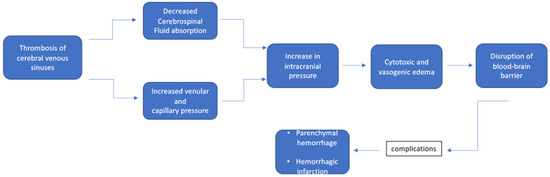
4. CSVT during Subsequent Pregnancy and Puerperium
References
- Saadatnia, M.; Fatehi, F.; Basiri, K.; Mousavi, S.A.; Mehr, G.K. Cerebral Venous Sinus Thrombosis Risk Factors. Int. J. Stroke 2009, 4, 111–123.
- Rizk, J.G.; Gupta, A.; Sardar, P.; Henry, B.M.; Lewin, J.C.; Lippi, G.; Lavie, C.J. Clinical Characteristics and Pharmacological Management of COVID-19 Vaccine–Induced Immune Thrombotic Thrombocytopenia With Cerebral Venous Sinus Thrombosis: A Review. JAMA Cardiol. 2021, 6, 1451.
- Long, B.; Koyfman, A.; Runyon, M.S. Cerebral Venous Thrombosis: A Challenging Neurologic Diagnosis. Emerg. Med. Clin. N. Am. 2017, 35, 869–878.
- Kristoffersen, E.S.; Harper, C.E.; Vetvik, K.G.; Zarnovicky, S.; Hansen, J.M.; Faiz, K.W. Incidence and Mortality of Cerebral Venous Thrombosis in a Norwegian Population. Stroke 2020, 51, 3023–3029.
- Ferro, J.M.; de Sousa, D.A. Cerebral Venous Thrombosis: An Update. Curr. Neurol. Neurosci. Rep. 2019, 19, 74.
- Mehta, A.; Danesh, J.; Kuruvilla, D. Cerebral Venous Thrombosis Headache. Curr. Pain Headache Rep. 2019, 23, 47.
- Duman, T.; Uluduz, D.; Midi, I.; Bektas, H.; Kablan, Y.; Goksel, B.K.; Milanlioglu, A.; Orken, D.N.; Aluclu, U.; Colakoglu, S.; et al. A Multicenter Study of 1144 Patients with Cerebral Venous Thrombosis: The VENOST Study. J. Stroke Cerebrovasc. Dis. 2017, 26, 1848–1857.
- Giordano, M.; Trotta, M.C.; Ciarambino, T.; D’amico, M.; Schettini, F.; Di Sisto, A.; D’auria, V.; Voza, A.; Malatino, L.S.; Biolo, G.; et al. Circulating miRNA-195-5p and -451a in Patients with Acute Hemorrhagic Stroke in Emergency Department. Life 2022, 12, 763.
- Giordano, M.; Trotta, M.C.; Ciarambino, T.; D’amico, M.; Galdiero, M.; Schettini, F.; Paternosto, D.; Salzillo, M.; Alfano, R.; Andreone, V.; et al. Circulating MiRNA-195-5p and -451a in Diabetic Patients with Transient and Acute Ischemic Stroke in the Emergency Department. Int. J. Mol. Sci. 2020, 21, 7615.
- Sidhom, Y.; Mansour, M.; Messelmani, M.; Derbali, H.; Fekih-Mrissa, N.; Zaouali, J.; Mrissa, R. Cerebral Venous Thrombosis: Clinical Features, Risk Factors, and Long-term Outcome in a Tunisian Cohort. J. Stroke Cerebrovasc. Dis. 2014, 23, 1291–1295.
- Hersh, D.S.; Shimony, N.; Groves, M.L.; Tuite, G.F.; Jallo, G.I.; Liu, A.; Garzon-Muvdi, T.; Huisman, T.A.G.M.; Felling, R.J.; Kufera, J.A.; et al. Pediatric cerebral venous sinus thrombosis or compression in the setting of skull fractures from blunt head trauma. J. Neurosurg. Pediatr. 2018, 21, 258–269.
- Wilcher, J.; Pannell, M. Dural Sinus (Cerebral Venous) Thrombosis in a Pediatric Trauma Patient: A Rare Complication After Closed Head Injury. Pediatr. Emerg. Care 2016, 32, 872–874.
- Son, H.-M. Massive cerebral venous sinus thrombosis secondary to Graves’ disease. Yeungnam Univ. J. Med. 2019, 36, 273–280.
- Rehman, A.; Husnain, M.G.; Mushtaq, K.; Eledrisi, M.S. Cerebral venous sinus thrombosis precipitated by Graves’ disease. BMJ Case Rep. 2018, 2018, bcr-2017-224143.
- Wang, H.-I.; Yiang, G.-T.; Hsu, C.-W.; Wang, J.-C.; Lee, C.-H.; Chen, Y.-L. Thyroid Storm in a Patient with Trauma—A Challenging Diagnosis for the Emergency Physician: Case Report and Literature Review. J. Emerg. Med. 2017, 52, 292–298.
- Karaoren, G.Y.; Sahin, O.T.; Erbesler, Z.A.; Bakan, N. Thyroid Storm Due To Head Injury. Turk. J. Trauma Emerg. Surg. 2014, 20, 305–307.
- Strada, L.; Gandolfo, C.; Del Sette, M. Cerebral sinus venous thrombosis in a subject with thyrotoxicosis and MTHFR gene polymorphism. Neurol. Sci. 2008, 29, 343–345.
- Verberne, H.J.; Fliers, E.; Prummel, M.F.; Stam, J.; Brandjes, D.P.; Wiersinga, W.M. Thyrotoxicosis as a Predisposing Factor for Cerebral Venous Thrombosis. Thyroid 2000, 10, 607–610.
- Franchini, M.; Lippi, G.; Targher, G. Hyperthyroidism and Venous Thrombosis: A Casual or Causal Association? A Systematic Literature Review. Clin. Appl. Thromb. Hemost. 2010, 17, 387–392.
- Bensalah, M.; Squizzato, A.; Kablia, S.O.; Menia, H.; Kemali, Z. Cerebral vein and sinus thrombosis and hyperthyrodism: A case report and a systematic review of the literature. Thromb. Res. 2011, 128, 98–100.
- Stuijver, D.J.; van Zaane, B.; Romualdi, E.; Brandjes, D.P.M.; Gerdes, V.E.A.; Squizzato, A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: A systematic review and meta-analysis. Thromb. Haemost. 2012, 108, 1077–1088.
- Mazur, P.; Sokołowski, G.; Hubalewska-Dydejczyk, A.; Płaczkiewicz-Jankowska, E.; Undas, A. Prothrombotic alterations in plasma fibrin clot properties in thyroid disorders and their post-treatment modifications. Thromb. Res. 2014, 134, 510–517.
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405.
- Fowlkes, S.; Murray, C.; Fulford, A.; De Gelder, T.; Siddiq, N. Myeloproliferative neoplasms (MPNs)-Part 1: An overview of the diagnosis and treatment of the “classical” MPNs. Can. Oncol. Nurs. J. 2018, 28, 262–268.
- Kralovics, R.; Passamonti, F.; Buser, A.S.; Teo, S.-S.; Tiedt, R.; Passweg, J.R.; Tichelli, A.; Cazzola, M.; Skoda, R.C. A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders. N. Engl. J. Med. 2005, 352, 1779–1790.
- Magadum, A.; Kishore, R. Cardiovascular Manifestations of COVID-19 Infection. Cells 2020, 9, 2508.
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445.
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418.
- Mai, V.; Tan, B.K.; Mainbourg, S.; Potus, F.; Cucherat, M.; Lega, J.-C.; Provencher, S. Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: A systematic review with meta-analysis. Vasc. Pharmacol. 2021, 139, 106882.
- Katsoularis, I.; Fonseca-Rodríguez, O.; Farrington, P.; Jerndal, H.; Lundevaller, E.H.; Sund, M.; Lindmark, K.; Connolly, A.-M.F. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after COVID-19: Nationwide self-controlled cases series and matched cohort study. BMJ 2022, 377, e069590.
- Kalita, J.; Misra, U.K.; Singh, V.K.; Kumar, S.; Jain, N. Does gender difference matter in cerebral venous thrombosis? J. Clin. Neurosci. 2022, 102, 114–119.
- Fraiman, P.; Junior, C.G.; Moro, E.; Cavallieri, F.; Zedde, M. COVID-19 and Cerebrovascular Diseases: A Systematic Review and Perspectives for Stroke Management. Front. Neurol. 2020, 11, 574694.
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell. Neurosci. 2018, 12, 386.
- Zhu, Q.; Xu, Y.; Wang, T.; Xie, F. Innate and adaptive immune response in SARS-CoV-2 infection-Current perspectives. Front. Immunol. 2022, 13, 1053437.
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38.
- Hernández-Fernández, F.; Valencia, H.S.; Barbella-Aponte, R.A.; Collado-Jiménez, R.; Ayo-Martín, Ó.; Barrena, C.; Molina-Nuevo, J.D.; García-García, J.; Lozano-Setién, E.; Alcahut-Rodriguez, C.; et al. Cerebrovascular disease in patients with COVID-19: Neuroimaging, histological and clinical description. Brain 2020, 143, 3089–3103.
- Kremer, S.; Lersy, F.; Anheim, M.; Merdji, H.; Schenck, M.; Oesterlé, H.; Bolognini, F.; Messie, J.; Khalil, A.; Gaudemer, A.; et al. Neurologic and neuroimaging findings in COVID-19 patients: A retrospective multicenter study. Neurology 2020, 95, e1868–e1882.
- Weetman, A.P. Disease Graves’. N. Engl. J. Med. 2000, 343, 1236–1248.
- Squizzato, A.; Romualdi, E.; Büller, H.R.; Gerdes, V.E.A. Thyroid Dysfunction and Effects on Coagulation and Fibrinolysis: A Systematic Review. J. Clin. Endocrinol. Metab. 2007, 92, 2415–2420.
- Lamy, C.; Hamon, J.B.; Coste, J.; Mas, J.L. For the French Study Group on Stroke and Pregnancy. Neurology 2000, 55, 269–274.
- Ginsberg, J.S.; Greer, I.; Hirsh, J. Use of Antithrombotic Agents During Pregnancy. Chest 2001, 119, 122S–131S.
- Ray, J.G.; Chan, W.S. Deep Vein Thrombosis During Pregnancy and the Puerperium: A Meta-Analysis of the Period of Risk and the Leg of Presentation. Obstet. Gynecol. Surv. 1999, 54, 169–175.
- Taous, A.; Berri, M.A.; Lamsiah, T.; Zainoun, B.; Ziadi, T.; Rouimi, A. Cerebral venous thrombosis revealing an ulcerative colitis. Pan Afr. Med. J. 2016, 23, 120.
- Cohen, J.B.; Comer, D.M.; Yabes, J.G.; Ragni, M.V. Inflammatory Bowel Disease and Thrombosis: A National Inpatient Sample Study. TH Open 2020, 4, e51–e58.
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2020, 56, 2769–2778.




