Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giorgia Natalia Iaconisi | -- | 2716 | 2023-06-22 17:15:37 | | | |
| 2 | Catherine Yang | Meta information modification | 2716 | 2023-06-25 04:27:05 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Production and Application of Hyaluronic Acid. Encyclopedia. Available online: https://encyclopedia.pub/entry/45976 (accessed on 07 February 2026).
Iaconisi GN, Lunetti P, Gallo N, Cappello AR, Fiermonte G, Dolce V, et al. Production and Application of Hyaluronic Acid. Encyclopedia. Available at: https://encyclopedia.pub/entry/45976. Accessed February 07, 2026.
Iaconisi, Giorgia Natalia, Paola Lunetti, Nunzia Gallo, Anna Rita Cappello, Giuseppe Fiermonte, Vincenza Dolce, Loredana Capobianco. "Production and Application of Hyaluronic Acid" Encyclopedia, https://encyclopedia.pub/entry/45976 (accessed February 07, 2026).
Iaconisi, G.N., Lunetti, P., Gallo, N., Cappello, A.R., Fiermonte, G., Dolce, V., & Capobianco, L. (2023, June 22). Production and Application of Hyaluronic Acid. In Encyclopedia. https://encyclopedia.pub/entry/45976
Iaconisi, Giorgia Natalia, et al. "Production and Application of Hyaluronic Acid." Encyclopedia. Web. 22 June, 2023.
Copy Citation
Hyaluronic acid (HA) is a glycosaminoglycan widely distributed in the human body, especially in body fluids and the extracellular matrix of tissues. It plays a crucial role not only in maintaining tissue hydration but also in cellular processes such as proliferation, differentiation, and the inflammatory response. HA has demonstrated its efficacy as a powerful bioactive molecule not only for skin antiaging but also in atherosclerosis, cancer, and other pathological conditions.
hyaluronic acid
biomaterial
bioactive molecule
metabolic pathways
bioproduction
fermentation processes
1. Introduction
HA is a linear glycosaminoglycan polymer present in the extracellular matrix of vertebrate tissues, including connective, epithelial, and neural tissues [1]. It is involved in several biological processes such as embryonic development, wound healing, cancer progression, angiogenesis, inflammation, and bone regeneration [2][3][4]. HA has been found to regulate essential cellular processes such as cell adhesion, proliferation, and differentiation [5][6].
In addition to belonging to vertebrate connective tissues, HA could be found in several bacterial strains (e.g., groups A and C streptococci and Pasteurella multocida) as a component of their capsules and mucus with the role of conferring adherence, protection, and molecular mimicry necessary to evade the host’s immune system during their infection process [7].
Over the past 20 years, HA has garnered an exponential interest due to the continuous scientific discoveries regarding its intrinsic properties and subsequent applications. Ongoing research continues to investigate the mechanisms underlying its biological activity.
HA applications cover several fields ranging from cosmetic formulations to viscosurgery, ophthalmology, orthopedic surgery, rheumatology, tissue regeneration, and targeted cancer therapy [8]. This broad range of applications is possible due to the specific bioactivity exhibited by HA based on its molecular weight (MW). Furthermore, in addition to the use of pure HA, an emerging trend is the combination of HA with other bioactive ingredients or materials for several applications such as facial volume restoration [9][10][11], osteoarthritis treatment [12][13][14] (NCT00653432, NCT05683327), cosmetic medicine [15][16][17][18][19], nasolabial fold reduction [20][21], cancer treatment [22][23][24][25] (NCT05024773), atherosclerosis diagnosis and treatment [26], psoriasis treatment [27][28], urinary tract infection treatment [29][30], tissue engineering [31][32][33][34], antioxidant and anti-inflammatory effects [35][36][37], and wound healing [38][39].
The aging population, the consequent increase in demand for antiaging products, and the rise in age-related diseases along with the introduction of technologically advanced products, the growing preference for non-invasive techniques, the adoption of aesthetic procedures, and the need for faster and natural-like results are all factors that have affected and will continue to impact the growth in research and the market size of HA in the coming years.
2. HA Microbial Metabolic Pathways
Several studies on HA biosynthesis focused on natural producer microorganisms such as group A and C streptococci and Pasteurella multocida, which produce HA as a component of their capsules and mucus [40][41].
Generally, HA is synthesized via two different metabolic pathways, resulting in the production of two precursors: UDP-GA and UDP-NAG [42] (Figure 1).
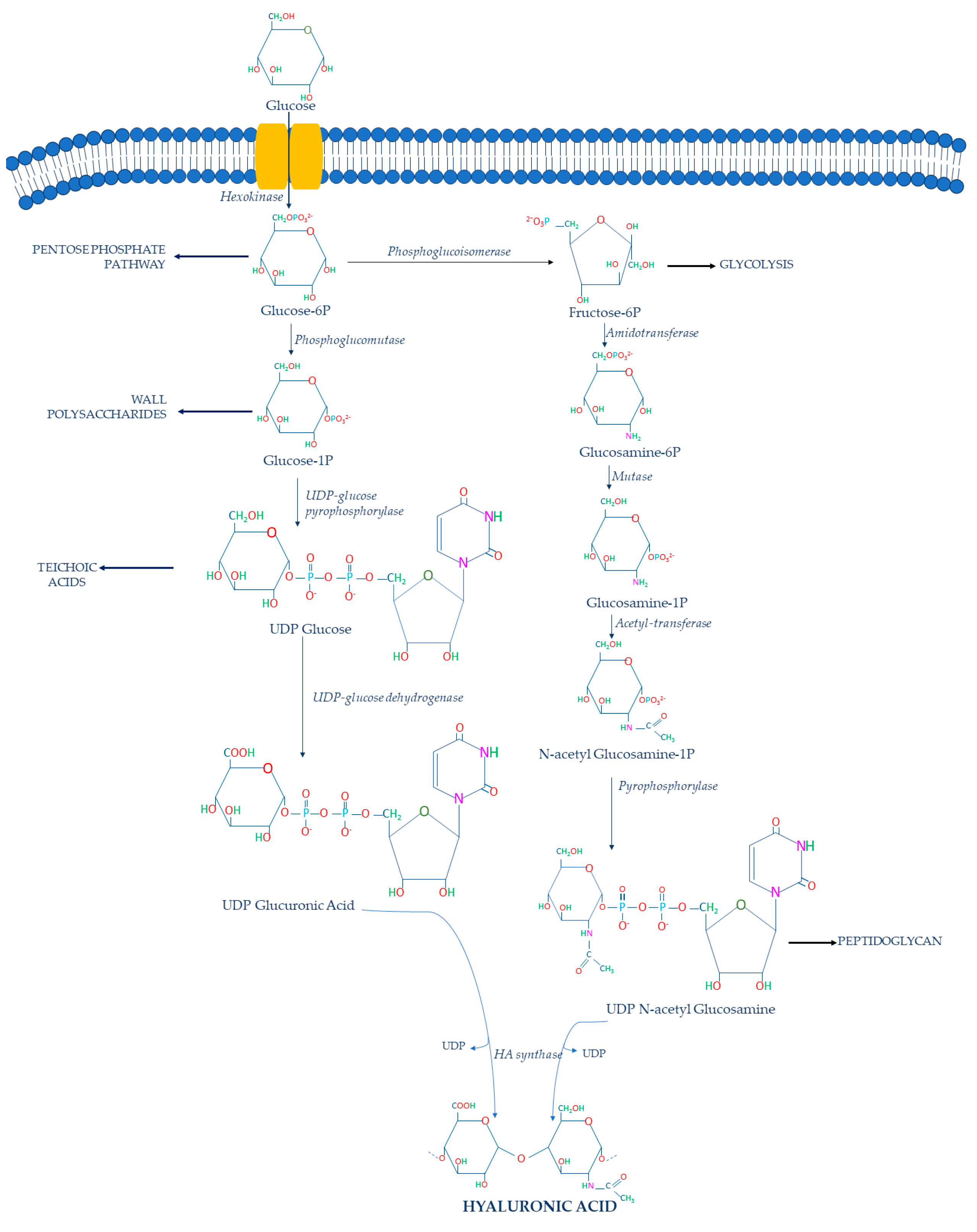
Figure 1. HA biosynthetic pathway in S. zooepidemicus.
Glucose is imported into the bacterial cell and phosphorylated by hexokinase, converting it into glucose-6-phosphate (glucose-6P) in the cytoplasm. Then, it is converted by the phosphoglucomutase in glucose-1-phosphate (glucose-1P), after which the UDP-glucose pyrophosphorylase catalyzes the formation of UDP-glucose. This UDP-glucose is subsequently oxidized by the UDP-glucose 6-dehydrogenase to obtain the first HA precursor: UDP-GA. However, other metabolic pathways compete with this conversion since the glucose-1P can be used for glycogen production [40] and the UDP-glucose can be converted to trehalose-6P [40]. These two metabolic pathways represent a metabolic limit for HA production [43].
The second HA precursor is produced through a second pathway in which glucose-6P is converted to fructose-6-phosphate (fructose-6P) by the phosphoglucoisomerase. UDP-glucose 6-dehydrogenase and phosphoglucoisomerase are the most important competing enzymes because the first one directs glucose-6P into the Pentose Phosphate Pathway, while the latter converts glucose-6P into fructose-6P via the Embden–Meyerhof–Parnas pathway [43]. Cells typically utilize the majority of glucose-6P for these two competing metabolic pathways rather than UDP-GA synthesis [44]. Furthermore, cells can use the phosphoglucoisomerase to convert fructose-6P into glucose-6P, which can be utilized in the Pentose Phosphate Pathway [40]. Then, glucosamine-6-phosphate (glucosamine-6P) is formed from fructose-6P by the action of amidotransferase. Glucosamine-6P is the substrate of the mutase that leads to the formation of the glucosamine-1-phosphate (glucosamine-1P).
Amidotransferase, on the other hand, competes with the UDP-NAG cascade by converting glucosamine-6P to fructose-6P [43]. Indeed, fructose-6P can be converted into fructose-1,6-bisphosphate through the ATP-dependent phosphofructokinase, which is utilized in the Embden–Meyerhof–Parnas pathway for energy generation and cell growth [43]. In addition, it must be considered that if cells have a limited amount of carbon sources to feed these two main metabolic pathways, a significant portion of cellular glucosamine-6-phosphate could be converted to fructose-6P and glucose-6P to meet the metabolic needs of cells [43]. Consequently, cells will have a limited reserve of glucosamine-6P to synthesize glucoamine-1P.
Glucosamine-1P is further acetylated and phosphorylated by pyrophosphorylase to form the second precursor: UDP-NAG. In this case, reduced flux to UDP-NAG due to competing pathways also is a significant limitation to HA synthesis [40]. Consequently, at the end of these metabolic pathways, UDP-GA and UDP-NAG are polymerized by the hyaluronic acid synthase (HAS), which catalyzes the formation of the HA chain [45]. The biosynthetic pathway of HA in bacteria shows that the HAS is a key enzyme that catalyzes the polymerization of HA chains. In particular, the streptococcal HAS is classified as a class I HAS because it is an integral membrane protein that catalyzes the addition of UDP-GA acid and UDP-NAG to the growing HA chain [46] (Figure 2A).
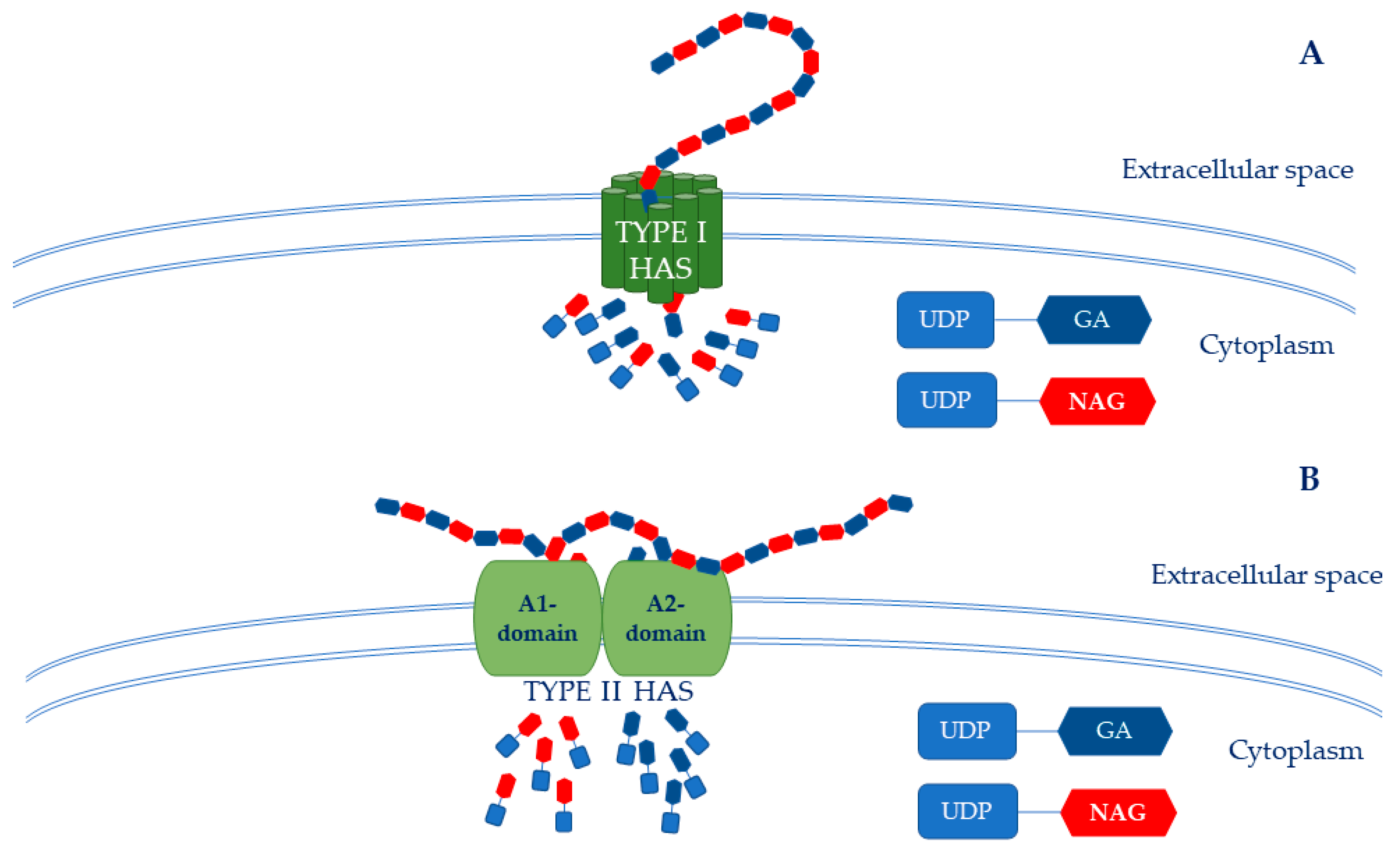
Figure 2. HAS structure. (A) The Type I HAS is an integral membrane protein that catalyzes the adding of UDP-GA and UDP-NAG to the HA growing chain. (B) The Type II HAS is a membrane-associated protein composed of A1 and A2 domains that work together as a single peptide. The A1 domain is a β-1,4-N-acetylglucosaminil-transferase, while the A2 domain is a β-1,3-glucuronyl-transferase.
Conversely, the HAS of P. multocida belongs to class II because it is a membrane-associated glycosyl transferase. This enzyme also has two independent active sites: (i) an “A1” domain that performs the UDP-NAG transferase activity, and (ii) and an “A2” domain that carries out the UDP-GA transferase activity (Figure 2B). Therefore, HAS catalyzes the sequential elongation of a growing HA chain at the non-reducing end with its β-1,3-glucuronyl-transferase and β-1,4-N-acetylglucosaminil-transferase domains, which work synergistically as a single polypeptide [46].
Hence, it is clear that HA production is an energy- and carbon-intensive process for the bacterial cell. Its metabolic pathway consumes large quantities of its donor substates, thereby linking HA synthesis to glucose metabolism. As already mentioned, glucose-6P is an important substrate of the Pentose Phosphate Pathway, while fructose-6P is an important intermediate of the glycolytic pathway. Moreover, some intermediates of HA biosynthesis are also required for cell wall biosynthesis [47]. In particular, glucose-1P is an important wall polysaccharide, UDP-glucose is used by streptococci for teichoic acid biosynthesis [48], and finally NAG is a component of the structural unit of peptidoglycan [49].
It is easy to see that HA synthesis is strongly influenced by the cellular availability of the two precursors [50] and thus to cells’ metabolic needs. Moreover, it has been observed that if each precursor is not supplied at sufficient rates, the amount of the synthesized HA and its MW are reduced [51]. The carbon source must be shared equally between the two pathways to correctly produce HA. Due to this, several studies were conducted in order to maximize hyaluronic acid production.
3. HA Fermentation Process in Engineered Host Microorganisms
As previously mentioned, industrial and scientific research is looking for optimized HA production methods with controlled parameters, high yields, low production costs, a high MW, and a high purity of the final product. HA bacterial production through metabolic engineering represents a promising strategy. Over the last years, several microorganisms have been engineered in order to produce HA. Different fermentation methods and culture media have been tested. Not only natural microbial producers of HA have been engineered in order to optimize the characteristics of the final product, but non-natural producers such as GRAS microorganisms (i.e., E. coli, B. subtilis, and B. megaterium) have been as well.
First of all, several researchers attempted to maximize HA production by tuning the microorganism culture conditions rather than engineering them. Parameters such as the initial pH of the broth, temperature, time of incubation, and agitation speed were evaluated as important parameters that influenced the yield of HA. Among several bacteria, S. equi spp. zooepidemicus was the most investigated. Güngör et al. found that the growing conditions that allowed them to improve the HA final yield (12 g/L) were pH 7.8, an incubation temperature of 33 °C, an incubation time of 24 h, and an agitation speed of about 187 rpm [52]. In another recent study, UV-induced mutagenesis on S. zooepidemicus allowed the researchers to further increase the HA yield, which reached about 4.2 g/L after 36 h of fermentation [53]. In this case, the optimal fermentation was obtained by incubating it at 37 °C and pH 7.4 in a fermentation broth that contained glucose as the carbon source, phosphate as the essential nutrient, peptone 250 as the nitrogen source, and dibasic potassium phosphate as the phosphate source [53]. Then, to enhance the efficiency of the hyaluronic acid production, a semi-continuous fermentation process that consisted of two-stage 3–L bioreactors was developed, and recombinant hyaluronidase SzHYal (300 U/L) was added into the second stage bioreactor to reduce the broth viscosity. In this way, an HA yield of 30 g/L was successfully attained [53]. Another strategy showed that as an alternative to glucose, molasses could be used as a carbon source in the fermentation medium of S. zooepidemicus [54]. Indeed, the presence of this alternative and cheaper sugar allowed an increase in the yield of the HA up to 0.2 g/L compared to standard conditions (43 mg/L) [54]. In addition to the pH, the incubation temperature, agitation speed, soil composition, and size of inoculum are key parameters of the fermentation process that affect the yield of the final product. Some researchers found that a streptococcal inoculum size of 10% had the greatest effect on the fermentation process and also that an agitation speed of 300 rpm could increase the HA yield [55]. This may have occurred because at this rate of speed, a greater subdivision of air bubbles could occur with a consequent larger surface area for the gas–liquid mass transfer, which therefore led to a reduction in the thickness of the gas and liquid films responsible for the resistance to the mass transport [55]. In this way, it is easy to understand that several parameters must be optimized in order to enhance the microbial production of HA starting from natural producer microorganisms.
With the aim of reducing extracted product endotoxins, recombinant non-natural microbial producers were designed, developed, and investigated in depth [40]. A variety of organisms including B. subtilis, B. megaterium, and E. coli have all been genetically engineered in order to produce HA [56][57][58]. Genetically modified E. coli strains have several potential advantages when compared to Streptococcus strains and therefore offer a promising alternative [40]. Additionally, compared to Streptococcus strains, several E. coli strains are non-pathogenic [43], and they can be grown at a high cell density using cheap and simple media. As a result, the fermentative process of E. coli could be improved through metabolic engineering [59]. For these reasons, many studies have been carried out with recombinant E. coli. In particular, a recent study compared the HA production in different E. coli strains (pLysY/lq, Rosetta2, Rosetta2 (DE3) pLysS, and Rosetta-gami B (DE3) pLysS) and B. megaterium (MS941) cells [57]. Firstly, the researchers used different fermentation conditions for E. coli cells. Initially, they engineered the bacterial cells by transforming them with different plasmids. The first introduction of the hasA gene of S. equi spp. zooepidemicus in E. coli Rosetta2 cells allowed them to obtain a yield of 8.8 mg/L [57]. Then, the introduction of a second set of genes, which were hasA, hasB and hasC genes in E. coli pLysY/lq cells, allowed them to reach a yield of 208.3 mg/L (by adding MgCl2, K2HPO4, and sorbitol to the fermentation broth) [57]. Nasser et al. also attempted to obtain HA from Rosetta2 (DE3) pLysS, with a successful yields of 346.7 mg/L in Super Optimal broth with Catabolite repression (SOC) medium and 500 mg/L in terrific broth (TB) medium [57]. Lastly, they analyzed the ability of Rosetta-gami B (DE3) pLysS to produce HA by transforming it with hasA (hyaluronic acid synthase gene), hasB (UDP-glucose dehydrogenase gene), hasC (UDP-glucose pyrophosphorylase gene), hasD (acetyltransferase gene), hasE (phosphoglucoisomerase gene) genes; in this way, they obtained 585 mg/L of HA [57].
In this case, it is also possible to note that the composition of the fermentation medium and the bacterial strain affect the HA yield. In a study by Nasser et al., it emerged that B. megaterium was able to produce a higher amount of HA rather than E. coli. Indeed, by expressing only the hasA gene along with the addition of xylose, MgCl2, K2HPO4 and sorbitol to Luria-Bertani (LB) fermentation broth, they were able to obtain 50 mg/L of HA. By expressing hasA, hasB, and hasC with the addition of sucrose to LB fermentation broth, the yield of HA was about 2116.7 mg/L. In TB broth instead, the yield was decreased (2016 mg/L). At this point, they modified the fermentation medium, and they observed that in A5 + sodium 2-hydroxy-3-(morpholin-4-yl)propane-1-sulfonate (MOPSO) medium (containing yeast extract and a mineral medium based on MOPSO buffer), the amount of HA was about 1990 mg/L. As a final step, they introduced hasA, hasB, hasC, hasD, and hasE genes in B. megaterium cells. In LB medium with sucrose, the HA yield was about 2350 mg/L; in A5+MOPSO medium, the HA amount was about 2436 mg/L [57].
Another study used E. coli Rosetta (DE3) as the host to obtain the production of HA by optimizing the fermentation medium and inserting the hasA and hasE genes [57]. In this case, the fermentation broth contained glucose as the carbon source, MgSO4 as the Mg2+ donor, and KH2PO4 and K2HPO4 as the phosphate sources [57]. This study further demonstrated the effect of fermentation temperature on HA yield, since the maximum HA biosynthesis was observed when fermentation was run at less than 37 °C. Finally, it was observed that the gene expression inductor concentration also could affect the HA yield. The highest HA concentration was obtained when the cell growth was at the lowest; that is, when the concentration of the gene expression inductor was the highest. Indeed, in these conditions, the cellular growth decreased and the HA concentration increased [57].
As previously mentioned, bacilli also can be used for HA production since they are safe, can be easily genetically tractable, and are able to grow in simple culture media [60]. In particular, in a recent study B. subtilis 3NA was engineered by overexpressing the hasA gene of S. zooepidemicus and its other endogenous genes involved in the HA biosynthetic pathway [58]. The fermentation medium contained glycerol as the carbon and energy source, H3PO4 as the phosphate source, NH4OH as the nitrogen source, MgSO4 as the Mg2+ donor and sulfur source, and finally KOH to buffer the pH [58]. Hence, the obtained HA yield was comparable with the streptococcal one [58].
Thus, all mentioned works allowed us to understand that the strain, exogenous genes, and fermentation conditions (i.e., soil composition, temperature, pH, concentration of the gene expression inductor, and agitation speed) are fundamental parameters that affect HA yield. The optimization of these parameters is fundamental for obtaining the highest HA amount from bacterial fermentation.
4. HA as a Powerful Bioactive Molecule
HA has been used for both medical and commercial applications thanks to its biocompatibility, biodegradability, and non-immunogenicity. Therefore, several HA-based products have been developed and are currently available on the market. HA showed its major potential for aesthetic applications but also was revealed to be promising in other biomedical fields. This is due to the fact that HA was revealed to be a versatile biomaterial with tunable properties that allowed the development of different kinds of devices (i.e., injectable formulations, gels, nanoparticles, sponges, and hydrogels) according to the injured tissue’s specific requirements. The main applications of HA as a powerful bioactive molecule are summarized in Figure 3.
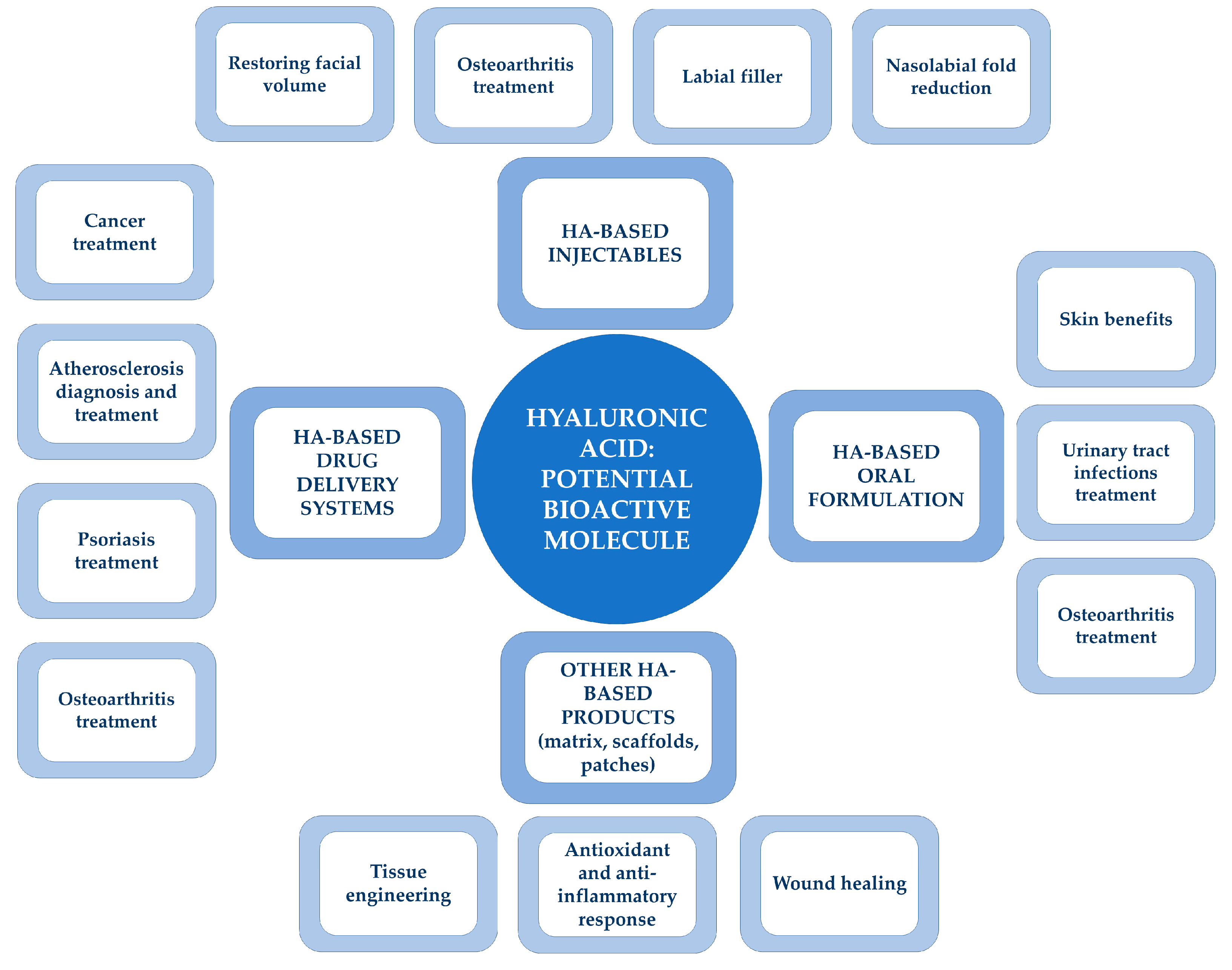
Figure 3. HA: a powerful bioactive molecule. Schematical representation of the most important HA applications in the health-related sector.
References
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715.
- Li, Z.; Tao, L.; Yinhong, X.; Zheng, Z.; Junying, C. A new classification method of nanotechnology for design integration in biomaterials. Nanotechnol. Rev. 2020, 9, 820–832.
- Xing, F.; Li, L.; Zhou, C.; Long, C.; Wu, L.; Lei, H.; Qingquan, K.; Fan, Y.; Xiang, Z.; Zhang, X. Regulation and directing stem cell fate by tissue engineering functional microenvironments: Scaffold physical and chemical cues. Stem Cells Int. 2019, 2019, 16.
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 622.
- Kandasamy, G.; Annenkov, V.; Krishnan, U.M. Nanoimmunotherapy—Cloaked defenders to breach the cancer fortress. Nanotechnol. Rev. 2018, 7, 317–340.
- Kang, J.H.; Kim, Y.Y.; Chang, J.Y.; Kho, H.S. Influences of hyaluronic acid on the anticandidal activities of lysozyme and the peroxidase system. Oral. Dis. 2011, 17, 577–583.
- Wessels, M.R.; Moses, A.E.; Goldberg, J.B.; Dicesare, T.J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 1991, 88, 8317–8321.
- Balazs, E. Hyaluronan as an ophthalmic viscoelastic device. Curr. Pharm. Biotechnol. 2008, 9, 236–238.
- Braccini, F.; Fabian, F.; Garcia, P.; Delmar, H.; Loreto, F.; Benadiba, L.; Nadra, K.; Kestemont, P. Comparative clinical study for the efficacy and safety of two different hyaluronic acid-based fillers with Tri-Hyal versus Vycross technology: A long-term prospective randomized clinical trial. J. Cosmet. Dermol. 2023, 22, 473–485.
- Ogilvie, P.; Benouaiche, L.; Philipp-Dormston, W.G.; Belhaouari, L.; Gaymans, F.; Sattler, G.; Harvey, C.; Schumacher, A. VYC-25L hyaluronic acid injectable gel is safe and effective for long-term restoration and creation of volume of the lower face. Aesthet. Surg. J. 2020, 40, 499–510.
- Kestemont, P.; Fanian, F.; Garcia, P.; Grand-Vincent, A.; Benadiba, L.; Delmar, H.; Bodokh, I.; Brun, P.; Braccini, F.; Desouches, C.; et al. Long-term efficacy and safety of a hyaluronic acid dermal filler based on Tri-Hyal technology on restoration of midface volume. J. Cosmet. Dermol. 2023. online ahead of print.
- Jin, Y.; Koh, R.H.; Kim, S.H.; Kim, K.M.; Park, G.K.; Hwang, N.S. Injectable anti-inflammatory hyaluronic acid hydrogel for osteoarthritic cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 115, 111096.
- Oliviero, F.; Ramonda, R.; Hoxha, A.; Scanu, A.; Galozzi, P.; Favero, M.; Frallonardo, P.; Punzi, L. Effect of an oral preparation containing hyaluronic acid, chondroitin sulfate, hydrolyzed collagen type II and hydrolyzed keratin on synovial fluid features and clinical indices in knee osteoarthritis. A pilot study. Reumatismo 2020, 72, 125–130.
- Kang, L.J.; Yoon, J.; Rho, J.G.; Han, H.S.; Lee, S.; Oh, Y.S.; Kim, H.; Kim, E.; Kim, S.J.; Lim, Y.T.; et al. Self-assembled hyaluronic acid nanoparticles for osteoarthritis treatment. Biomaterials 2021, 275, 120967.
- Narins, R.S.; Brandt, F.S.; Lorenc, Z.P.; Maas, C.S.; Monheit, G.D.; Smith, S.R.; McIntyre, S. A randomized, multicenter study of the safety and efficacy of Dermicol-P35 and non-animal-stabilized hyaluronic acid gel for the correction of nasolabial folds. Dermol. Surg. 2007, 33, 213–221.
- Downie, J.; Mao, Z.; Rachel Lo, T.W.; Barry, S.; Bock, M.; Siebert, J.P.; Bowman, A.; Ayoub, A. A double-blind, clinical evaluation of facial augmentation treatments: A comparison of PRI 1, PRI 2, Zyplast and Perlane. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 1636–1643.
- Bertossi, D.; Sbarbati, A.; Cerini, R.; Barillari, M.; Favero, V.; Picozzi, V.; Ruzzenente, O.; Salvagno, G.; Guidi, G.C.; Nocini, P. Hyaluronic acid: In vitro and in vivo analysis, biochemical properties and histological and morphological evaluation of injected filler. Eur. J. Dermol. 2013, 23, 449–455.
- Göllner, I.; Voss, W.; von Hehn, U.; Kammerer, S. Ingestion of an Oral Hyaluronan Solution Improves Skin Hydration, Wrinkle Reduction, Elasticity, and Skin Roughness: Results of a Clinical Study. J. Evid. Based Complement. Altern. Med. 2017, 22, 816–823.
- Michelotti, A.; Cestone, E.; De Ponti, I.; Pisati, M.; Sparta, E.; Tursi, F. Oral intake of a new full-spectrum hyaluronan improves skin profilometry and ageing: A randomized, double-blind, placebo-controlled clinical trial. Eur. J. Dermol. 2021, 31, 798–805.
- Chung, C.; Lee, J.H. A Single-Center, Randomized, Double-Blind Clinical Trial to Compare the Efficacy and Safety of a New Monophasic Hyaluronic Acid Filler and Biphasic Filler in Correcting Nasolabial Fold. Aesthetic Plast. Surg. 2021, 45, 2902–2908.
- Yi, K.H.; Lee, J.J.; Hur, H.W.; Bae, H.; Kim, H.J. Hyaluronic acid filler injection for deep nasolabial folds: A novel intraoral approach. Clin. Anat. 2022, 35, 820–823.
- Deng, C.; Zhang, Q.; Fu, Y.; Sun, X.; Gong, T.; Zhang, Z. Coadministration of oligomeric hyaluronic acid-modified liposomes with tumor-penetrating peptide-iRGD enhances the antitumor efficacy of doxorubicin against melanoma. ACS Appl. Mater. Interfaces 2017, 9, 1280–1292.
- Zhang, X.; Zhao, M.; Cao, N.; Qin, W.; Zhao, M.; Wu, J.; Lin, D. Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater. Sci. 2020, 8, 1885–1896.
- Hurle, R.; Guazzoni, G.; Colombo, P.; Santoro, A.; De Cobelli, O.; Trapani, E.D.; Nohales, G.; Carlos, L.; Duran-Merino, R.; Lazzeri, M. Oncofid-P-B: A novel treatment for BCG unresponsive carcinoma in situ (CIS) of the bladder: Results of a prospective European Multicentre study at 15 months from treatment start. Urol. Oncol. 2022, 40, 11.e9–11.e15.
- Gonzalez-Fernandez, P.; Rodriguez-Nogales, C.; Jordan, O.; Allémann, E. Combination of mesenchymal stem cells and bioactive molecules in hydrogels for osteoarthritis treatment. Eur. J. Pharm. Biopharm. 2022, 172, 41–52.
- Lee, G.Y.; Kim, J.H.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Choi, K.; Lee, B.H.; Park, J.H.; Kim, I.S. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials 2015, 53, 341–348.
- Huang, C.; Gou, K.; Yue, X.; Zhao, S.; Zeng, R.; Qu, Y.; Zhang, C. A novel hyaluronic acid-based dissolving microneedle patch loaded with ginsenoside Rg3 liposome for effectively alleviate psoriasis. Mater. Des. 2022, 224, 111363.
- Lee, W.H.; Rho, J.G.; Yang, Y.; Lee, S.; Kweon, S.; Kim, H.M.; Yoon, J.; Choi, H.; Lee, E.; Kim, S.H.; et al. Hyaluronic Acid Nanoparticles as a Topical Agent for Treating Psoriasis. ACS Nano 2022, 16, 20057–20074.
- Torella, M.; Del Deo, F.; Grimaldi, A.; Iervolino, S.A.; Pezzella, M.; Tammaro, C.; Gallo, P.; Rappa, C.; De Franciscis, P.; Colacurci, N. Efficacy of an orally administered combination of hyaluronic acid, chondroitin sulfate, curcumin and quercetin for the prevention of recurrent urinary tract infections in postmenopausal women. Eur. J. Obs. Gynecol. Reprod. Biol. 2016, 207, 125–128.
- Dinh, A.; Duran, C.; Hamami, K.; Afif, M.; Bonnet, F.; Donay, J.L.; Lafaurie, M.; Chartier-Kastler, E. Hyaluronic acid and chondroitin sulphate treatment for recurrent severe urinary tract infections due to multidrug-resistant gram-negative bacilli in a patient with multiple sclerosis: Case report and literature review. Open. Forum Infect. Dis. 2022, 9, 245.
- Xing, F.; Zhou, C.; Hui, D.; Du, C.; Wu, L.; Wang, L.; Wang, W.; Pu, X.; Gu, L.; Liu, L.; et al. Hyaluronic acid as a bioactive component for bone tissue regeneration: Fabrication, modification, properties, and biological functions. Nanotechnol. Rev. 2020, 9, 1059–1079.
- Choi, S.; Lee, J.S.; Shin, J.; Lee, M.S.; Kang, D.; Hwang, N.S.; Lee, H.; Yang, H.S.; Cho, S.W. Osteoconductive hybrid hyaluronic acid hydrogel patch for effective bone formation. J. Control. Release 2020, 327, 571–583.
- Saravanakumar, K.; Park, S.; Santosh, S.S.; Ganeshalingam, A.; Thiripuranathar, G.; Sathiyaseelan, A.; Vijayasarathy, S.; Swaminathan, A.; Priya, V.V.; Wang, M.H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022, 222 Pt B, 2744–2760.
- Tsanaktsidou, E.; Kammona, O.; Kiparissides, C. Recent developments in hyaluronic acid-based hydrogels for cartilage tissue engineering applications. Polymers 2022, 14, 839.
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699.
- Andrade del Olmo, J.; Pérez-Álvarez, L.; Sáez Martínez, V.; Benito Cid, S.; Pérez González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Drug delivery from hyaluronic Acid–BDDE injectable hydrogels for antibacterial and anti-inflammatory applications. Gels 2022, 8, 223.
- Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabañas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A Novel Hyaluronic Acid Matrix Ingredient with Regenerative, Anti-Aging and Antioxidant Capacity. Int. J. Mol. Sci. 2023, 24, 4774.
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67.
- Della Sala, F.; Longobardo, G.; Fabozzi, A.; di Gennaro, M.; Borzacchiello, A. Hyaluronic acid-based wound dressing with antimicrobial properties for wound healing application. Appl. Sci. 2022, 12, 3091.
- Mao, Z.; Shin, H.D.; Chen, R. A recombinant E. coli bioprocess for hyaluronan synthesis. Appl. Microbiol. Biotechnol. 2009, 84, 63–69.
- Rehm, B.H. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592.
- Chong, B.F.; Blank, L.M.; Mclaughlin, R.; Nielsen, L.K. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005, 66, 341–351.
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Fact. 2011, 10, 1–9.
- Pires, A.M.B.; Santana, M.H.A. Metabolic effects of the initial glucose concentration on microbial production of hyaluronic acid. Appl. Biochem. Biotechnol. 2010, 162, 1751–1761.
- Zhang, Y.; Luo, K.; Zhao, Q.; Qi, Z.; Nielsen, L.K.; Liu, H. Genetic and biochemical characterization of genes involved in hyaluronic acid synthesis in Streptococcus zooepidemicus. Appl. Microbiol. Biotechnol. 2016, 100, 3611–3620.
- Mandawe, J.; Infanzon, B.; Eisele, A.; Zaun, H.; Kuballa, J.; Davari, M.D.; Jakob, F.; Elling, L.; Schwaneberg, U. Directed Evolution of Hyaluronic Acid Synthase from Pasteurella multocida towards High-Molecular-Weight Hyaluronic Acid. ChemBioChem 2018, 19, 1414–1423.
- Shah, M.V.; Badle, S.S.; Ramachandran, K.B. Hyaluronic acid production and molecular weight improvement by redirection of carbon flux towards its biosynthesis pathway. Biochem. Eng. J. 2013, 80, 53–60.
- Ma, Z.; Fan, H.J.; Lu, C.P. Molecular cloning and analysis of the UDP-Glucose Pyrophosphorylase in Streptococcus equi subsp. zooepidemicus. Mol. Biol. Rep. 2011, 38, 2751–2760.
- Skarzynski, T.; Mistry, A.; Wonacott, A.; Hutchinson, S.E.; Kelly, V.A.; Duncan, K. Structure of UDP-N-acetylglucosamine enolpyruvyl transferase, an enzyme essential for the synthesis of bacterial peptidoglycan, complexed with substrate UDP-N-acetylglucosamine and the drug fosfomycin. Structure 1996, 4, 1465–1474.
- Weigel, P.H. Hyaluronan synthase: The mechanism of initiation at the reducing end and a pendulum model for polysaccharide translocation to the cell exterior. Int. J. Cell Biol. 2015, 2015, 1–15.
- Chen, S.J.; Chen, J.L.; Huang, W.C.; Chen, H.L. Fermentation process development for hyaluronic acid production by Streptococcus zooepidemicus ATCC 39920. Korean J. Chem. Eng. 2009, 26, 428–432.
- Güngör, G.; Gedikli, S.; Toptaş, Y.; Akgün, D.E.; Demirbilek, M.; Yazıhan, N.; Çelik, P.A.; Denkbaş, E.B.; Çabuk, A. Bacterial hyaluronic acid production through an alternative extraction method and its characterization. J. Chem. Technol. Biotechnol. 2019, 94, 1843–1852.
- Zhang, Y.; Dong, J.; Xu, G.; Han, R.; Zhou, J.; Ni, Y. Efficient production of hyaluronic acid by Streptococcus zooepidemicus using two-stage semi-continuous fermentation. Bioresour. Technol. 2023, 377, 128896.
- Saraphanchotiwitthaya, A.; Sripalakit, P. Production of Hyaluronic Acid from Molasses by Streptococcus thermophilus TISTR 458. Trends Sci. 2022, 19, 2192.
- Shoparwe, N.F.; Kew, W.S.; Mohamad, M.; Ameram, N.; Makhtar, M.M.Z. Optimization And Kinetic Analysis On The Production Of Hyaluronic Acid By Streptococcus Zooepidemicus In A Batch System. IOP Conf. 2020, 596, 012046.
- Nasser, H.; Eikmanns, B.J.; Tolba, M.M.; El-Azizi, M.; Abou-Aisha, K. The Superiority of Bacillus megaterium over Escherichia coli as a Recombinant Bacterial Host for Hyaluronic Acid Production. Microorganisms 2022, 10, 2347.
- Lai, Z.W.; Rahim, R.A.; Ariff, A.B.; Mohamad, R. Comparison of hyaluronic acid biosynthesis by the recombinant Escherichia coli strains in different mode of bioreactor operation. J. Microbiol. 2021, 2021, 905–910.
- Cerminati, S.; Leroux, M.; Anselmi, P.; Peirú, S.; Alonso, J.C.; Priem, B.; Menzella, H.G. Low cost and sustainable hyaluronic acid production in a manufacturing platform based on Bacillus subtilis 3NA strain. Appl. Microbiol. Biotechnol. 2021, 105, 3075–3086.
- Yu, H.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for biosynthesis of hyaluronic acid. Metab. Eng. 2008, 10, 24–32.
- Westbrook, A.W.; Ren, X.; Oh, J.; Moo-Young, M.; Chou, C.P. Metabolic engineering to enhance heterologous production of hyaluronic acid in Bacillus subtilis. Metab. Eng. 2018, 47, 401–413.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
25 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




