Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sui Kiat Chang | -- | 2437 | 2023-06-19 15:02:19 | | | |
| 2 | Rita Xu | Meta information modification | 2437 | 2023-06-20 03:19:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Alasalvar, C.; Chang, S.K.; Kris-Etherton, P.M.; Sullivan, V.K.; Petersen, K.S.; Guasch-Ferré, M.; Jenkins, D.J.A. Dried Fruits. Encyclopedia. Available online: https://encyclopedia.pub/entry/45798 (accessed on 07 February 2026).
Alasalvar C, Chang SK, Kris-Etherton PM, Sullivan VK, Petersen KS, Guasch-Ferré M, et al. Dried Fruits. Encyclopedia. Available at: https://encyclopedia.pub/entry/45798. Accessed February 07, 2026.
Alasalvar, Cesarettin, Sui Kiat Chang, Penny M. Kris-Etherton, Valerie K. Sullivan, Kristina S. Petersen, Marta Guasch-Ferré, David J. A. Jenkins. "Dried Fruits" Encyclopedia, https://encyclopedia.pub/entry/45798 (accessed February 07, 2026).
Alasalvar, C., Chang, S.K., Kris-Etherton, P.M., Sullivan, V.K., Petersen, K.S., Guasch-Ferré, M., & Jenkins, D.J.A. (2023, June 19). Dried Fruits. In Encyclopedia. https://encyclopedia.pub/entry/45798
Alasalvar, Cesarettin, et al. "Dried Fruits." Encyclopedia. Web. 19 June, 2023.
Copy Citation
Dried fruits contain many bioactive compounds broadly classified as phytochemicals including phenolics, flavonoids, carotenoids, proanthocyanidins, stilbenes, chalcones/dihydrochalcones, and phytoestrogens. These compounds have antioxidant effects that may benefit health. Dried fruits are also a diverse group of foods with varying fibre contents. The evaluation of the biological activity of these bioactive compounds, including their bioaccessibility and bioavailability, may contribute to the understanding of the health effects of dried fruits.
dried fruits
gut health and microbiome
dietary guidance
health benefits
phytochemicals
1. Introduction
Dried fruits are enjoyed by populations worldwide as a shelf-stable, convenient alternative to fresh fruit. Epidemiological evidence suggests dried fruit consumption is associated with lower risk of cardiovascular disease (CVD), type 2 diabetes (T2D), as well as obesity, various cancers, and other chronic diseases, although the evidence is limited and sometimes contradictory. Nonetheless, dried fruits are nutrient dense and a good source of bioactives/phytochemicals [1].
The biological action of bioactives/phytochemicals in dried fruits is dependent on the food matrix release (e.g., bioaccessibility), bioavailability, and metabolism by colonic microbiota [2]. In 2014, the European Food Safety Authority (EFSA) authorized a health claim for dried plums/prunes and gastrointestinal health [3]. This claim states that “Dried plums/prunes can contribute to normal bowel function”. To obtain the claimed effect, about 100 g/day (~8–12 prunes, depending on their size) of prunes should be consumed. More recently, the effects of dried fruits and the constituent phytochemicals on microbiota composition and functionality have been active areas of investigation. It is now recognized that microbiota contributes to metabolic health and, when aberrant, the development of cardiometabolic diseases. Thus, identifying dietary strategies to promote metabolic health through microbial modulation is a priority.
Evidence from epidemiological and clinical studies suggests that dried fruit intake may improve glucose metabolism and other cardiovascular risk factors, as well as a lower risk for osteoporosis [4][5]. The intake of dried fruits has also been proposed as a strategy to meet fruit recommendations, improve diet quality, and address nutrient deficiencies [6][7].
2. Bioactives/Phytochemicals, Dietary Fibre, and Antioxidant Activity in Dried Fruits
Dried fruits contain a variety of bioactive compounds/phytochemicals such as flavonoids (anthocyanins, flavan-3-ols, flavonols, and flavones), proanthocyanidins (dimer, trimer, 4–6 m, and 7–10 m), phenolic acids (hydroxycinnamic acids and hydroxybenzoic acids), carotenoids (α-carotene, β-carotene, β-cryptoxanthin, lutein, and zeaxanthin), and stilbenes as well as phytoestrogens (isoflavones, lignans, and coumestan) and chalcones/dihydrochalcones [1][8][9]. Among these bioactive phytochemicals, phenolic compounds are the major group (Table 1). Alasalvar et al. [9] reported various phenolic compounds (anthocyanins, flavan-3-ols, flavonols, flavones, phenolic acids, proanthocyanidins, chalcones/dihydrochalcones, and stilbenes) in nine dried fruits (apples, apricots, cranberries, dates, figs, peaches, pears, prunes, and raisins). Some dried fruits (such as apricots, cranberries, dates, figs, prunes, and raisins) have the most diverse phenolic profiles. Little information is available about the exact phenolic profiles of dried apples, peaches, and pears. With regard to carotenoids, which are plant pigments responsible for yellow, orange, and bright red hues in many fruits and vegetables, α-carotene, β-carotene, β-cryptoxanthin, and lutein + zeaxanthin are present in dried fruits except raisins (seedless), albeit in varying quantities. Of these, β-carotene is the most abundant in apricots (2163 μg/100 g), peaches (1074 μg/100 g), and prunes (394 μg/100 g) [10]. Phytoestrogens consist of isoflavones, lignans, and coumestans. Apricots, dates, prunes, and raisins have been reported to contain phytoestrogens. Total phytoestrogen content ranged from 30.3 µg/100 g in raisins (seedless) to 445 µg/100 g in apricots. No phytoestrogens have been reported in dried apples, cranberries, figs, and peaches (Table 1) [11]. Detailed quantitative analysis on different classes of phenolic compounds, carotenoids, and phytoestrogens in different forms and varieties of dried fruits are needed.
Dried fruits are a good source of dietary fibre (3.7–9.8 g/100 g) (Table 1). Consumption of dried fruits (around 20–30 g per/day recommended by many countries) provides 10–16% of the recommended daily intake of fibre (14 g/day), depending on the fruit [10][12][13].
The oxygen radical absorbance capacity (ORAC), a measure of antioxidant activity, of dried fruits is relatively high, although it varies by dried fruit type as well as by cultivar/variety (Table 1). For example, raisins (seedless) have the lowest ORAC values (3037 µmol trolox equivalents (TE)/100 g), whereas raisins (Golden seedless) have the highest ORAC value ((10,450 µmol TE)/100 g). Similarly, ORAC values vary appreciably for dates cultivars of Deglet noor and Medjool [14].
Table 1. Reported bioactives/phytochemicals, dietary fibre, and antioxidant activity in selected dried fruits.
| Phenolics | Carotenoids (μg/100 g) |
Phytoestrogens (μg/100 g) |
Dietary Fibre (g/100 g) |
Antioxidant Activity (μmol of TE/100 g) a |
|
|---|---|---|---|---|---|
| Apples | Flavan-3-ols Flavonols Phenolic acids Chalcones/dihydrochalcones |
Lutein + zeaxanthin (18) | - | 8.7 | 6681 |
| Apricots | Flavan-3-ols Flavonols Flavones Phenolic acids Chalcones/dihydrochalcones |
β-Carotene (2163) | Isoflavones (39.8) Lignans (401) Coumestan (4.2) |
7.3 | 3234 |
| Cranberries | Anthocyanins Flavan-3-ols Flavonols Phenolic acids Proanthocyanidins |
β-Carotene (27) Lutein + zeaxanthin (138) |
- | 5.3 | - |
| Dates | Anthocyanins Flavonols Phenolic acids Proanthocyanidins |
β-Carotene (6) Lutein + zeaxanthin (75) |
Isoflavones (5.1) Lignans (324) Coumestan (0.8) |
8.0 | 2387–3895 b |
| Figs | Anthocyanins Flavan-3-ols Flavonols Flavones Phenolic acids Proanthocyanidins |
β-Carotene (6) Lutein + zeaxanthin (32) |
- | 9.8 | 3383 |
| Peaches | Anthocyanins Flavan-3-ols Flavonols Phenolic acids |
α-Carotene (3) β-Carotene (1074) β-Cryptoxanthin (444) Lutein + zeaxanthin (559) |
- | 8.2 | 4222 |
| Pears | Flavan-3-ols Phenolic acids Chalcones/dihydrochalcones |
β-Carotene (2) Lutein + zeaxanthin (50) |
- | 7.5 | 9496 |
| Prunes | Flavan-3-ols Flavonols Phenolic acids |
α-Carotene (57) β-Carotene (394) β-Cryptoxanthin (93) Lutein + zeaxanthin (148) |
Isoflavones (4.2) Lignans (178) Coumestan (1.8) |
7.1 | 8578 |
| Raisins | Anthocyanins Flavan-3-ols Flavonols Flavones Phenolic acids Stilbenes |
- | Isoflavones (8.1) Lignans (22) Coumestan (0.2) |
3.7 | 3037–10,450 c |
| References | [1][9] | [10] | [11] | [10] | [14] |
a Based on oxygen radical absorbance capacity (ORAC). b Between Deglet noor and Medjool cultivars. c Among white, seedless, and Golden seedless raisins.
Several studies have reported the bioactive compounds and antioxidant activities of dried fruits are higher than those of their corresponding fresh counterparts [15][16][17][18]. This is due to bioactive compounds and antioxidants becoming concentrated after the drying process. However, losses (e.g., carotenoids and anthocyanins) or changes in some compounds occur during drying and storage. Therefore, drying types and duration, as well as storage and packaging are of great importance in terms of functional/nutritional quality and flavour (taste and aroma) of the final product for consumption.
3. Bioaccessibility and Bioavailability of Compounds in Dried Fruits
The bioaccessibility and bioavailability of compounds in dried fruits have been investigated using in vitro models. These models mimic human in vitro GI digestion (e.g., mouth (oral or salivary digestion), stomach (gastric digestion), small intestine (intestinal digestion), and colon or large intestine (colonic digestion)) [19][20][21][22]. Bioaccessibility refers to the level of a compound released from the food matrix during GI digestion that becomes available for absorption (bioavailability) in the intestine [23]. To exert health effects, ingested compounds, including phytochemicals and micronutrients (vitamins and minerals) contained in food, must be released from the food matrix in the GI tract and become bioavailable [21].
Evidence suggests that the phenolics contained within dried fruits are bioaccessible. Recently, Scrob et al. [24] investigated the bioaccessibility of constituents in six dried fruits (dates, raisins, coconuts, cranberries, prunes, and bananas) and demonstrated the highest bioaccessibility of phenolics was observed in prunes and the lowest in cranberries and dates. Total sugars content increased after in vitro digestion of coconuts, dates, and raisins, but it decreased for bananas, cranberries, and prunes. In vitro digestion led to an increase in the antioxidant activity for most dried fruits. This study showed prunes, coconuts, bananas, and raisins are sources of high bioaccessible phenolics. However, the contribution of dried fruit consumption to the recommended dietary allowances (%) was less considering the bioaccessible fraction compared to the total content.
Polar phenol bioaccessibility of dates using a static model of in vitro digestion was also investigated by Panagopoulou et al. [22]. Simulated GI digestion revealed date polar phenols were found to be bioaccessible to an extent depending on the polar phenol class, the nature of the polar phenols, and the specific date matrix. A 37–70% release was observed post-oral digestion, in terms of total phenolic content, which further increased post-gastric digestion (>100%).
Ma et al. [20] investigated the biological activities of kiwifruits and kiwifruit products including dried slices under simulated GI in vitro digestion. Dried slices showed the lowest biological activity compared to those of other kiwifruit products (such as raw fruit, juice, vinegar, wine, yogurt, and jelly). However, dried slices and jam had the highest quantity of minerals (per unit weight). Thus, consuming dried slices and jam could supply more mineral elements than other forms of the fruit [20].
The impact of GI digestion on the total phenolic content (TPC) and antioxidant activities of dried apricots, figs, and raisins was evaluated by Kamiloglu et al. [25]. There was an increase in TPC (0.4–4.5-fold) for all samples after the gastric digestion. The antioxidant activities of dried apricots and figs were increased as determined by various antioxidant activity assays.
In conclusion, in vitro GI digestion studies have some advantages including being fast and inexpensive, without human ethics concerns. However, these digestion systems (static and dynamic) might not completely mimic human physiology. In vitro models need to be compared with in vivo models (particularly, human intervention studies) to better understand the biological effects. These comparative data are essential for demonstrating the biological relevance of bioactive compounds in the context of nutrition and human health [1][25].
4. Dried Fruits, Gut Health, and Microbiota
Diet is an important modulator of the gut microbiota and its metabolite production. The multiple interactions between food components and gut microbiota as well as the modification of the gut microbiota composition and activities by food components contribute to human health [26][27].
4.1. In Vivo Animal Studies
A recent chapter by Muñoz and Lamuela-Raventós [28] reviewed the effects of different dried fruits on gut health and microbiota composition using in vitro and in vivo studies. These in vivo studies were conducted using rat, fish, or broiler chick models. The effects of dried fruits on the modulation of gut microbiota from preclinical studies published following the chapter by Muñoz and Lamuela-Raventós [28] are discussed in this section. Among dried fruits, gut health and the microbiota data are available for goji berries, prunes, and dried cranberries.
In a recent study conducted by Cremonesi et al. [29], New Zealand white rabbits fed chow with 3% goji berries had enrichment of Ruminococcaceae, Lachnospiraceae, Lactobacillaceae, and the genus Lactobacillus, all of which are considered to be beneficial bacteria, compared to a control group fed regular chow. In addition, the supplementation of goji berries enhanced lactic acid fermentation that contributes to the caecal fermentation [29]. Similarly, in a 10-week study involving mice, Tian et al. [30] demonstrated that supplementation of goji berries at 1.5 or 3% modulated the gut microbiota composition by enhancing the growth of beneficial bacteria such as Verrucomicrobia, Bacteroidetes, Bacteroidales S24-7 group, Anaerotruncus, Coprococcus 1, Ruminococcaceae UCG-014, and Akkermansia, while suppressing the growth of harmful bacteria such as Firmicutes, Helicobacter, Bacteroides, and Mucispirillum. Meanwhile, administration of goji berries promoted the growth of short-chain fatty acid (SCFA)-producing bacteria, increasing the production of SCFAs [30]. Finally, Kang et al. [31] studied interleukin (IL)-10-deficient mice and showed feeding with goji berries (1% of dry feed weight) for 14 days enhanced the abundance of Bifidobacteria and butyrate-producing bacteria, Clostridium leptum, and its dominant constituent Fecalibacterium prazusnitzii, compared to control chow fed mice. This resulted in an increase in faecal butyrate content [31].
In another study, the effects of freeze-dried cranberries in dextran sodium sulphate-treated (DSST) male CD-1 mice (to induce colitis) were evaluated [32]. This study showed supplementation with 1.5% (w/w) freeze-dried cranberries (equivalent to 7.5 g of whole cranberry powder) alleviated colitis in DSST mice by reducing the levels of numerous pro-inflammatory cytokines. In addition, treatment with freeze-dried cranberries alleviated the reduced α-diversity of the gut microbiota induced by DSST [32]. Specifically, treatment with freeze-dried cranberries enhanced the abundance of beneficial bacteria, such as Bifidobacterium and Lactobacillus while reducing the abundance of harmful bacteria, such as Suterella and Bilophila [32].
4.2. Human Clinical Trials
Wijayabahu et al. [33] conducted a human clinical trial evaluating the effect of three servings (28.3 g per serving) of sun-dried raisins daily for 14 days on gut microbiota composition in healthy adults. Overall gut microbiota composition was not different after raisin consumption, but specific operational taxonomic units (OTUs) were affected. For example, OTUs matching Faecalibacterium prausnitzii and Ruminococcaceae were significantly enhanced, while OTUs matching Klebsiella spp. and Prevotella spp. were reduced significantly. These taxa, Faecalibacterium prausnitzii and Ruminococcaceae, are important for the breakdown of complex carbohydrates in the gut microbiota [33]. Meanwhile, the reduction in OTUs matching Klebsiella sp. and Prevotella sp. indicated a reduced risk for urinary tract infections and chronic inflammation, respectively [34].
In another randomized, double-blind, cross-over, controlled trial, healthy adults consumed 30 g/day of freeze-dried whole cranberry powder or a placebo for 5 days [35]. Cranberry powder consumption decreased the abundance of Firmicutes, while increasing the abundance of Bacteroidetes. In addition, the consumption of freeze-dried cranberry powder reduced the production of secondary bile acids and prevented the reduction in SCFAs, relative to the control [35]. Bekiares et al. [36] demonstrated that intake of 42 g/d of sweetened dried cranberries (SDC) for 14 days increased the Firmicutes:Bacteroidetes ratio and the relative abundance of Akkermansia. The authors recommended that further studies be conducted using well-controlled study designs and larger sample sizes to better understand the effect of cranberries on the relative abundance of Akkermansia [36].
The effect of prunes on bowel function has also been investigated [37]. Healthy adults (n = 120) consumed either 80 g or 120 g of prunes daily for 4 weeks, with stool weight and frequency as the primary study outcomes. Participants who consumed both 80 g and 120 g of prunes daily had higher stool weight and frequency than the control group. Supplementation with prunes significantly enhanced the relative abundance of Bifidobacteria compared to the control group. However, supplementation of prunes did not affect the levels of SCFA or stool pH in the subjects studied. The authors postulated that the effect of prunes on the gut microbiota could be mediated by fibre content, sorbitol, or phytochemicals in prunes [37]. Hence, more research should be carried out to confirm this result. A recent randomized, open-label, controlled trial evaluated the effects of prune consumption in adult women after undergoing benign gynaecologic surgery [38]. Participants (n = 77) consumed 12 prunes with 100 g docusate sodium (widely used as medicine as laxative and as stool softener) twice daily vs. docusate alone for 3 days. Participants who consumed 12 prunes twice daily had an increased likelihood of a bowel movement and earlier hospital discharge than the control group [38].
Dates have also been tested in a randomized, controlled, cross-over, clinical trial for gut microbiota, and GI function [39]. Healthy adult participants consumed 50 g of dates per day or maltodextrin-dextrose as a control for 21 days, with a 14-day washout period. Adults who consumed prunes daily had higher stool weight and bowel movement frequency than the control group. Supplementation with dates did not cause any significant alterations in the SCFA levels or in the growth of selected bacteria [39].
Most studies conducted to date have examined the effects of dried fruits on microbiota composition, whereas studies on metabolite production and functionality are scarce. Figure 1 summarises the potential mechanisms by which intake of dried fruits may modulate gut microbiota to influence health. Phytochemicals from dried fruits undergo significant biotransformation by gut microbiota, and the resulting metabolites may influence health [40]. Future studies, including in vitro, animal, human, and mechanistic studies are needed to address this research gap.
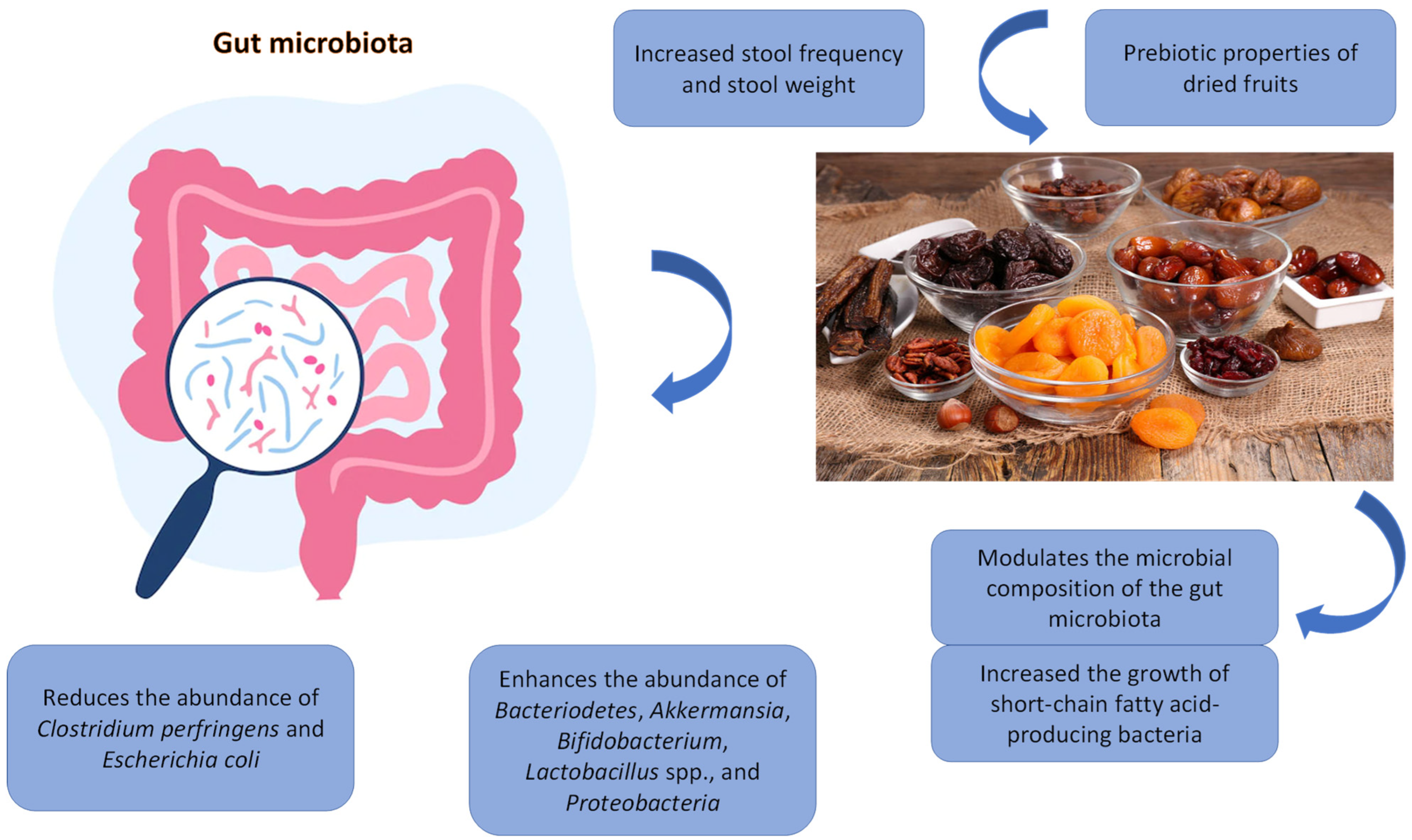
Figure 1. Potential mechanisms of action of dried fruit-related gut microbiota modulation.
References
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of Dried Fruits: Phytochemicals, Antioxidant Efficacies, and Health Benefits. J. Funct. Foods 2016, 21, 113–132.
- Carughi, A.; Gallaher, D.; Mandalari, G. Bioavailability of Nutrients and Phytochemicals from Dried Fruits. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 369–396.
- European Food Safety Authority (EFSA). Scientific Opinion on the Substantiation of a Health Claim Related to Prunes and Contribution to Normal Bowel Function Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2014, 12, 3892.
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E.; Sabaté, J. Health Benefits of Nuts and Dried Fruits: An Overview. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 1–9.
- Arjmandi, B.H.; George, K.S. Bone Health and Osteoprotection. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 469–486.
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. Available online: https://www.dietaryguidelines.gov (accessed on 23 January 2023).
- Health Promotion Knowledge Gateway. Food-Based Dietary Guidelines in Europe. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/topic/food-based-dietary-guidelines-europe_en (accessed on 18 January 2023).
- Alasalvar, C.; Salas-Salvadó, J.; Ros, E. Bioactives and Health Benefits of Nuts and Dried Fruits. Food Chem. 2020, 314, 126192.
- Alasalvar, C.; Chang, S.K.; Shahidi, F. Dried Fruits: Nutrients, Natural Antioxidants, and Phytochemicals. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 335–368.
- U.S. Department of Agriculture (USDA). National Nutrient Database for Standard Reference Legacy Release. 2018. Available online: https://ndb.nal.usda.gov/ndb/search/list (accessed on 1 March 2023).
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen Content of Foods Consumption in Canada, Including Isoflavones, Lignans, and Coumestan. Nutr. Cancer 2006, 54, 184–201.
- U.S. Department of Agriculture (USDA). Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005.
- Silva Caldas, A.P.; Bressan, J. Dried Fruits as Components of Health Dietary Patters. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 513–526.
- U.S. Department of Agriculture (USDA). Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2.0; U.S. Department of Agriculture: Beltsville, MD, USA, 2010.
- Ishiwata, K.; Yamaguchi, T.; Takamura, H.; Matoba, T. DPPH Radical-Scavenging Activity and Polyphenol Content in Dried Fruits. Food Sci. Technol. Res. 2004, 10, 152–156.
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried Fruits: Excellent In Vitro and In Vivo antioxidants. J. Am. Coll. Nutr. 2005, 24, 44–50.
- Rababah, T.M.; Ereifej, K.; Howard, L. Effect of Ascorbic Acid and Dehydration on Concentrations of Total Phenolics, Antioxidant Capacity, Anthocyanins, and Color in Fruits. J. Agric. Food Chem. 2005, 53, 4444–4447.
- Threlfall, R.; Morris, J.; Meullenet, J.F. Product Development and Nutraceutical Analysis to Enhance the Value of Dried Fruit. J. Food Qual. 2007, 30, 552–566.
- McClements, D.J.; Li, Y. Review of In Vitro Digestion Models for Rapid Screening of Emulsion-based Systems. Food Funct. 2010, 1, 32–59.
- Ma, T.; Lan, T.; Geng, T.; Ju, Y.; Cheng, G.; Que, Z.; Gao, G.; Fang, Y.; Sun, X. Nutritional Properties and Biological Activities of Kiwifruit (Actinidia) and Kiwifruit Products under Simulated Gastrointestinal In Vitro Digestion. Food Nutr. Res. 2019, 63, 1674.
- Scrob, T.; Hosu, A.; Cimpoiu, C. The Influence of In Vitro Gastrointestinal Digestion of Brassica Oleracea Florets on the Antioxidant Activity and Chlorophyll, Carotenoid and Phenolic Content. Antioxidants 2019, 8, 212.
- Panagopoulou, E.A.; Chiou, A.; Kasimatis, T.-D.; Bismpikis, M.; Mouraka, P.; Karathanos, V.T. Dried Dates: Polar Phenols and Their Fate during in Vitro Digestion. J. Food Meas. Charact. 2021, 15, 1899–1906.
- Schmite, B.d.F.P.; Bitobrovec, A.; Hacke, A.C.M.; Pereira, R.P.; Los Weinert, P.; Dos Anjos, V.E. In Vitro Bioaccessibility of Al, Cu, Cd, and Pb Following Simulated Gastro-Intestinal Digestion and Total Content of These Metals in Different Brazilian Brands of Yerba Mate Tea. Food Chem. 2019, 281, 285–293.
- Scrob, T.; Covaci, E.; Hosu, A.; Tanaselia, C.; Casoni, D.; Torok, A.I.; Frentiu, T.; Cimpoiu, C. Effect of In Vitro Simulated Gastrointestinal Digestion on Some Nutritional Characteristics of Several Dried Fruits. Food Chem. 2022, 385, 132713.
- Kamiloglu, S.; Pasli, A.A.; Ozcelik, B.; Capanoglu, E. Evaluating the In Vitro Bioaccessibility of Phenolics and Antioxidant Activity during Consumption of Dried Fruits with Nuts. LWT-Food Sci. Technol. 2014, 56, 284–289.
- Moles, L.; Otaegui, D. The Impact of Diet on Microbiota Evolution and Human Health. Is Diet an Adequate Tool for Microbiota Modulation? Nutrients 2020, 12, 1654.
- Zhang, N.; Ju, Z.; Zuo, T. Time for Food: The Impact of Diet on Gut Microbiota and Human Health. Nutrition 2018, 51, 80–85.
- Muñoz, M.M.; Lamuela-Raventós, R.M. Gut Health and Microbiota. In Health Benefits of Nuts and Dried Fruits; Alasalvar, C., Salas-Salvadó, J., Ros, E., Sabaté, J., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020; pp. 487–495.
- Cremonesi, P.; Curone, G.; Biscarini, F.; Cotozzolo, E.; Menchetti, L.; Riva, F.; Marongiu, M.L.; Castiglioni, B.; Barbato, O.; Munga, A. Dietary Supplementation with Goji Berries (Lycium barbarum) Modulates the Microbiota of Digestive Tract and Caecal Metabolites in Rabbits. Animals 2022, 12, 121.
- Tian, B.; Zhang, Z.; Zhao, J.; Ma, Q.; Liu, H.; Nie, C.; Ma, Z.; An, W.; Li, J. Dietary Whole Goji Berry (Lycium barbarum) Intake Improves Colonic Barrier Function by Altering Gut Microbiota Composition in Mice. Int. J. Food Sci. Technol. 2021, 56, 103–114.
- Kang, Y.; Yang, G.; Zhang, S.; Ross, C.F.; Zhu, M. Goji Berry Modulates Gut Microbiota and Alleviates Colitis in IL-10-deficient Mice. Mol. Nutr. Food Res. 2018, 62, 1800535.
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary Cranberry Suppressed Colonic Inflammation and Alleviated Gut Microbiota Dysbiosis in Dextran Sodium Sulfate-Treated Mice. Food Funct. 2019, 10, 6331–6341.
- Wijayabahu, A.T.; Waugh, S.G.; Ukhanova, M.; Mai, V. Dietary Raisin Intake Has Limited Effect on Gut Microbiota Composition in Adult Volunteers. Nutr. J. 2019, 18, 14.
- Clemente, J.C.; Manasson, J.; Scher, J.U. The Role of the Gut Microbiome in Systemic Inflammatory Disease. BMJ 2018, 360, j5145.
- Rodríguez-Morató, J.; Matthan, N.R.; Liu, J.; de la Torre, R.; Chen, C.-Y.O. Cranberries Attenuate Animal-Based Diet-Induced Changes in Microbiota Composition and Functionality: A Randomized Crossover Controlled Feeding Trial. J. Nutr. Biochem. 2018, 62, 76–86.
- Bekiares, N.; Krueger, C.G.; Meudt, J.J.; Shanmuganayagam, D.; Reed, J.D. Effect of Sweetened Dried Cranberry Consumption on Urinary Proteome and Fecal Microbiome in Healthy Human Subjects. Omi. J. Integr. Biol. 2017, 22, 145–153.
- Lever, E.; Scott, S.M.; Louis, P.; Emery, P.W.; Whelan, K. The Effect of Prunes on Stool Output, Gut Transit Time and Gastrointestinal Microbiota: A Randomised Controlled Trial. Clin. Nutr. 2019, 38, 165–173.
- Rasouli, M.A.; Dancz, C.E.; Dahl, M.; Volpe, K.A.; Horton, C.J.; Ozel, B.Z. Effect of Prunes on Gastrointestinal Function after Benign Gynecological Surgery: A Randomized Control Trial. Langenbeck’s Arch. Surg. 2022, 407, 3803–3810.
- Eid, N.; Osmanova, H.; Natchez, C.; Walton, G.; Costabile, A.; Gibson, G.; Rowland, I.; Spencer, J.P.E. Impact of Palm Date Consumption on Microbiota Growth and Large Intestinal Health: A Randomised, Controlled, Cross-over, Human Intervention Study. Br. J. Nutr. 2015, 114, 1226–1236.
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659.
More
Information
Subjects:
Food Science & Technology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.1K
Revisions:
2 times
(View History)
Update Date:
20 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




