
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Roberta Cassano | -- | 2308 | 2023-06-19 10:45:41 | | | |
| 2 | Catherine Yang | + 1 word(s) | 2309 | 2023-06-20 03:05:33 | | |
Video Upload Options
Phenolic compounds are bioactive phytochemicals showing a wide range of pharmacological activities, including anti-inflammatory, antioxidant, immunomodulatory, and anticancer effects. Moreover, they are associated with fewer side effects compared to most currently used antitumor drugs. Combinations of phenolic compounds with commonly used drugs have been largely studied as an approach aimed at enhancing the efficacy of anticancer drugs and reducing their deleterious systemic effects. In addition, some of these compounds are reported to reduce tumor cell drug resistance by modulating different signaling pathways. However, often, their application is limited due to their chemical instability, low water solubility, or scarce bioavailability. Nanoformulations, including polyphenols in combination or not with anticancer drugs, represent a suitable strategy to enhance their stability and bioavailability and, thus, improve their therapeutic activity.
1. Introduction
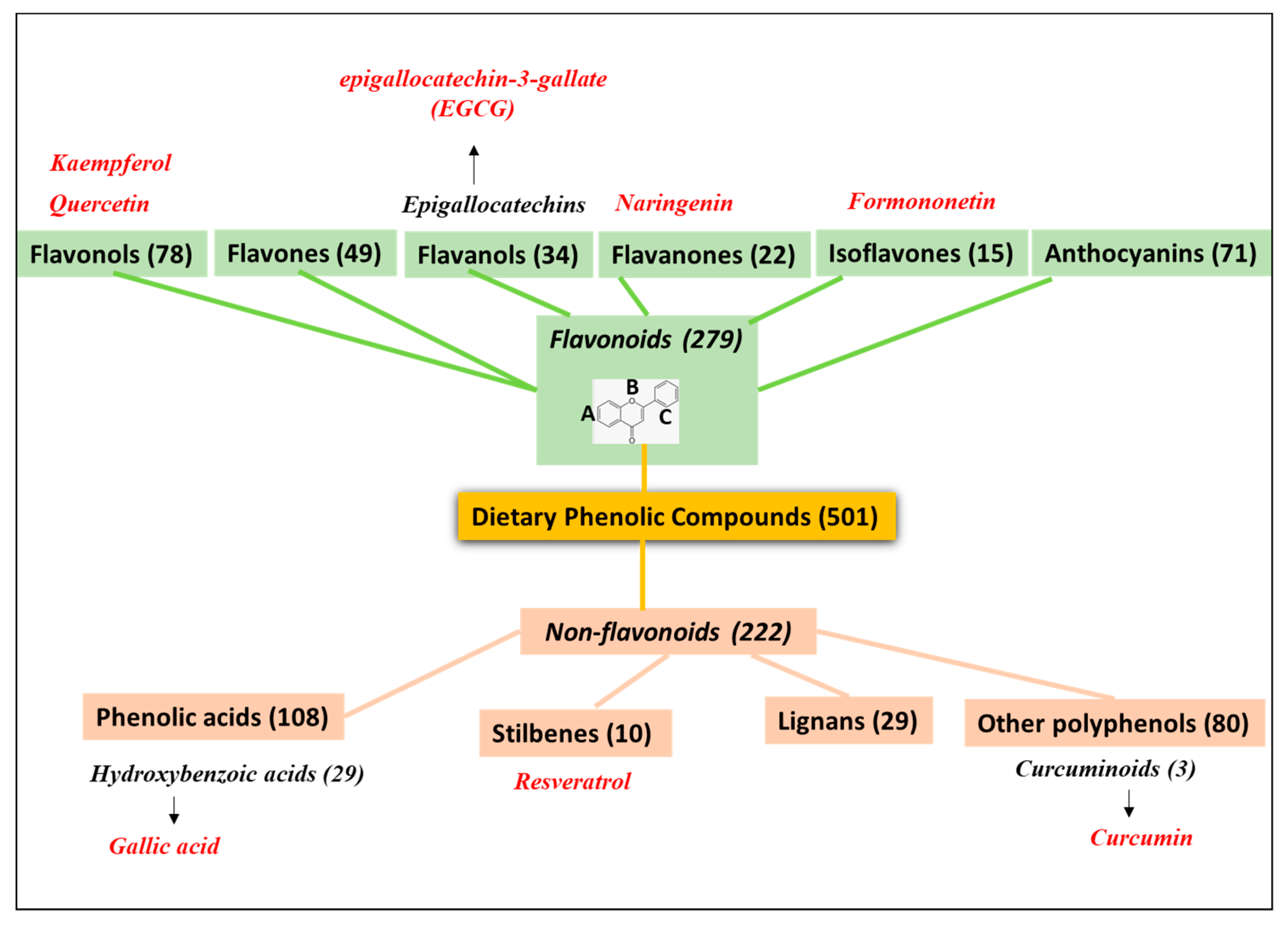
2. PheCs Included in Drug Nanodelivery Systems for Tumor Targeting
3. Hyaluronic Acid: An Efficient Carrier for the Specific Delivery of Antineoplastic Drugs and Natural Bioactive Products
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Canceratlas. Available online: https://canceratlas.cancer.org/wp-content/uploads/2019/10/ACS_CA3_Book.pdf (accessed on 27 January 2023).
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126.
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Majrashi, T.A.; Alshehri, S.A.; Alsayari, A.; Bin Muhsinah, A.; Alrouji, M.; Alshahrani, A.M.; Shamsi, A.; Atiya, A. Insight into the Biological Roles and Mechanisms of Phytochemicals in Different Types of Cancer: Targeting Cancer Therapeutics. Nutrients 2023, 15, 1704.
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29.
- Gan, R.Y.; Chan, C.L.; Yang, Q.Q.; Li, H.B.; Zhang, D.; Ge, Y.Y.; Gunaratne, A.; Ge, J.; Corke, H. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Feng, H., Nemzer, B., DeVries, J.W., Eds.; Woodhead Publishing: Cambridge, UK; AACC International Press: Cambridge, UK, 2019; pp. 191–246. ISBN 9780128115251.
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S.
- Phenol-Explorer. Available online: http://phenol-explorer.eu/compounds (accessed on 17 January 2023).
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750.
- Ma, Y.; Liu, J.; Cui, X.; Hou, J.; Yu, F.; Wang, J.; Wang, X.; Chen, C.; Tong, L. Hyaluronic Acid Modified Nanostructured Lipid Carrier for Targeting Delivery of Kaempferol to NSCLC: Preparation, Optimization, Characterization, and Performance Evaluation In Vitro. Molecules 2022, 27, 4553.
- Guo, Y.; Liu, S.; Luo, F.; Tang, D.; Yang, T.; Yang, X.; Xie, Y. A Nanosized Codelivery System Based on Intracellular Stimuli-Triggered Dual-Drug Release for Multilevel Chemotherapy Amplification in Drug-Resistant Breast Cancer. Pharmaceutics 2022, 14, 422.
- Naseer, F.; Ahmad, T.; Kousar, K.; Kakar, S.; Gul, R.; Anjum, S.; Shareef, U. Formulation for the Targeted Delivery of a Vaccine Strain of Oncolytic Measles Virus (OMV) in Hyaluronic Acid Coated Thiolated Chitosan as a Green Nanoformulation for the Treatment of Prostate Cancer: A Viro-Immunotherapeutic Approach. Int. J. Nanomed. 2023, 18, 185–205.
- Quagliariello, V.; Gennari, A.; Jain, S.A.; Rosso, F.; Iaffaioli, R.V.; Barbarisi, A.; Barbarisi, M.; Tirelli, N. Double-responsive hyaluronic acid-based prodrugs for efficient tumour targeting. Mater. Sci. Eng. C 2021, 131, 112475.
- Qian, J.; Liu, S.; Yang, T.; Xiao, Y.; Sun, J.; Zhao, J.; Zhang, Z.; Xie, Y. Polyethyleneimine- -Tocopherol Hydrogen Succinate/Hyaluronic Acid-Quercetin (PEI-TOS/HA-QU) Core–Shell Micelles Delivering Paclitaxel for Combinatorial Treatment of MDR Breast Cancer. J. Biomed. Nanotechnol. 2021, 17, 382–398.
- Xiong, Q.; Wang, Y.; Wan, J.; Yuan, P.; Chen, H.; Zhang, L. Facile preparation of hyaluronic acid-based quercetin nanoformulation for targeted tumor therapy. Int. J. Biol. Macromol. 2020, 147, 937–945.
- Liu, S.; Li, R.; Qian, J.; Sun, J.; Li, G.; Shen, J.; Xie, Y. Combination Therapy of Doxorubicin and Quercetin on Multidrug-Resistant Breast Cancer and Their Sequential Delivery by Reduction-Sensitive Hyaluronic Acid-Based Conjugate/d-α-Tocopheryl Poly(ethylene glycol) 1000 Succinate Mixed Micelles. Mol. Pharm. 2020, 17, 1415–1427.
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2019, 234, 4959–4969.
- Chen, M.-L.; Lai, C.-J.; Lin, Y.-N.; Huang, C.-M.; Lin, Y.-H. Multifunctional nanoparticles for targeting the tumor microenvironment to improve synergistic drug combinations and cancer treatment effects. J. Mater. Chem. B 2020, 8, 10416–10427.
- Ding, J.; Liang, T.; Min, Q.; Jiang, L.-P.; Zhu, J.-J. “Stealth and Fully-Laden” Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9938–9948.
- Chu, P.-Y.; Tsai, S.-C.; Ko, H.-Y.; Wu, C.-C.; Lin, Y.-H. Co-Delivery of Natural Compounds with a Dual-Targeted Nanoparticle Delivery System for Improving Synergistic Therapy in an Orthotopic Tumor Model. ACS Appl. Mater. Interfaces 2019, 11, 23880–23892.
- Song, Q.; Zhang, G.; Wang, B.; Cao, G.; Li, D.; Wang, Y.; Zhang, Y.; Geng, J.; Li, H.; Li, Y. Reinforcing the Combinational Immuno-Oncotherapy of Switching “Cold” Tumor to “Hot” by Responsive Penetrating Nanogels. ACS Appl. Mater. Interfaces 2021, 13, 36824–36838.
- Huang, W.-Y.; Lai, C.-H.; Peng, S.-L.; Hsu, C.-Y.; Hsu, P.-H.; Chu, P.-Y.; Feng, C.-L.; Lin, Y.-H. Targeting Tumor Cells with Nanoparticles for Enhanced Co-Drug Delivery in Cancer Treatment. Pharmaceutics 2021, 13, 1327.
- Bao, J.; Zhao, Y.; Xu, J.; Guo, Y. Design and construction of IR780- and EGCG-based and mitochondrial targeting nanoparticles and their application in tumor chemo-phototherapy. J. Mater. Chem. B 2021, 9, 9932–9945.
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33.
- Dong, Z.; Wang, Y.; Guo, J.; Tian, C.; Pan, W.; Wang, H.; Yan, J. Prostate Cancer Therapy Using Docetaxel and Formononetin Combination: Hyaluronic Acid and Epidermal Growth Factor Receptor Targeted Peptide Dual Ligands Modified Binary Nanoparticles to Facilitate the in vivo Anti-Tumor Activity. Drug Des. Dev. Ther. 2022, 16, 2683–2693.
- Xiong, Y.; Yang, G.; Zhou, H.; Li, W.; Sun, J.; Tao, T.; Li, J. A ROS-Responsive Self-Assembly Driven by Multiple Intermolecular Interaction Enhances Tumor-Targeted Chemotherapy. J. Pharm. Sci. 2021, 110, 1668–1675.
- Chu, A.J. Quarter-Century Explorations of Bioactive Polyphenols: Diverse Health Benefits. Front. Biosci. 2022, 27, 134.
- Di Giacomo, S.; Percaccio, E.; Gullì, M.; Romano, A.; Vitalone, A.; Mazzanti, G.; Gaetani, S.; Di Sotto, A. Recent Advances in the Neuroprotective Properties of Ferulic Acid in Alzheimer’s Disease: A Narrative Review. Nutrients 2022, 14, 3709.
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221.
- Bento-Silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; Sahlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.-M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2020, 59, 1275–1293.
- Shao, Y.; Luo, W.; Guo, Q.; Li, X.; Zhang, Q.; Li, J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des. Dev. Ther. 2019, 13, 2043–2055.
- de Oliveira, M.M.; Nakamura, C.V.; Auzély-Velty, R. Boronate-ester crosslinked hyaluronic acid hydrogels for dihydrocaffeic acid delivery and fibroblasts protection against UVB irradiation. Carbohydr. Polym. 2020, 247, 116845.
- Chen, Z.; Hao, W.; Gao, C.; Zhou, Y.; Zhang, C.; Zhang, J.; Wang, R.; Wang, Y.; Wang, S. A polyphenol-assisted IL-10 mRNA delivery system for ulcerative colitis. Acta Pharm. Sin. B 2022, 12, 3367–3382.
- Samanta, S.; Rangasami, V.K.; Sarlus, H.; Samal, J.R.; Evans, A.D.; Parihar, V.S.; Varghese, O.P.; Harris, R.A.; Oommen, O.P. Interpenetrating gallol functionalized tissue adhesive hyaluronic acid hydrogel polarizes macrophages to an immunosuppressive phenotype. Acta Biomater. 2022, 142, 36–48.
- Mishra, A.P.; Swetanshu; Singh, P.; Yadav, S.; Nigam, M.; Seidel, V.; Rodrigues, C.F. Role of the Dietary Phytochemical Curcumin in Targeting Cancer Cell Signalling Pathways. Plants 2023, 12, 1782.
- Minnelli, C.; Laudadio, E.; Galeazzi, R.; Barucca, G.; Notarstefano, V.; Cantarini, M.; Armeni, T.; Mobbili, G. Encapsulation of a Neutral Molecule into a Cationic Clay Material: Structural Insight and Cytotoxicity of Resveratrol/Layered Double Hydroxide/BSA Nanocomposites. Nanomaterials 2019, 10, 33.
- Al-Jubori, A.A.; Sulaiman, G.M.; Tawfeeq, A.T.; Mohammed, H.A.; Khan, R.A.; Mohammed, S.A.A. Layer-by-Layer Nanoparticles of Tamoxifen and Resveratrol for Dual Drug Delivery System and Potential Triple-Negative Breast Cancer Treatment. Pharmaceutics 2021, 13, 1098.
- Shi, Q.; Wang, X.; Tang, X.; Zhen, N.; Wang, Y.; Luo, Z.; Zhang, H.; Liu, J.; Zhou, D.; Huang, K. In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci. Nutr. 2021, 9, 3530–3537.
- Shin, G.R.; Kim, H.E.; Ju, H.J.; Kim, J.H.; Choi, S.; Choi, H.S.; Kim, M.S. Injectable click-crosslinked hydrogel containing resveratrol to improve the therapeutic effect in triple negative breast cancer. Mater. Today Bio 2022, 16, 100386.
- Seok, H.-Y.; Rejinold, N.S.; Lekshmi, K.M.; Cherukula, K.; Park, I.-K.; Kim, Y.-C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross-linked zein nanogels for curcumin delivery to cancer cells: In vitro and in vivo evaluation. J. Control. Release 2018, 280, 20–30.
- Wang, Z.; Sau, S.; Alsaab, H.O.; Iyer, A.K. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1441–1454.
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces 2021, 197, 111404.
- Liu, M.; Wang, B.; Guo, C.; Hou, X.; Cheng, Z.; Chen, D. Novel multifunctional triple folic acid, biotin and CD44 targeting pH-sensitive nano-actiniaes for breast cancer combinational therapy. Drug Deliv. 2019, 26, 1002–1016.
- Wang, B.; Zhang, W.; Zhou, X.; Liu, M.; Hou, X.; Cheng, Z.; Chen, D. Development of dual-targeted nano-dandelion based on an oligomeric hyaluronic acid polymer targeting tumor-associated macrophages for combination therapy of non-small cell lung cancer. Drug Deliv. 2019, 26, 1265–1279.
- Xi, Y.; Jiang, T.; Yu, Y.; Yu, J.; Xue, M.; Xu, N.; Wen, J.; Wang, W.; He, H.; Shen, Y.; et al. Dual targeting curcumin loaded alendronate-hyaluronan- octadecanoic acid micelles for improving osteosarcoma therapy. Int. J. Nanomed. 2019, 14, 6425–6437.
- Zhao, M.-D.; Li, J.-Q.; Chen, F.-Y.; Dong, W.; Wen, L.-J.; Fei, W.; Zhang, X.; Yang, P.-L.; Zhang, X.-M.; Zheng, C.-H. Co-Delivery of Curcumin and Paclitaxel by “Core-Shell” Targeting Amphiphilic Copolymer to Reverse Resistance in the Treatment of Ovarian Cancer. Int. J. Nanomed. 2019, 14, 9453–9467.
- Li, Y.; Wu, J.; Lu, Q.; Liu, X.; Wen, J.; Qi, X.; Liu, J.; Lian, B.; Zhang, B.; Sun, H.; et al. GA&HA-Modified Liposomes for Co-Delivery of Aprepitant and Curcumin to Inhibit Drug-Resistance and Metastasis of Hepatocellular Carcinoma. Int. J. Nanomed. 2022, 17, 2559–2575.
- Liu, L.; Yang, S.; Chen, F.; Cheng, K.-W. Polysaccharide-Zein Composite Nanoparticles for Enhancing Cellular Uptake and Oral Bioavailability of Curcumin: Characterization, Anti-colorectal Cancer Effect, and Pharmacokinetics. Front. Nutr. 2022, 9, 846282.
- Wu, J.; Qi, C.; Wang, H.; Wang, Q.; Sun, J.; Dong, J.; Yu, G.; Gao, Z.; Zhang, B.; Tian, G. Curcumin and berberine co-loaded liposomes for anti-hepatocellular carcinoma therapy by blocking the cross-talk between hepatic stellate cells and tumor cells. Front. Pharmacol. 2022, 13, 961788.
- Zamani, M.; Aghajanzadeh, M.; Jashnani, S.; Shahangian, S.S.; Shirini, F. Hyaluronic acid coated spinel ferrite for combination of chemo and photodynamic therapy: Green synthesis, characterization, and in vitro and in vivo biocompatibility study. Int. J. Biol. Macromol. 2022, 219, 709–720.
- Li, X.; He, Y.; Zhang, S.; Gu, Q.; McClements, D.J.; Chen, S.; Liu, X.; Liu, F. Lactoferrin-Based Ternary Composite Nanoparticles with Enhanced Dispersibility and Stability for Curcumin Delivery. ACS Appl. Mater. Interfaces 2023, 15, 18166–18181.
- Gómez-Garduño, J.; León-Rodríguez, R.; Alemón-Medina, R.; Pérez-Guillé, B.E.; Soriano-Rosales, R.E.; González-Ortiz, A.; Chávez-Pacheco, J.L.; Solorio-López, E.; Fernandez-Pérez, P.; Rivera-Espinosa, L. Phytochemicals That Interfere With Drug Metabolism and Transport, Modifying Plasma Concentration in Humans and Animals. Dose-Response 2022, 20, 15593258221120485.
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal–Phenolic Networks as Versatile Coating Materials for Biomedical Applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695.
- Dai, Q.; Geng, H.; Yu, Q.; Hao, J.; Cui, J. Polyphenol-Based Particles for Theranostics. Theranostics 2019, 9, 3170–3190.
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243.
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279.
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928.
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205.
- Chen, D.; Lian, S.; Sun, J.; Liu, Z.; Zhao, F.; Jiang, Y.; Gao, M.; Sun, K.; Liu, W.; Fu, F. Design of novel multifunctional targeting nano-carrier drug delivery system based on CD44 receptor and tumor microenvironment pH condition. Drug Deliv. 2016, 23, 798–803.
- Labie, H.; Perro, A.; Lapeyre, V.; Goudeau, B.; Catargi, B.; Auzély, R.; Ravaine, V. Sealing hyaluronic acid microgels with oppositely-charged polypeptides: A simple strategy for packaging hydrophilic drugs with on-demand release. J. Colloid Interface Sci. 2019, 535, 16–27.
- Johnson, P. CD44 and its Role in Inflammation and Inflammatory Diseases. Inflamm. Allergy-Drug Targets 2009, 8, 208–220.
- Mesrati, M.H.; Syafruddin, S.E.; Mohtar, M.A.; Syahir, A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules 2021, 11, 1850.
- Ponomarev, A.; Gilazieva, Z.; Solovyeva, V.; Allegrucci, C.; Rizvanov, A. Intrinsic and Extrinsic Factors Impacting Cancer Stemness and Tumor Progression. Cancers 2022, 14, 970.
- Wu, K.; Xu, H.; Tian, Y.; Yuan, X.; Wu, H.; Liu, Q.; Pestell, R.G. The role of CD44 in epithelial–mesenchymal transition and cancer development. OncoTargets Ther. 2015, 8, 3783–3792.
- Chen, L.; Fu, C.; Zhang, Q.; He, C.; Zhang, F.; Wei, Q. The role of CD44 in pathological angiogenesis. FASEB J. 2020, 34, 13125–13139.
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618.
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 1–23.
- de la Rosa, J.M.R.; Pingrajai, P.; Pelliccia, M.; Spadea, A.; Lallana, E.; Gennari, A.; Stratford, I.J.; Rocchia, W.; Tirella, A.; Tirelli, N. Binding and Internalization in Receptor-Targeted Carriers: The Complex Role of CD44 in the Uptake of Hyaluronic Acid-Based Nanoparticles (siRNA Delivery). Adv. Healthc. Mater. 2019, 8, e1901182.
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236.




