Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tatyana A. Rutckova | -- | 3190 | 2023-06-15 23:30:24 | | | |
| 2 | Peter Tang | Meta information modification | 3190 | 2023-06-16 05:09:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Popov, A.; Kozlovskaya, E.; Rutckova, T.; Styshova, O.; Vakhrushev, A.; Kupera, E.; Tekutyeva, L. Matrix Metalloproteinases in Cancer Development. Encyclopedia. Available online: https://encyclopedia.pub/entry/45685 (accessed on 08 February 2026).
Popov A, Kozlovskaya E, Rutckova T, Styshova O, Vakhrushev A, Kupera E, et al. Matrix Metalloproteinases in Cancer Development. Encyclopedia. Available at: https://encyclopedia.pub/entry/45685. Accessed February 08, 2026.
Popov, Aleksandr, Emma Kozlovskaya, Tatyana Rutckova, Olga Styshova, Aleksey Vakhrushev, Elena Kupera, Ludmila Tekutyeva. "Matrix Metalloproteinases in Cancer Development" Encyclopedia, https://encyclopedia.pub/entry/45685 (accessed February 08, 2026).
Popov, A., Kozlovskaya, E., Rutckova, T., Styshova, O., Vakhrushev, A., Kupera, E., & Tekutyeva, L. (2023, June 15). Matrix Metalloproteinases in Cancer Development. In Encyclopedia. https://encyclopedia.pub/entry/45685
Popov, Aleksandr, et al. "Matrix Metalloproteinases in Cancer Development." Encyclopedia. Web. 15 June, 2023.
Copy Citation
Matrikines (MKs) can be a rich source of functional nutrition components and additional therapy, thereby contributing to human health care and reducing the risk of developing serious diseases, including cancer. Functionally active MKs as products of enzymatic transformation by matrix metalloproteinases (MMPs) are used for various biomedical purposes. Due to the absence of toxic side effects, low species specificity, relatively small size, and presence of various targets at the cell membranes, MKs often exhibit antitumor properties and, therefore, are promising agents for antitumor combination therapy.
matrikines (MKs)
collagen
antitumor activity
matrix metalloproteinases (MMPs)
1. Introduction
Peptide therapy with matrikines (MKs), biologically active compounds produced via partial proteolysis of collagen proteins and glycosaminoglycans of the extracellular matrix (ECM), has become increasingly popular on the pharmaceutical market. This is because they act as endogenous regulators of many physiological and pathological processes in the body. As a result, more than 60 peptide drugs approved by FDA (Food and Drug Administration, USA) are already used in practical medicine, and more than 600 have undergone clinical and preclinical trials. Accordingly, there is an increasing need for strategies to improve the properties of peptides, such as a longer half-life, higher bioavailability and increased efficiency [1][2].
The main advantage of regulatory MKs over chemical drugs is that being analogs of endogenous compounds, they rarely cause side reactions and exhibit a positive therapeutic effect in relatively small doses. Their activity is determined by the composition and amino acid sequence. The size of active peptides can vary from three to twenty amino acid residues. Due to their endogenous origin, low species specificity, relatively small size and the presence of various targets on the cell membranes, MKs often exhibit multifunctional properties and act as promising agents for targeted therapy of various pathologies, including tumor diseases [3][4][5]. However, despite some progress in the study of the antitumor properties of MKs, the arsenal of truly effective pharmacological agents based on them is extremely small.
A few years ago, the name “matrikines” was coined to refer to peptide products of partial proteolysis of ECM macromolecules capable of regulating cellular activity [6][7]. However, recent data indicate that some of these peptides may modulate proliferation, migration or apoptosis and play an important role in controlling tumor progression. Different modules of proteins that make up ECM macromolecules present new signals to cells in contact with them, capable of activating various intracellular signaling pathways and thus modulating numerous cellular functions. Taken together, it appears that many MKs are able to modulate the growth or invasive properties of tumor cells. In addition to its architectural role, ECM should now be seen as an integrated and dynamic system in which modules constitute a large array of signals to the surrounding cells. MKs produced by partial proteolysis of ECM macromolecules constitute a new family of messengers that control cell activity. Violations in the regulation of this system are often involved in modulating the growth of certain tumors in the connective tissues [5][6][7].
2. Sources of Matrikines
MKs originate from the fragmentation of ECM proteins of different compositions and structures, which regulate cellular activities by interacting with specific receptors. The structure and function of ECM change dramatically in the course of solid cancer development. Therefore, features of the structure and function of various sources of MKs are of great interest.
2.1. Collagen-Derived MKs
Collagen is the most abundant protein in mammals and accounts for over 90% of the total ECM proteins. Collagen fibers provide tensile strength to the ECM and scaffold for cell-to-cell communication. Twenty-eight different collagens containing 46 distinct polypeptide chains have been identified in vertebrates. Collagen polypeptides possess a primary structural Gly-Pro-X repeat (with X often being another proline) responsible for the characteristic right-handed helix secondary structure. The quaternary structure of collagen is a triple helix that contains two α1 chains and one α2 chain. Interstrand hydrogen bonding between proline and hydroxyproline hides the abundant glycine residues (at least one-third of the protein residues) within this trimeric collagen molecule, making them largely inaccessible in solution and thus protected from enzymatic degradation. This triple-helix strand can then be further assembled into either nonfibrillar collagen (type IV) or fibrillar collagen (types I, II, III, and V), with each fibril containing many collagen molecules [8][9][10][11][12][13]. Protection of the core glycine residues from proteolysis is important to maintain the structural integrity of collagen and to escape many inherited disorders of ECM stability [14].
Numerous studies have demonstrated the presence of strong regulatory potential in almost all collagen proteins and ECM peptidoglycans, which make up 25% of the mass of all extracellular proteins [8][9][10]. At the same time, it is important to understand how these MK precursors interact with the cell membrane receptors, regulating numerous signaling pathways both under normal conditions and in various pathologies, including tumorigenesis [11].
It is important to emphasize that even minor changes in the content of collagens and proteoglycans in the ECM of cancer cells ultimately make a significant contribution to their resistance to therapeutic agents and tumor recurrence. Unlike normal epithelial cells, cancer cells do not need to be connected to the ECM in order to survive and proliferate. Due to the resistance to anoikis (a special case of apoptotic cell death) and without proper contact with the ECM, cancer cells can migrate throughout the body and metastasize. This is the reason for targeting pathogenic switches in integrins, tumor antigens, growth factors, and metabolic and signaling pathways acquired by the tumor during malignancy with many potential anticancer MKs. These MKs are in various phases of preclinical and clinical trials. The combinatorial approaches of modern chemotherapy can maximize therapeutic efficacy and minimize the activation of alternative signaling pathways that, on the contrary, may contribute to drug resistance. In this regard, a comprehensive analysis of the mechanisms of action of potential drugs and lead compounds among MKs can provide a rational basis for clinical applications of the respective agents for patients with distinct drug sensitivity profiles [10][11][12]. The pathophysiological functions of collagen in various types of cancer illustrate its dual role and pinpoint therapeutic strategies that can be used in clinical practice (Figure 1) [13].
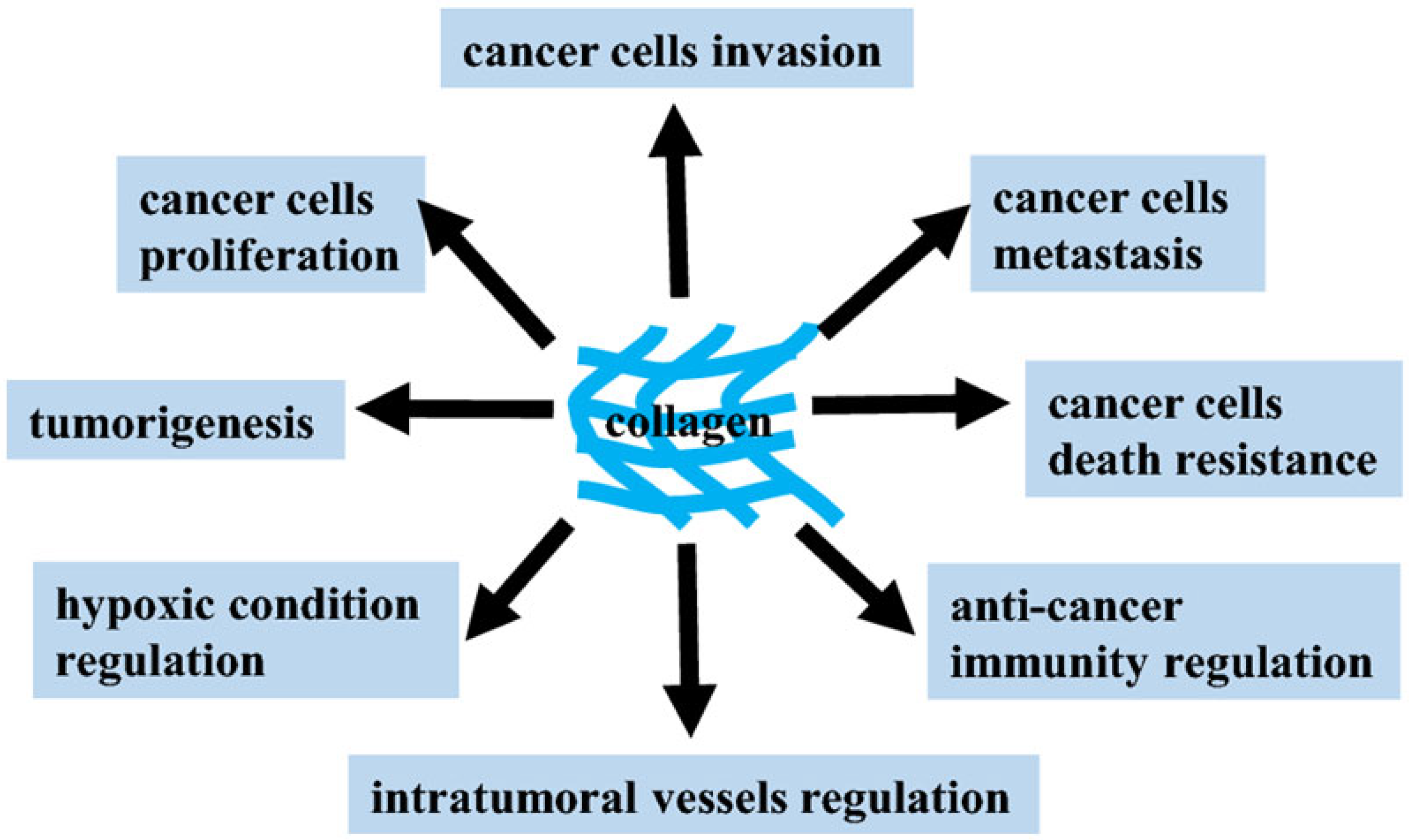
Figure 1. Potential contribution of collagens to the regulation and development of the tumor process [13].
The therapeutic use of a mimetic 20-amino acid peptide derived from type IV collagen has recently been studied in the treatment of breast cancer. The peptide decreased proliferation, adhesion, and migration of endothelial and tumor cells in vitro. In addition, there was a 75% inhibition of triple-negative human cancer cell MDA-MB-231 xenograft growth relative to control when the peptide was administered intraperitoneally for 27 days at 10 mg/kg. The treatment also resulted in an increase in caspase-3 activity and a reduction of microvascular density. The multiple modes of action of this peptide, both anti-angiogenic and anti-tumorigenic, make it a viable candidate as a therapeutic agent for monotherapy or in combination with other compounds [15].
2.2. Elastin-Derived MKs
Elastin is a polymer of tropoelastin that consists of lysine- and alanine-rich domains along with hydrophobic domains that are rich in glycine, valine, and proline repeats. In addition to compromising the structural integrity, proteolytic degradation of elastin enhances an ongoing pulmonary injury by liberating matrikines. Elastin fragments under 50 kDa were capable of potently inducing monocyte chemotaxis. Elastin hexamer VGVAPG repeat was found to be chemotactic to both monocytes and fibroblasts. Further studies demonstrated that this peptide upregulated various MMPs in endothelial cells, fibroblasts, and tumor cells. The conformation of the VGVAPG peptide is critical to its biological function, and the S-Gal receptor (also known as elastin-binding protein [EBP]) is thought to be the primary elastin receptor for this fragment. A variety of proteases degrade elastin, and their tight regulation is critical for the maintenance of health. Elastin-derived peptides are likely elicited by the elastases, macrophage metalloelastase (MMP12) and neutrophil elastase. The destruction of elastin promotes the development and progression of pathological conditions, including chronic obstructive pulmonary disease, atherosclerosis, vascular aneurysms, and cancer [14]. For example, in lung and colon cancer, the degradation of the matrix and fragmentation of elastin was found mainly to occur at the invasive front, and the expression levels of MMP also correlated to the metastatic potential of these cancers [16].
2.3. Hyaluronan-Derived MKs
Hyaluronan (HA) is an anionic glycosaminoglycan found throughout the ECM. HA comprises a large constituent of the basement membrane in solid organs. It is synthesized as a large polysaccharide repeat composed of N-acetyl-d-glycosamine and d-glucuronic acid, widely present in soft tissues. There are three HA synthase isoforms (HAS1–3) and six human hyaluronidase enzymes that degrade HA by hydrolysis of saccharide linkages. Exogenous hyaluronidases produced by bacteria or concentrated in snake and scorpion venom are pathologic factors that mediate tissue invasion and necrosis. The molecular weight of HA is a primary determinant of its function as a matrikine (MK). Like collagen and elastin, the common high-molecular-weight (>500 kDa) HA is important for the tissue’s structural characteristics. The low-molecular-weight (<100 kDa) HA isoforms can ligate receptors to mediate cellular signaling activity. Thus, the availability of tissue-active HA is regulated by the degree of HA hydrolysis or synthesis. Among HA’s effects, the induction of proteases or other matrix lytic enzymes is particularly relevant to its pathologic role. Low–molecular-weight HA induces transcriptional upregulation and release of active forms of MMP12 (which cleaves elastin) and MMP9 (which cleaves collagen), each of which can facilitate further MKs generation. HA activity impacts multiple aspects of cell migration, invasion, angiogenesis, and cellular differentiation [14]. Syndecans are transmembrane proteoglycans with heparan and chondroitin sulfate chains attached to their extracellular domain. They may also exist as soluble extracellular domains. Similar to many proteoglycans, they interact with a multitude of ligands, such as growth factors, adhesion receptors, proteinases, cytokines, chemokines and other ECM proteins to initiate downstream signaling responsible for proliferation, adhesion, angiogenesis, and inflammation. Elevated levels of syndecan expression in cancer can correlate with poor outcomes: Syndecan-1 in breast cancer and Syndecan-2 in colorectal cancer are highly associated with metastasis. HA binds to the cluster of differentiation 44 protein (CD44), a transmembrane receptor that participates in many physiological and pathological processes by activating key signaling cascades. Ligation of CD44 initiates the expression of genes related to tumor growth, proliferation, and survival. CD44 ligation with HA induces cytoskeletal rearrangements and membrane ruffling that leads to active cell migration. Further, CD44 serves as a marker for several types of stem cells [16].
2.4. Laminin-Derived MKs
Laminins are large-molecular-weight heterotrimeric glycoproteins that are designated with three number codes that correspond to their constituent α, β, and γ chains (e.g., laminin 111 or 543). These proteins are found in large quantities in tissue basement membranes, where the C-terminus of the laminin α subunit interacts with basolateral cellular plasma membranes, and the N-termini of all three subunits assemble through coiled-coil domains and project into the basement membrane to interact with other ECM components such as collagen or HA. These glycoproteins transduce both biochemical and mechanical signals between ECM and cells to influence cell survival, differentiation, and migration. Laminins are degraded by serine protease and metalloprotease family members. Laminin 332 (also known as laminin 5) is a component of multiple basement membranes and is implicated in hemidesmosome formation. The cleavage of the γ2 chain of laminin 332 by multiple MMPs (MMP3, -12, -13, -14, and -20) creates biologically active peptides that bind and ligate EGFR to mediate mitogenic epithelial activation and cell migration. MMP14-dependent γ2 cleavage in the lung generates fragments with EGF mimetic activity that induces regenerative alveologenesis. Apart from metalloprotease activity, the serine protease neutrophil elastase has been shown to cleave all three chains of laminin 332, resulting in global neutrophil chemotaxis. More specifically, the neutrophil elastase-mediated cleavage of the γ2 chain releases the γ2 peptide 597–618. This peptide neighbors the MMP2 cleavage site and is chemotactic for polymorphonuclear leukocytes in vitro, though the specific receptor for this peptide remains uncharacterized [14].
Of the synthetic MKs, peptide PCK3145 corresponding to amino acids 31–45 of prostate secretory protein 94 can reduce experimental skeletal metastases and prostate tumor growth. These anti-metastatic and anti-tumoral effects of PCK3145 are partially explained by the in vivo and in vitro decrease in matrix metalloproteinase (MMP)-9 extracellular levels through as yet unidentified molecular mechanisms. It can be assumed that PCK3145 rapidly triggers intracellular signaling through cell surface laminin receptors. This leads to decreased HuR expression and subsequent destabilization of MMP-9 transcripts. HuR protein is a nucleocytoplasmic protein that plays an important role in the regulation of mRNA stability. Dysregulation of its expression has been linked to carcinogenesis. This is the first molecular evidence demonstrating the intracellular signaling and anti-metastatic mechanism of action of PCK3145 that leads to the inhibition of MMP-9 secretion [16][17].
Thus, a complex ensemble of MKs from various sources modulate processes vital for normal and cancer cells, such as proliferation, migration, invasion, autophagy, and angiogenesis, managing tumor aggressiveness and metastatic potential of various malignant neoplasms. In the future, it is necessary to solve the complex problem of managing tumor processes and directing their development toward normalization using MKs and various new therapeutic approaches.
3. The Role of Various MPPs in Cancer Development
MMPs are a large family of Ca2+-dependent Zn2+-containing endopeptidases, which are responsible for the tissue remodeling and degradation of the ECM, including collagens, elastins, gelatin, matrix glycoproteins, and proteoglycan [18]. MMPs are secreted by various cells (fibroblasts, macrophages, smooth muscle cells of the vascular wall, neutrophils, chondrocytes, osteoblasts, keratocytes, etc.) and hydrolyze all components of the extracellular matrix (EM): all collagens and procollagens, proteoglycans, elastin, fibronectin, laminin, as well as adhesive and other connective tissue proteins. Under physiological conditions, there is a balance between the synthesis and breakdown of collagen, which prevents the process of tumor malignancy. Any change in the structure of the ECM means a violation of the stable balance between the rates of synthesis and degradation of its proteins. The degradation of ECM components is carried out by endogenous matrix MMPs. These enzymes play a crucial role in the development of such physiological processes as tissue remodeling, migration, adhesion, cell differentiation and proliferation. It is now known that an increase in MMP activity does not necessarily imply the promotion of tumor growth or metastasis. Moreover, some MMPs have been shown to play a protective role in cancer. It is important to note that the ECM changes strongly in tumors, and these changes can be both protumor and antitumor in nature. There is evidence that MMP-1, -3, -7, -9, -14, -16 and -19 can degrade and regulate endothelial vascular growth factor (VEGF) bioavailability and vascularization in cancer. Exposure of ECM components such as collagen IV, XVIII, perlecan, and heparan sulfate proteoglycan 2 (HSPG2) by various MMPs (-1, -2, -3, -9 or -13) can initiate the production of anti-angiogenic products, such as tumstatin, endostatin, angiostatin and endorepellin [19].
Overexpression and dysregulation of MMPs are often associated with various neoplastic diseases. Therefore, the regulation and inhibition of MMPs is an important therapeutic approach to combat these pathologies, and MMP inhibitors can be used as antitumor agents. However, to date, more than 50 different MMP inhibitors have been found to be ineffective in clinical trials [20][21]. Nevertheless, further research in this direction with the aim of developing new selective MMP inhibitors in the fight against cancer remains relevant. The initial concept of the involvement of MMPs in the development of malignancies was that inhibition of their proteolytic activity reduces ECM remodeling and prevents cell invasion and cancer metastasis [22]. However, often things are much more complicated. For example, increased expression and activity of MMP-8 are associated with good outcomes in oral squamous cell carcinoma and skin cancer but poor outcomes in ovarian, digestive tract, and hepatocellular cancer [23].
Previous generation MMP inhibitors have been developed and tested based only on the extracellular role of MMPs. These inhibitors were non-selective and have not been clinically tested in cancer therapy, mainly due to therapeutic dose-limiting toxicity. Collagen peptide-drug conjugates represent the current generation of targeted therapeutics, like other well-known antibody-drug conjugates. The main advantages of these drugs are increased cell permeability and relatively high selectivity. BT1718 is a drug designed to target and inhibit the function of membrane metalloproteinase type 1 (MT1-MMP) and MMP-2. This drug is usually well tolerated during the course of antitumor therapy, and current data on the pharmacokinetics in blood plasma and tumors is consistent with the proposed preclinical mechanism for targeted delivery of the toxin to the tumor. MT1-MMP and MMP-2 are involved in the breakdown of proteins normally surrounding the cell; however, within cancer cells, these proteinases promote their growth and invasion. The content of MT1-MMP and MMP-2 in normal cells is usually low but can reach higher levels in cancer cells. A growing body of evidence shows that MT1-MMP and MMP-2 are present and active in different subcellular structures. This is important because the discovery of new roles for MT1-MMP and MMP-2 outside the ECM will be of great importance in anticancer therapy [24].
The first clinical trials of the hybrid drug BT1718 showed promising results. However, future studies are needed to identify new intracellular substrates and epigenetic functions of MT1-MMP and MMP-2 in cancer. For example, nuclear MT1-MMP and nuclear/nucleolar MMP-2 may be new therapeutic targets in metastatic cancers. This will help develop more specific inhibitors that will increase the therapeutic efficacy of their interventions in neoplastic diseases [25]. It should be noted that MMP-14 is also the driving force behind tissue destruction during cancer invasion and metastasis and has a significant effect on intercellular communication, regulating the activity of many plasma membrane-anchored and extracellular proteins. For this reason, MMP-14 is an important target for screening new selective inhibitors [26].
There is ample evidence that MMPs are strongly involved in tumor invasion and metastasis tumor growth processes. For example, the results of increased expression of individual MMPs in tumors and the association of specific MMPs with poor prognosis. Recent studies highlight and enhance the role of specific MMPs as key players in tumor growth processes [27].
The tumor microenvironment is formed by cells as well as ECM components and their complex interactions in and around a solid tumor mass. MMPs can degrade ECM components to release MKs. These bioactive peptides often regulate tumor progression and metastasis and can be used for diagnostic purposes. For example, the degradation of perlecan by MMP leads to the formation of several fragments, in particular, the angiostatic endorepellin. Subsequent proteolytic cleavage of endorepellin by proteases and cathepsin L releases a laminin G-like domain that binds the α2β1 integrin. In addition, plasma membrane-associated proteins, such as the G-protein-coupled adhesion B1 receptor and orphan G-protein-coupled receptor protein, can also be cleaved by MMP-14. As a result, such biologically active molecules with a matrikin-like function as angiogenesis-inhibiting vasculostatin-40 are formed. Conversely, MMP activity is regulated by MKs. For example, lamstatin (NC1), the C-terminal domain of the α5-chain of type IV collagen, inhibits tumor cell migration by suppressing both αvβ3 integrin and MMP-14 [28]. Lumican (a small, leucine-rich proteoglycan) binds to MMP-14, selectively inhibits protease activity, and prevents collagen degradation, limiting tumor invasion and progression [29].
Therefore, the study of the main functional mechanisms of the interaction of MKs with MMPs is extremely useful for the development of new selective antitumor agents, markers and treatments. Each MMP has important specific functions both in normal conditions and during tumor development. Given the established role of MMPs in cancer progression and metastasis, it is reasonable to continue to consider them as potential therapeutic targets in the development of cancer therapies.
References
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765.
- Popov, A.M.; Krivoshapko, O.N. Bio-medical properties of peptides from marine organisms. Rus. J. Biopharm. 2014, 6, 3–11.
- Meisel, H. Food-derived bioactive proteins and peptides as potential components of nutraceuticals. Curr. Pharm. Des. 2007, 13, 771–772.
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557.
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467.
- Maquart, F.-X. Les matrikines anti-tumorales: Intérêt potentiel en cancérologie. Anti-tumoral matrikines: Potential interest in oncology. Bull. L’Acad. Natl. Méd. 2010, 194, 633–645.
- Maquart, F.-X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202.
- Korhonen, H.; Pihlanto, A. Food-derived bioactive peptides—Opportunities for designing future foods. Curr. Pharm. Des. 2003, 9, 1297–1308.
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100.
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323.
- Nagaprashantha, L.; Vartak, N.; Awasthi, S.; Awasthi, S.; Singhal, S.S. Novel anti-cancer compounds for developing combinatorial therapies to target anoikis-resistant tumors. Pharm. Res. 2012, 29, 621–636.
- Elango, J.; Hou, C.; Bao, B.; Wang, S.; Maté Sánchez de Val, J.E.; Wenhui, W. The molecular interaction of collagen with cell receptors for biological function. Polymers 2022, 14, 876.
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Trans. Med. 2019, 17, 309.
- Gaggar, A.; Weathington, N. Bioactive extracellular matrix fragments in lung health and disease. J. Clin. Investig. 2016, 126, 3176–3184.
- Rosca, E.V.; Penet, M.F.; Mori, N.; Koskimaki, J.E.; Lee, E.; Pandey, N.B.; Bhujwalla, Z.M.; Popel, A.S. A biomimetic collagen derived peptide exhibits anti-angiogenic activity in triple negative breast cancer. PLoS ONE 2014, 9, e111901.
- Raskov, H.; Gaggar, S.; Tajik, A.; Orhan, A.; Gögenur, I. The matrix reloaded-the role of the extracellular matrix in cancer. Cancers 2023, 15, 2057.
- Annabi, B.; Bouzeghrane, M.; Currie, J.-C.; Dulude, H.; Daigneault, L.; Garde, S.; Rabbani, S.A.; Panchal, C.; Wu, J.J.; Béliveau, R. Inhibition of MMP-9 secretion by the anti-metastatic PSP94-derived peptide PCK3145 requires cell surface laminin receptor signaling. Anticancer Drugs 2006, 17, 429–438.
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical–biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268.
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix metalloproteinases: From molecular mechanisms to physiology, pathophysiology, and pharmacology. Pharm. Rev. 2022, 74, 712–768.
- Sanyal, S.; Amin, S.A.; Banerjee, P.; Gayen, S.; Jha, T. A review of MMP-2 structures and binding mode analysis of its inhibitors to strategize structure-based drug design. Bioorg. Med. Chem. 2022, 74, 117044.
- Rosenbaum, E.; Zahurak, M.; Sinibaldi, V.; Carducci, M.A.; Pili, R.; Laufer, M.; DeWeese, T.L.; Eisenberger, M.A. Marimastat in the treatment of patients with biochemically relapsed prostate cancer: A prospective randomized, double-blind, phase I/II trial. Clin. Cancer Res. 2005, 11, 4437–4443.
- Parganlija, D.; Gehlert, S.; Herrera, F.; Rittweger, J.; Bloch, W.; Zange, J. Enhanced blood supply through lower body negative pressure duringslow-paced, high load leg press exercise alters the response of muscle AMPK and circulating angiogenic factors. Front. Physiol. 2020, 11, 781.
- Cox, T.R. The matrix in cancer. Nat. Rev. Cancer 2021, 21, 217–238.
- Maybee, D.V.; Ink, N.L.; Ali, M.A.M. Novel roles of MT1-MMP and MMP-2: Beyond the extracellular milieu. Int. J. Mol. Sci. 2022, 23, 9513.
- Fu, C.; Yu, L.; Miao, Y.; Liu, X.; Yu, Z.; Wei, M. Peptide-drug conjugates (PDCs): A novel trend of research and development on targeted therapy, hype or hope? Acta Pharm. Sin. B 2023, 13, 498–516.
- Yao, C.; Wu, S.; Kong, J.; Sun, Y.; Bai, Y.; Zhu, R.; Li, Z.; Sun, W.; Zheng, L. Angiogenesis in hepatocellular carcinoma: Mechanisms and anti-angiogenic therapies. Cancer Biol. Med. 2023, 20, 25–43.
- Brown, G.T.; Murray, G.I. Current mechanistic insights into the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2015, 237, 273–281.
- Karousou, E.; Parnigoni, A.; Moretto, P.; Passi, A.; Viola, M.; Vigetti, D. Hyaluronan in the cancer cells microenvironment. Cancers 2023, 15, 798.
- Appunni, S.; Rubens, M.; Ramamoorthy, V.; Anand, V.; Khandelwal, M.; Saxena, A.; McGranaghan, P.; Odia, Y.; Kotecha, R.; Sharma, A. Lumican, pro-tumorigenic or anti-tumorigenic: A conundrum. Clin. Chim. Acta 2021, 514, 1–7.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
891
Revisions:
2 times
(View History)
Update Date:
16 Jun 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




