
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ting-an Zhang | -- | 2264 | 2023-06-15 12:56:08 | | | |
| 2 | Catherine Yang | Meta information modification | 2264 | 2023-06-16 03:18:47 | | |
Video Upload Options
Red mud is a highly alkaline solid waste discharged in the alumina production process. The comprehensive utilization of Bayer red mud is mainly divided into the following aspects. (1) Building materials include the use of red mud for the production of cement or concrete; road cornerstones or pavement materials in road construction; and geopolymers, ceramics, or composites. (2) Applications in the environmental field include the use of red mud to remove heavy metals and improve acidic soils. (3) Applications in the chemical industry include the use of red mud to produce dyes, catalysts, coagulants, or adsorbents. (4) Recovery of valuable components from red mud includes recovery of alkali and extraction of elements, such as aluminum, iron, titanium, and scandium, and important metals, such as vanadium and gallium.
1. Building Material
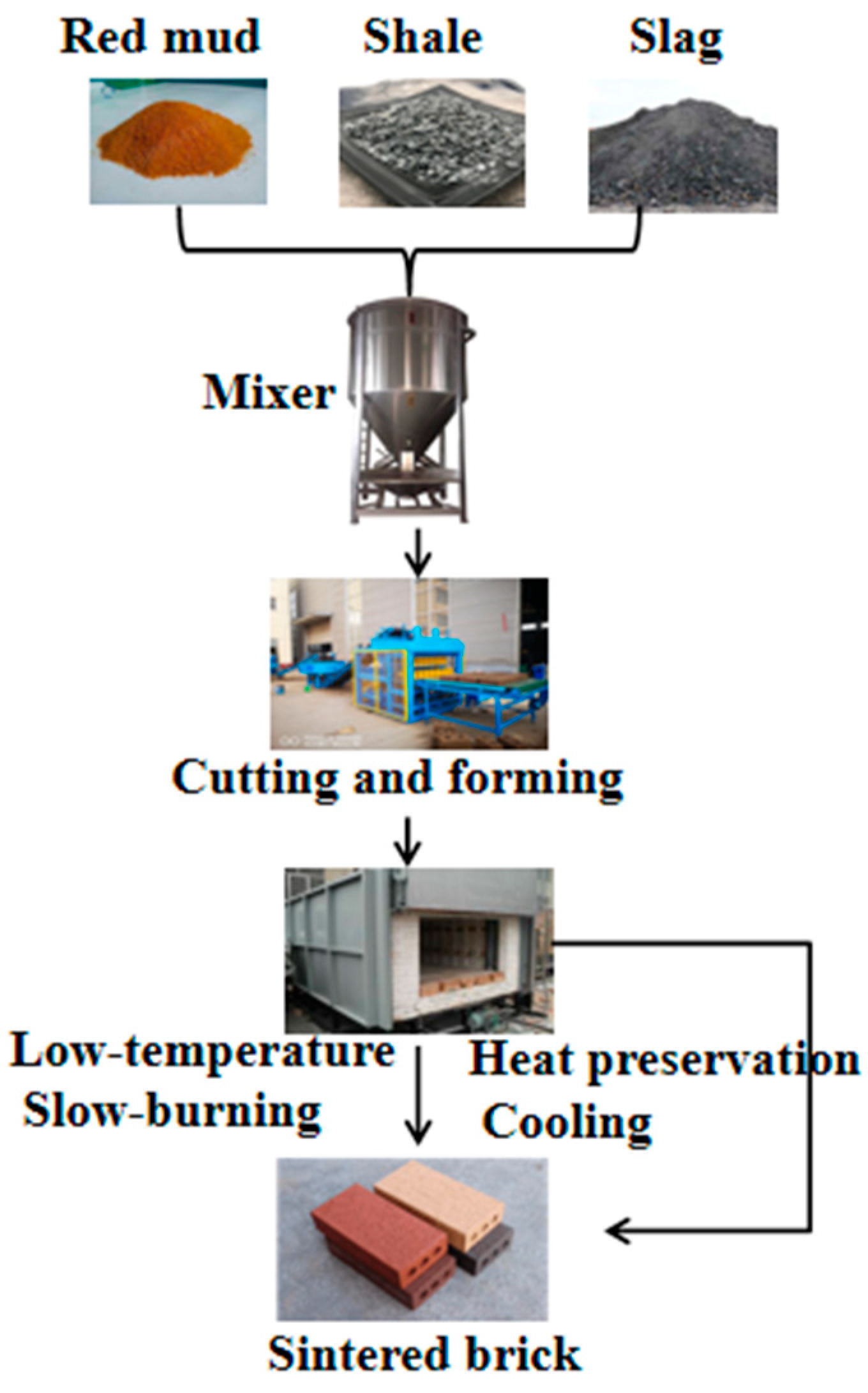
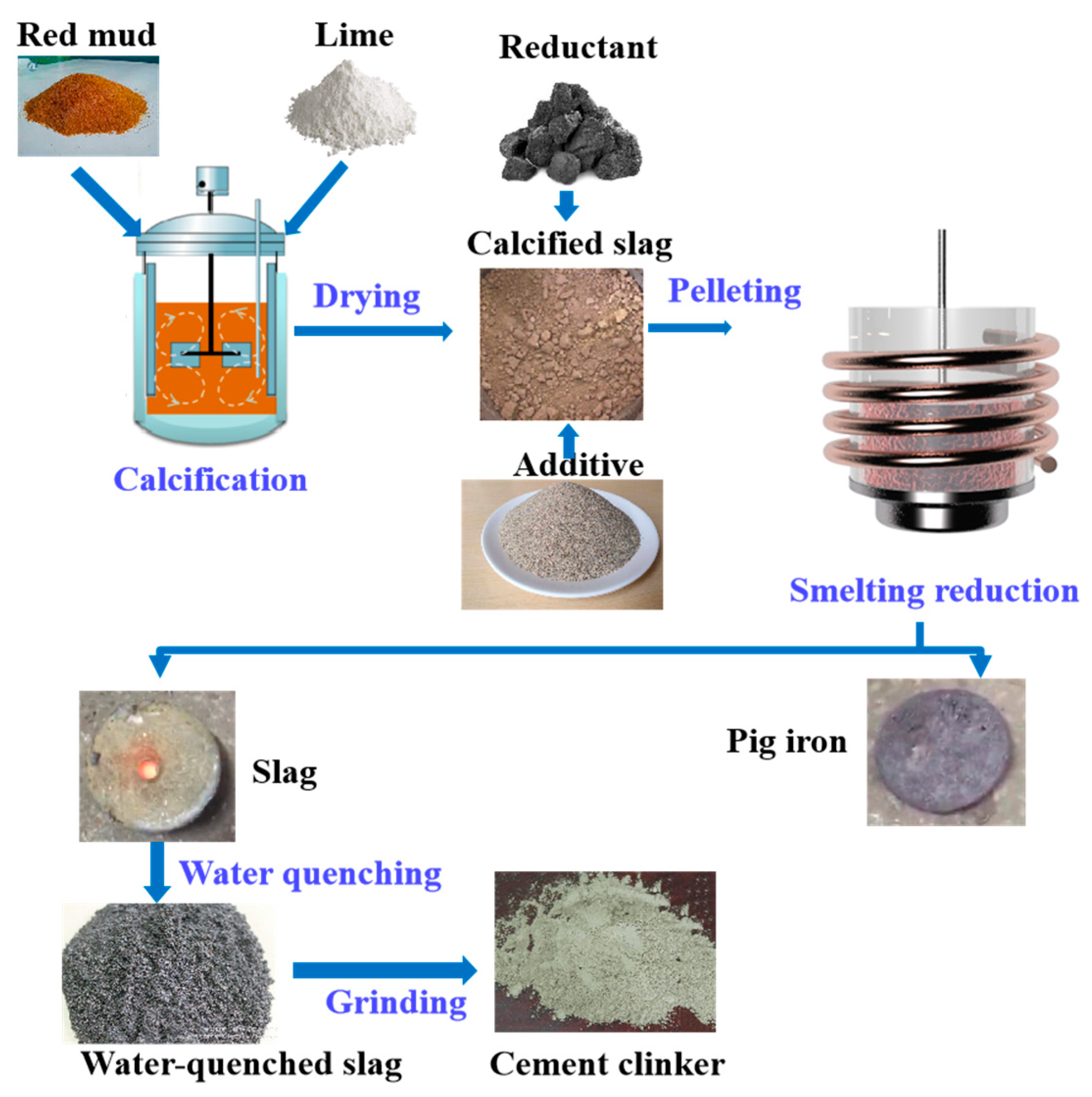
2. Agriculture and Environment
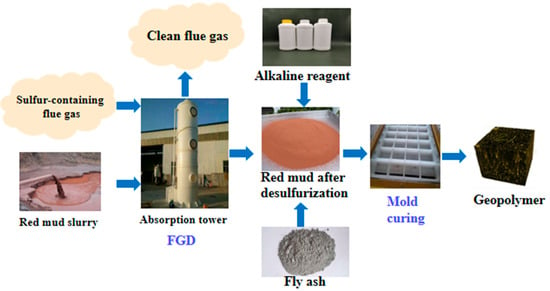
3. Chemical Industry
References
- Zhao, J.; Wang, L.; Xie, X. Preparation of Sintering-expanded Haydite with Red Mud from Byer Process. Multipurp. Util. Miner. Resour. 2009, 4, 41–45.
- Lu, H. Present Situation and Prospect of Comprehensive Utilization of Red Mud. Hunan Nonferrous Met. 2022, 38, 60–64, (In Chinese with English Abstract).
- He, S.; Jiang, S.; Wang, W. Research progress of utilizing red mud as resource of building material in China. Light Met. 2007, 12, 1–5.
- Pontikes, Y.; Angelopoulos, G.N. Bauxite residue in cement and cementitious applications: Current status and a possible way forward. Resour. Conserv. Recycl. 2013, 73, 53–63.
- Wang, Y.; Zhang, T.; Zhang, Y.; Lv, G.; Zhang, W. Transformation and characterization of cement clinker prepared from new structured red mud by sintering. JOM J. Miner. Met. Mater. Soc. 2019, 71, 2505–2512.
- Yu, S.; Dong, F.; Yang, X. Brief description of the industrial method of comprehensive utilization of red mud. China Met. Bull. 2019, 192–193, (In Chinese with English Abstract).
- Jin, K.; Wang, J.; Lv, C.; Jia, Y. The Analysis of Efflorescence about Sintered Red Mud Brick. Guangzhou Chem. Ind. 2013, 41, 72–74, (In Chinese with English Abstract).
- Thakur, R.S.; Sant, B.R. Utilization of red mud: Part I—Analysis and utilization as raw material for absorbents, building materials, catalysts, fillers, paints and pigments. J. Sci. Ind. Res. 1983, 42, 87–108.
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of iron rich cements using red mud. Cem. Concr. Res. 1997, 27, 1037–1046.
- Singh, M.; Upadhayay, S.N.; Prasad, P.M. Preparation of special cements from red mud. Waste Manag. 1996, 16, 665–670.
- Tsakiridis, P.E. Agatzini-Leonardou, S.; Oustadakis, P. Red mud addition in the raw meal for the production of Portland cement clinker. J. Hazard. Mater. 2004, 116, 103–110, (In Chinese with English Abstract).
- Vangelatos, I.; Angelopoulos, G.N.; Boufounos, D. Utilization of ferroalumina as raw material in the production of Ordinary Portland Cement. J. Hazard. Mater. 2009, 168, 473–478.
- Hindalco. Available online: http://www.hindalco.com/media/Press-releases/hindalcosupply-1.2-mn-mt-of-red-mud-to-ultratech-two-flagship-aditya-birla-group (accessed on 18 March 2022).
- Zhang, T.; Wang, Y.; Lu, G.; Liu, Y.; Zhang, W.; Zhao, Q. Comprehensive Utilization of Red Mud: Current Research Status and a Possible Way Forward for Nonhazardous Treatment. TMS Annu. Meeting Light Met. 2018, 2018, 135–141.
- Zhang, T.; Wang, K.; Liu, Y.; Lyu, G.; Li, X.; Chen, X. A review of comprehensive utilization of high-iron red mud of China. TMS Annu. Meeting Light Met. 2020, 2020, 65–71.
- Liu, X.; Zhang, N. Utilization of red mud in cement production: A review. Waste Manag. Res. 2011, 29, 1053–1063.
- Pappu, A.; Saxena, M.; Asolekar, S.R. Solid wastes generation in India and their recycling potential in building materials. Build. Environ. 2007, 42, 2311–2320.
- Agarwal, G.; Speyer, R.F. Devitrifying cupola slag for use in abrasive products. JOM 1992, 44, 32–37.
- Peng, F.; Liang, K.M.; Hua, S.; Hu, A.M. Nanocrystal glass-ceramics obtained by crystallization of vitrified red mud. Chemosphere 2005, 59, 899–903.
- Yang, J.; Zhang, D.; Jian, H.; He, B.; Bo, X. Preparation of glass-ceramics from red mud in the aluminium industries. Ceram. Int. 2008, 34, 125–130.
- Wang, Z.; Han, M.; Zhang, Y.; Zhou, F. Study on the Dealkalization Technics of Bayer-process Red Mud with CO2 by Carbonation. Bull. Chin. Ceram. Soc. 2013, 32, 1851–1855, (In Chinese with English Abstract).
- Wang, Z.; Lu, F.; Gu, X.; Peng, N.; Hu, C. Status of research on red mud dealkalization. Guizhou Agric. Mech. 2020, 15–18, (In Chinese with English Abstract).
- Zhu, X.; Li, W.; Guan, X. An active dealkalization of red mud with roasting and water leaching. J. Hazard. Mater. 2015, 286, 85–91.
- Wang, K.; Liu, Y.; Dou, Z.; Lv, G.; Li, X.; Zhang, T. A Novel Method of Extracting Iron from High-Iron Red Mud and Preparing Low-Carbon Cement Clinker from Tailings. JOM 2022, 74, 2750–2759.
- Brunori, C.; Cremisini, C.; Massanisso, P.; Pinto, V.; Torricelli, L. Reuse of a treated red mud bauxite waste: Studies on environmental compatibility. J. Hazard. Mater. 2005, 117, 55–63.
- Hamdy, M.K.; Williams, F.S. Bacterial amelioration of bauxite residue waste of industrial alumina plants. J. Ind. Microbiol. Biotechnol. 2001, 27, 228–233.
- Alva, A.K.; Huang, B.; Paramasivam, S.; Sajwan, K.S. Evaluation of root growth limiting factors in spodic horizons of spodosols. J. Plant Nutr. 2002, 25, 2001–2014.
- Ciccu, R.; Ghiani, M.; Serci, A.; Fadda, S.; Peretti, R.; Zucca, A. Heavy metal immobilization in the mining-contaminated soils using various industrial wastes. Miner. Eng. 2003, 16, 187–192.
- Snars, K.; Gilkes, R.J. Evaluation of bauxite residues (red muds) of different origins for environmental applications. Appl. Clay Sci. 2009, 46, 13–20.
- Feigl, V.; Ujaczki, E.; Vaszita, E.; Molnar, M. Influence of red mud on soil microbial communities: Application and comprehensive evaluation of the biology ecoplate approach as a tool in soil microbiological studies. Sci. Total Environ. 2017, 595, 903–911.
- Summers, R.N.; Guise, N.R.; Smirk, D.D.; Summers, K.J. Bauxite residue (red mud) improves pasture growth on sandy soils in Western Australia. Aust. J. Soil Res. 1996, 34, 569–581.
- Snars, K.E.; Gilkes, R.J.; Wong, M.T.F. The liming effect of bauxite processing residue (red mud) on sandy soils. Soil Res. 2004, 42, 321–328.
- Menzies, N.W.; Snars, K.E.; Kopittke, G.R.; Kopittke, P.M. Amelioration of cadmium contaminated soils using cation exchangers. J. Plant Nutr. 2009, 32, 1321–1335.
- Khairul, M.A.; Jafar, Z.; Moghtaderi, B. The composition, recycling and utilization of Bayer red mud. Resour. Conserv. Recycl. 2018, 141, 483–498.
- Fois, E.; Lallai, A.; Mura, G. Sulfur Dioxide Absorption in a Bubbling Reactor with Suspensions of Bayer Red Mud. Ind. Eng. Chem. Res. 2007, 46, 6770–6776.
- Summers, R.N.; Pech, J.D. Nutrient and metal content of water, sediment and soils amended with bauxite residue in the catchment of the Peel Inlet and Harvey Estuary, Western Australia. Agric. Ecosyst. Environ. 1997, 64, 219–232.
- Jing, Y.; Jing, Y.; Yang, Q. Basic properties and engineering properties of red mud. Light Met. 2001, 27, 20–23, (In Chinese with English Abstract).
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burke, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582.
- Li, B.; Wu, H.; Wang, Z.; Wang, J.; Li, M.; Ning, P. Research Progress of Desulfurization and Denitrification of Alkaline Solid Waste Red Mud. Bull. Chin. Ceram. Soc. 2019, 38, 1401–1407+1419, (In Chinese with English Abstract).
- Wang, X.; Zhang, Y.; Lv, F.; An, Q.; Lu, R.; Hu, P.; Jiang, S. Removal of alkali in the red mud by SO2 and simulated flue gas under mild conditions. Environ. Prog. Sustain. Energy 2015, 34, 81–87.
- Luo, D.; Li, Z.; Du, Q.; Zhang, J.; Zhang, X. Research progress on comprehensive utilization of red mud. Technol. Innov. Appl. 2020, 2009, 5–76, (In Chinese with English Abstract).
- Chen, Y.; Li, J.Q.; Huang, F.; Zhou, J.; Liu, W. The Performance Research on Absorbing SO2 Waste. J. Guizhou Univ. Technol. Nat. Sci. Ed. 2017, 30–37, (In Chinese with English Abstract).
- Nie, Q.; Hu, W.; Huang, B.; Shu, X.; He, Q. Synergistic utilization of red mud for flue-gas desulfurization and fly ash-based geopolymer preparation. J. Hazard. Mater. 2019, 369, 503–511.
- Yan, Y.; Chang, Z.; Fu, Y. Advances in research on red mud utilization. China Energy Environ. Prot. 2020, 42, 134–138, (In Chinese with English Abstract).
- Han, Y.; Wang, J.; Tang, M. Adsorption of Hexavalent Chromium in Wastewater on Modified Red Mud. Environ. Prot. Chem. Ind. 2005, 25, 132–136, (In Chinese with English Abstract).
- Liu, Y.J.; Naidu, R.; Ming, H. Red mud as an amendment for pollutants in solid and liquid phases. Geoderma 2011, 163, 1–12.
- Sutar, H. Progress of Red Mud Utilization: An Overview. Am. Chem. Sci. J. 2014, 4, 255–279.
- Samal, S.; Ray, A.K.; Bandopadhyay, A. Proposal for resources, utilization and processes of red mud in India-A review. Int. J. Miner. Process. 2013, 118, 43–55.
- Fang, H.; Liang, W.; Ren, S.; Yang, F.; Ma, L. Preparation of modified red mud-based catalysts and their catalytic combustion performance for toluene. China Environ. Sci. 2021, 41, 5764–5770, (In Chinese with English Abstract).
- Sushil, S.; Batra, V.S. Catalytic applications of red mud, an aluminium industry waste: A review. Appl. Catal. B Environ. 2008, 81, 64–77.
- Li, F. Superficial Modification of Porous Ceramicas Filter Media on the Basis of Red Mud and Its Application in the Water Treatment. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2008.
- Xu, X.; Di, Y.; Wu, J.; Lei, Z.; Hong, J.; Lu, J.; Liu, X.; Deng, Q. Study on Preparing Porous Ceramic Filter Material Msde from Solid Waste. J. Wuhan Univ. Technol. 2004, 26, 12–15, (In Chinese with English Abstract).




