
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cesareo Saiz Jimenez | -- | 988 | 2023-06-15 02:54:18 | | | |
| 2 | Jessie Wu | -1 word(s) | 987 | 2023-06-15 03:07:18 | | | | |
| 3 | Jessie Wu | -5 word(s) | 982 | 2023-06-15 03:32:10 | | | | |
| 4 | Jessie Wu | Meta information modification | 982 | 2023-06-15 03:32:41 | | | | |
| 5 | Jessie Wu | + 2 word(s) | 984 | 2023-06-15 03:34:18 | | | | |
| 6 | Jessie Wu | + 14 word(s) | 998 | 2023-06-15 03:35:33 | | | | |
| 7 | Cesareo Saiz Jimenez | Meta information modification | 998 | 2023-06-17 09:08:42 | | | | |
| 8 | Jessie Wu | Meta information modification | 998 | 2023-06-19 08:53:52 | | |
Video Upload Options
The genus Crossiella contains two species, C. equi, causing nocardioform placentitis in horses, and C. cryophila, an environmental bacterium.
1. Introduction
“Rare actinobacteria” are non-Streptomyces actinobacteria whose isolation frequency is much lower than Streptomyces strains, commonly isolated by conventional methods [1][2]. Tiwari and Gupta [3][4] reported 120 new genera of “rare actinobacteria” in the first decade of the 21th century. A total of 40 out of 120 genera were isolated from soils, with comparatively lower percentages from other environments: marine and freshwater sediments, marine animals, plants, buildings, etc. A few reports included the rare genera Actinomadura, Nonomuraea, Micromonospora, Streptosporangium, Nocardiopsis, and Pseudonocardia as the most frequent in diverse environments [5][6][7][8][9]. It is noteworthy an abundance of “rare actinobacteria” in extreme environments, as exemplified in Atacama [7] and other deserts [10][11][12][13]. However, Crossiella has not been included among “rare actinobacteria” thus far. Researchers have found that Crossiella is an abundant genus in most studied Spanish caves, whether they are gypsum, karstic or volcanic [14][15][16][17][18][19][20][21], and in other terrestrial and aquatic environments.
2. The Genus Crossiella in Caves
The genus Crossiella contains two species, C. equi, causing nocardioform placentitis in horses, and C. cryophila, an environmental bacterium. Table 1 shows the occurrence of Crossiella in different Spanish caves. The high relative abundance of this genus is in moonmilks (Figure 1), either from karstic (Pindal) or volcanic (Fuente de la Canaria and Bucara II) caves, as well as in colored biofilms (Pindal, Altamira, Castañar, Covadura, Yeso) [[17][18][19][20][21], and unpublished data]. The relative humidity is near 100% in these caves. In addition, other mineral/biological formations, such as a pink formation in Bucara II, exhibit high relative abundance (38.9%). Similarly, formations such as mucous formations or brown deposits also reach relatively high abundances (6.7-12.8%) [19]. Interestingly, low percentages of Crossiella found in the sediments under the moonmilk indicates an aerobic behaviour for this genus [22]. Crossiella was also found in phototrophic biofilms from Nerja Cave [20].
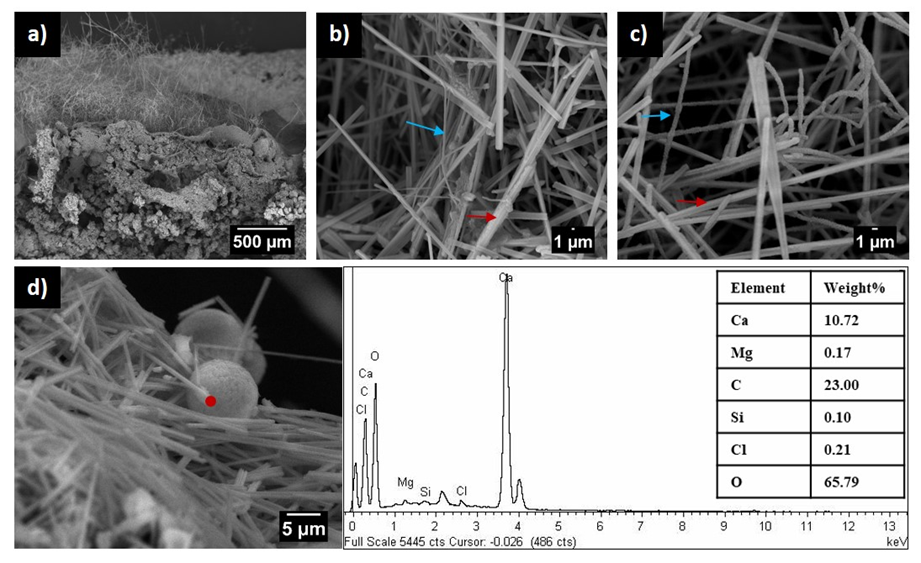
Figure 1. Scanning electron micrographs of moonmilk deposits in Pindal Cave, Spain. a) Longitudinal view of sediment covered by moonmilk. b-c) Crystalline calcite fibers (red arrow) and Actinomycetota filaments (blue arrow). d) Scanning electron micrographs and EDX spectra of crystalline calcite fibers. Note the swelling of filaments in c), similar to those reported for Crossiella cryophila [22].
| Cave | Relative Abundance | Genus | Type of Sample | References |
|---|---|---|---|---|
| Pindal | 16.0–27.1 | Crossiella | Moonmilk | [16][18][21] |
| 1.4–1.7 | Crossiella | Sediment under moonmilk | ||
| 11.3–11.7 | Crossiella | Top-layer sediments | ||
| 6.0–9.0 | Crossiella | Sediments | ||
| 5.3–7.9 | Crossiella | Yellow biofilm | ||
| 2.0–8.0 7.0–8.0 |
Crossiella Crossiella |
Grey biofilms Pink biofilms |
||
| Fuente de la Canaria | 12.6–12.8 | Crossiella | Mucous formations | [19] |
| 12.3 | Crossiella | Moonmilk | ||
| 6.7 | Crossiella | Brown and yellow deposits | ||
| Bucara II | 38.9 24.9 |
Crossiella Crossiella |
Pink deposit Moonmilk |
[19] |
| Nerja | 0.1–1.5 | Crossiella | Phototrophic biofilms | [20] |
| Castañar | 15.0 | Crossiella | Grey biofilm | [21] |
| Altamira | >20.0 27.0 38.0 |
Crossiella Crossiella Crossiella |
Grey biofilms White biofilms Yellow biofilms |
[23] |
| Covadura | 26.4–54.1 21.8–51.9 4.5–19.7 |
Crossiella Crossiella Crossiella |
White biofilm Yellow biofilm Sediments |
Unpublished data |
| Yeso | 1.3–13.3 | Crossiella | Sediments | Unpublished data |
| Thyssen Museum basement |
16.6 64.2 2.8–7.4 |
Crossiella Crossiella Crossiella |
White biofilm Grey biofilm Sediment |
[24] |
Crossiella, at a relative abundance of 15.0% for was found in grey biofilms from Castañar Cave [21]. Similar grey biofilms were observed in Altamira Cave [23] and the Thyssen Museum, reaching a relative abundance of 64.2% [24]. Data from a geomicrobiological study of a Roman Nymphaeum located in the archaeological basement of Thyssen Museum, Malaga, Spain, were also included in Table 1 due to its interest.
The environmental conditions of this archaeological basement are special because they mix the characteristics of an environment heavily influenced by the natural underlying karst system with those of an enclosure located in a urban building. Apart from caves, it was remarkable that a subterranean environment, the Roman mortar pavement of the archaeological basement, was colonized by grey biofilms with a high relative abundance of Crossiella. This environment is characterized by permanent darkness, absence of visits and high relative humidity.
Table 1 shows the occurrence of Crossiella in moonmilks, grey, yellow, pink and white biofilms, and sediments from different caves and subterranean environments. Crossiella is abundant in different types of rocks, either in volcanic (Fuente de la Canaria, Bucara II), karstic (Pindal, Nerja, Castañar, Altamira) or gypsum (Covadura, Yeso) caves.
In addition to the studies in Table 1, authors have reported the occurrence of Crossiella using methodological approaches other than NGS. Stomeo et al. [25] found metabolically active Crossiella in white biofilms from Ardales Cave, Malaga, Spain; Portillo and Gonzalez [26] identified Crossiella as the major metabolically active bacterium in the black crust of a shelter located in Aragon, Spain, and Sanchez-Moral [27] reported Crossiella in Altamira Cave.
Table 2 shows the wide occurrence of Crossiella in caves in the USA, France and China. Less frequent records were found in caves in Italy, Pakistan, Portugal, Serbia and Thailand, among other countries [28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50].
Table 2. Occurrence and relative abundance (>1%) of Crossiella in caves all over the world.
| Karstic Caves | Relative Abundance% | Genus | Type of Sample (Method) | References |
|---|---|---|---|---|
| Heshang | n.d. | Crossiella | Weathered rocks (NGS) | [28] |
| Laugerie-Haute | 4.0 | Crossiella | Salt efflorescences (clones) | [29] |
| Sorcerers | 30.0 | Crossiella | Salt efflorescences (NGS) | [30] |
| Pillier | n.d. | Crossiella | Wall rock (NGS) | [31] |
| Yixing Shanjuan | 3.9 | Crossiella | Speleothem (NGS) | [32] |
| Shuanghe | 9.5 | Crossiella | Rock (NGS) | [33] |
| Manao-Pee | 4.1 | Crossiella | Soil (NGS) | [34] |
| KN14 | 27.1–52.3 | Crossiella | Rock/Clay (NGS) | [35] |
| RN5 | 1.0–17.9 | Crossiella | Rock/Clay/Mud (NGS) | [35] |
| Maijishan Grottoes | n.d. | Crossiella | Walls paintings (NGS) | [36] |
| Heshang | n.d. | Crossiella | Weathered rocks (NGS) | [37] |
| Kashmir and Tiser | 11.9–36.6 | Crossiella | Soil (NGS) | [38] |
| Zhijin | 4.1 | Crossiella | Wall rock (NGS) | [39] |
| Rouffignac | ~70.0 | Crossiella | Wall rock (NGS) | [40] |
| Stiffe | 9.9 | Crossiella | Biofilms (NGS) | [41] |
| Heshang | n.d. | Crossiella | Weathered rocks (NGS) | [42] |
| Cave Church | 0.1–4.9 | Crossiella | Fresco (NGS) | [43] |
| Volcanic Caves | ||||
| Azorean caves | 18.6 | Crossiella | Biofilms (clones) | [44] |
| Hawaiian caves | n.d. | Crossiella | Biofilms (NGS) | [45] |
| Californian caves | n.d. | Crossiella | Biofilms (NGS) | [46] |
| Idahoan caves | n.d. | Crossiella | Biofilms (NGS) | [47] |
| Sicilian caves | 62.5–77.6 | Crossiella | Biofilms (NGS) | [48] |
| Other Cave Types | ||||
| Carlsbad Cavern | n.d. | Crossiella | Rocks (clones) | [49] |
| Imawarì Yeuta | n.d. | Crossiella | Patina/Speleothems (NGS) | [50] |
Apart from the high abundance in Spanish caves, the high relative abundance of Crossiella in Italian caves is also remarkable. In this regard, Nicolosi et al. [48] recorded high relative abundances in four Etna volcano caves. One of them ranged from 62.5 to 77.6%. Other notable abundances were found in the salt efflorescence of a French shelter [30] and in caves in the USA [35], France [40], Pakistan [38], and the Azores, Portugal [44].
References
- Subramani, R.; Aalbersberg, W. Culturable rare Actinomycetes: diversity, isolation and marine natural product discovery. Appl. Microbiol. Biotechnol. 2013, 97, 9291–9321.
- Tiwari, K.; Gupta, R.K. Rare actinomycetes: a potential storehouse for novel antibiotics. Crit. Rev. Biotechnol. 2012, 32, 108–132.
- Tiwari, K.; Gupta, R.K. Diversity and isolation of rare actinomycetes: an overview. Crit. Rev. Microbiol. 2013, 39, 256–294.
- Seong, C.N.; Choi, J.H.; Baik, K-S. An improved selective isolation of rare actinomycetes from forest soil. J. Microbiol. 2001, 39, 17–23.
- Bredholdt, H.; Galatenko, O.A.; Engelhardt, K.; Fjærvik, E.; Terekhova, L.P.; Zotchev, S.B. Rare actinomycete bacteria from the shallow water sediments of the Trondheim Fjord, Norway: isolation, diversity and biological activity. Environ. Microbiol. 2007, 9, 2756–2764..
- Fang, B-Z.; Salam, N.; Han, M-X.; Jiao, J-Y.; Cheng, J.; Wei, D-Q.; Xiao M.; Li, W-J. Insights on the effects of heat pretreatment, pH, and calcium salts on isolation of rare actinobacteria from karstic caves. Front. Microbiol. 2017, 8, 1535.
- Goodfellow, M.; Nouioui, I.; Sanderson, R.; Xie, F.; Bull, A.T. Rare taxa and dark microbial matter: novel bioactive actinobacteria abound in Atacama Desert soils. Anton. Leeuw. 2018, 111, 1315–1332.
- Benhadj, M.; Gacemi-Kirane, D.; Menasria, T.; Guebla, K.; Ahmane, Z. Screening of rare actinomycetes isolated from natural wetland ecosystem (Fetzara Lake, northeastern Algeria) for hydrolytic enzymes and antimicrobial activities. J. King Saud Univ. Sci. 2019, 31, 706–712.
- Zamora-Quintero, A.Y.; Torres-Beltrán, M.; Guillén Matus, D.G.; Oroz-Parra, I.; Millán-Aguiñaga, N. Rare actinobacteria isolated from the hypersaline Ojo de Liebre Lagoon as a source of novel bioactive compounds with biotechnological potential. Microbiology 2022, 168, 001144.
- Tiwari, K.; Upadhyay, D.J.; Mösker, E.; Süssmuth, R.; Gupta, R.K. Culturable bioactive actinomycetes from the Great Indian Thar Desert. Ann. Microbiol. 2015, 65, 1901–1914.
- Mohammadipanah, F.; Wink, J. Actinobacteria from arid and desert habitats: Diversity and biological activity. Front. Microbiol. 2016, 6, 1541.
- Gacem, M.A.; Ould‑El‑Hadj‑Kheli, A.; Abd‑Elsalam, K.A.; Wink, J. Actinobacteria in the Algerian Sahara: Diversity, adaptation mechanism and special unexploited biotopes for the isolation of novel rare taxa. Biologia 2001, 76, 3787–3799.
- Hui, M.L.-Y.; Tan, L.T.-H.; Letchumanan, V.; He, Y.-W.; Fang, C.-M.; Chan, K.-G.; Law, J.W.-F.; Lee, L.-H. The extremophilic Actinobacteria: From microbes to medicine. Antibiotics 2021, 10, 682.
- Gonzalez-Pimentel, J.L.; Dominguez-Moñino, I.; Jurado, V.; Laiz, L.; Caldeira, A.T.; Saiz-Jimenez, C. The rare actinobacterium Crossiella sp. is a potential source of new bioactive compounds with activity against bacteria and fungi. Microorganisms 2022, 10, 1575.
- Gonzalez-Pimentel, J.L.; Martin-Pozas, T.; Jurado, V.; Miller, A.Z.; Caldeira, A.T.; Fernandez-Lorenzo, O.; Sanchez-Moral, S.; Saiz-Jimenez, C. Prokaryotic communities from a lava tube cave in La Palma Island (Spain) are involved in the biogeochemical cycle of major elements. PeerJ 2021, 9, e11386.
- Martin-Pozas, T.; Cuezva, S.; Fernandez-Cortes, A.; Cañaveras, J.C.; Benavente, D.; Jurado, V.; Saiz-Jimenez, C.; Janssens, I.; Seijas, N.; Sanchez-Moral, S. Role of subterranean microbiota in the carbon cycle and greenhouse gas dynamics. Sci. Total Environ. 2022, 831, 154921.
- Cuezva, S.; Martin-Pozas, T.; Fernandez-Cortes, A.; Cañaveras, J.C.; Janssens, I.; Sanchez-Moral, S. 2020. On the role of cave-soil in the carbon cycle. A first approach. EGU General Assembly 2020. Abstract EGU2020-21793.
- Martin-Pozas, T.; Fernandez-Cortes, A.; Cuezva, S.; Cañaveras, J.C.; Benavente, D.; Duarte, E.; Saiz-Jimenez, C.; Sanchez-Moral, S. New insights into the structure, microbial diversity and ecology of yellow biofilms in a Paleolithic rock art cave (Pindal Cave, Asturias, Spain). Sci. Total Environ. 2023 (submitted).
- González-Pimentel, J.L. Microorganismos de las cuevas volcánicas de La Palma (Islas Canarias). Diversidad y potencial uso biotecnológico. Ph D thesis. Universidad Pablo Olavide, Sevilla, Spain, 2019.
- Jurado, V.; del Rosal, Y.; Gonzalez-Pimentel, J.L.; Hermosin, B.; Saiz-Jimenez, C. Biological control of phototrophic biofilms in a show cave: The case of Nerja Cave. Appl. Sci. 2020, 10, 3448.
- Martin-Pozas, T. Papel de los microorganismos en procesos de captación y emisión de gases de efecto invernadero en ambientes subterráneos. Ph D thesis. Universidad Complutense de Madrid, 2023.
- Labeda, D.P. Crossiella gen. nov., a new genus related to Streptoalloteichus. Int. J. Syst. Evol. Microbiol. 2001, 51, 575–579.
- González-Riancho Fernández, C. Análisis descriptivo y funcional de las colonias microbianas visibles que crecen en la cueva de Altamira, enfocado al diseño de medidas de control. Ph D thesis. Universidad de Cantabria, 2021.
- Sánchez-Moral, S.; Martín-Pozas, T., Seijas Morales, N., Fernández-Cortés, A., Benavente García, D., Cañaveras Jiménez, J.C.; Cuezva, S. Instalación de sensores en sótano arqueológico del Museo Carmen Thyssen de Málaga para la toma de datos, el análisis y adopción de medidas correctoras del deterioro del recinto. Unpublished Report MNCN, Madrid, 2021.
- Stomeo, F.; Portillo, M.C.; Gonzalez, J.M.; Laiz, L.; Saiz-Jimenez, C. Pseudonocardia in white colonizations in two caves with Paleolithic paintings. Int. Biodeter. Biodegr. 2008, 62, 483–486.
- Portillo, M.C., Gonzalez, J.M. 2011. Microbial community diversity and the complexity of preserving cultural heritage. In: Charola, A.E., McNamara, C., Koestler, R.J. (Eds.), Biocolonization of Stone: Control and Preventive Methods. Smithsonian Institution, Scholarly Press, Washington, 2011, pp. 19–28.
- Sánchez-Moral, S. Estudio integral del estado de conservación de la cueva de Altamira y su arte paleolítico (2007 - 2009). Perspectivas futuras de conservación. Monografías Nº 24. Museo Nacional y Centro de Investigación de Altamira, 2014.
- Yun, Y.; Wang, H.; Man, B.; Xiang, X.; Zhou, J., Qiu, X.; Duan, Y.; Engel, A.S. The relationship between ph and bacterial communities in a single karst ecosystem and its implication for soil acidification. Front. Microbiol. 2016, 7, 1955.
- Lepinay, C.; Mihajlovski, A.; Seyer, D.; Touron, S.; Bousta, F.; Di Martino. P. Biofilm communities survey at the areas of salt crystallization on the walls of a decorated shelter listed at UNESCO World cultural Heritage. Int. Biodeter. Biodegr. 2017, 122, 116–127.
- Lepinay, C., Mihajlovski, A., Touron, S., Seyer, D., Bousta, F., Di Martino. P. Bacterial diversity associated with saline efflorescences damaging the walls of a French decorated prehistoric cave registered as a World Cultural Heritage Site. Int. Biodeter. Biodegr. 2018, 130, 55–64.
- Alonso, L.; Pommier, T.; Kaufmann, B.; Dubost, A.; Chapulliot, D.; Doré, J.; Douady, C.J.; Moënne-Loccoz, Y. Anthropization level of Lascaux Cave microbiome shown by regional‐scale comparisons of pristine and anthropized caves. Mol. Ecol. 2019, 28, 3383–3394.
- Li, M.; Fang, C.; Kawasaki, S.; Huang, M.; Achal, V. Bio-consolidation of cracks in masonry cement mortars by Acinetobacter sp. SC4 isolated from a karst cave. Int. Biodeter. Biodegr. 2019, 141, 94-100.
- Long, Y.; Jiang, J.; Hu, X.; Zhou, J.; Hu, J.; Zhou, S. Actinobacterial community in Shuanghe Cave using culture-dependent and -independent approaches. World J. Microbiol. Biotechnol. 2019, 35, 153.
- Wiseschart, A.; Mhuantong, W.; Tangphatsornruang, S.; Chantasingh, D.; Pootanakit, K. Shotgun metagenomic sequencing from Manao-Pee cave, Thailand, reveals insight into the microbial community structure and its metabolic potential. BMC Microbiol. 2019, 19, 144.
- Frazier, V.E. Carbon Metabolism in Cave Subaerial Biofilms. Master Thesis, University of Tennessee, USA, 2020.
- He, D.; Wu, F.; Ma, W.; Zhang, Y.; Gu, J-D.; Duan, Y.; Xu, R.; Feng, H.; Wang, W.; Li, S-W. Insights into the bacterial and fungal communities and microbiome that causes a microbe outbreak on ancient wall paintings in the Maijishan Grottoes. Int. Biodeter. Biodegr. 2021, 163, 105250.
- Ma, L.; Huang, X.; Wang, H.; Yun, Y.; Cheng, X.; Liu, D.; Lu, X.; Qiu, X. Microbial interactions drive distinct taxonomic and potential metabolic responses to habitats in karst cave ecosystem. Microbiol. Spect. 2021, 9, e01152-21.
- Zada, S.; Xie, J.; Yang, M.; Yang, X.; Sajjad, W.; Rafiq, M.; Hasan, F.; Hu, Z.; Wang, H. Composition and functional profiles of microbial communities in two geochemically and mineralogically different caves. Appl. Microbiol. Biotechnol. 2021, 105, 8921–8936.
- Ai, J.; Guo, J.; Li, Y.; Zhong, X.; Lv, Y.; Li, J.; Yang, A. The diversity of microbes and prediction of their functions in karst caves under the influence of human tourism activities—a case study of Zhijin Cave in Southwest China. Environ. Sci. Pollut. Res. 2022, 29, 25858–25868.
- Buresova-Faitova, A.; Kopecky, J.; Sagova-Mareckova, M.; Alonso, L.; Vautrin, F.; Moënne-Loccoz, Y.; Rodriguez-Nava, V. Comparison of Actinobacteria communities from human‐impacted and pristine karst caves. MicrobiologyOpen 2022, 11, e1276.
- Djebaili, R., Mignini, A., Vaccarelli, I., Pellegrini, M., Spera, D.M., Del Gallo, M., D’Alessandro, A.M. Polyhydroxybutyrate-producing cyanobacteria from lampenflora: The case study of the “Stiffe” caves in Italy. Front. Microbiol. 2022, 13, 933398.
- Cheng, X.; Xiang, X.; Yun, Y.; Wang, W.; Wang, H.; Bodelier P.L.E. Archaea and their interactions with bacteria in a karst ecosystem. Front. Microbiol. 2023, 14, 1068595.
- Dimkic, I.; Copic, M.; Petrovic, M.; Stupar, M.; Savkovic, Ž.; Kneževic, A.; Simic, G.S.; Grbic, M.L.; Unkovic, N. Bacteriobiota of the cave church of Sts. Peter and Paul in Serbia - Culturable and non-culturable communities’ assessment in the bioconservation potential of a peculiar fresco painting. Int. J. Mol. Sci. 2023, 24, 1016.
- Riquelme, C.; Rigal, F.; Hathaway, J.J.M.; Northup, D.E.; Spilde, M.N.; Borges, P.A.V.; Gabriel, R.; Amorin, I.R.; Dapkevicius, M.L.N.E. Cave microbial community composition in oceanic islands: disentangling the effect of different colored mats in diversity patterns of Azorean lava caves. FEMS Microbiol. Ecol. 2015, 91, fiv141.
- Spilde, M.N.; Northup, D.E.; Caimi, N.A.; Boston, P.J.; Stone, F.D.; Smith, S. Microbial mat communities in Hawaiian lava caves. Int. Symp. on Vulcanospeleology 2016, pp. 1–7.
- Lavoie, K.H.; Winter, A.S.; Read, K.J.H.; Hughes, E.M.; Spilde, M.N.; Northup, D.E. Comparison of bacterial communities from lava cave microbial mats to overlying surface soils from Lava Beds National Monument, USA. PLoS ONE 2017, 12, e0169339.
- Weng, M.M.; Zaikova, E.; Millan, M.; Williams, A.J.; McAdam, A.C.; Knudson, C.A.; Fuqua, S.R.; Wagner, N.Y.; Craft, K.; Nawotniak, S.K., et al. Life underground: Investigating microbial communities and their biomarkers in Mars-analog lava tubes at Craters of the Moon National Monument and Preserve. J. Geophys. Res. Planets 2022, 127, e2022JE007268.
- Nicolosi, G.; Gonzalez-Pimentel, J.L.; Piano, E.; Isaia, M.; Miller, A.Z. First insights into the bacterial diversity of Mount Etna volcanic caves. Microb. Ecol. 2023. https://doi.org/10.1007/s00248-023-02181-2.
- Barton H.A.; Taylor N M.; Kreate M.P.; Springer A.C.; Oehrle S.A.; Bertog J.L. 2007. The impact of host rock geochemistry on bacterial community structure in oligotrophic cave environments. Int. J. Speleol. 2007, 36, 93-104.
- Ghezzi, D.; Sauro, F.; Columbu, A.; Carbone, C.; Hong, P.-Y.; Vergara, F.; De Waele, J.; Cappelletti, M. Transition from unclassified Ktedonobacterales to Actinobacteria during amorphous silica precipitation in a quartzite cave environment. Sci. Rep. 2021, 11, 3921.




